Abstract
Traumatic brain injury (TBI) is followed by an energy crisis that compromises the capacity of the brain to cope with challenges, and often reduces cognitive ability. New research indicates that events that regulate energy homeostasis crucially impact synaptic function and this can compromise the capacity of the brain to respond to challenges during the acute and chronic phases of TBI. The goal of the present study is to determine the influence of the phenolic yellow curry pigment curcumin on molecular systems involved with the monitoring, balance, and transduction of cellular energy, in the hippocampus of animals exposed to mild fluid percussion injury (FPI). Young adult rats were exposed to a regular diet (RD) without or with 500 ppm curcumin (Cur) for four weeks, before an FPI was performed. The rats were assigned to four groups: RD/Sham, Cur/Sham, RD/FPI, and Cur/FPI. We found that FPI decreased the levels of AMP-activated protein kinase (AMPK), ubiquitous mitochondrial creatine kinase (uMtCK) and cytochrome c oxidase II (COX-II) in RD/FPI rats as compared to the RD/sham rats. The curcumin diet counteracted the effects of FPI and elevated the levels of AMPK, uMtCK, COX-II in Cur/FPI rats as compared to RD/sham rats. In addition, in the Cur/sham rats, AMPK and uMtCK increased compared to the RD/sham. Results show the potential of curcumin to regulate molecules involved in energy homeostasis following TBI. These studies may foster a new line of therapeutic treatments for TBI patients by endogenous upregulation of molecules important for functional recovery.
Keywords: curcumin, energy homeostasis, traumatic brain injury
Traumatic brain injury (TBI) is associated with costly health problems and results in impairment of cognitive abilities. TBI involves large transient increases in excitatory neurotransmitter efflux (Biegon, 2004) resulting in excitotoxicity, ionic imbalance, ATP depletion, proteolysis, and oxidative stress (Ansari et al., 2008). TBI is followed by an energy crisis that compromises the capacity of the brain to cope with challenges, and often reduces cognitive ability. Energy homeostasis is fundamental for the survival of both the cell and the organism. New research indicates that events that regulate energy homeostasis have crucial impact on synaptic function, and that this can compromise the capacity of the brain to respond to challenges during the acute and chronic phases of TBI.
The significance of Curcuma longa Linn (turmeric) in health and nutrition has changed considerably since the discovery of the antioxidant property of naturally occurring phenolic compounds, curcuminoids. Curcumin has numerous pharmacological activities like anti-inflammatory activity, antiprotozoal activity, nematocidal activity, antibacterial activity, anti-mutagenic activity and hepatoprotective activity. A single epidemiological study suggested that curcumin, as one of the most prevalent nutritional and medicinal compounds used by the Indian population, is responsible for the reduced (4.4-fold) prevalence of Alzheimer's disease (AD) in India compared to United States (Ganguli et al., 2000). Studies of animal models have also suggested that curcumin may be beneficial in neurodegenerative conditions such as AD (Lim et al., 2001; Yang et al., 2005; Begum et al., 2008) and focal cerebral ischemia (Thiyagarajan and Sharma, 2004; Zhao et al., 2008). Recent finding suggests that curcumin stimulates proliferation of embryonic progenitor cells and neurogenesis in the adult hippocampus (Kim et al., 2008). In addition, the curcumin treatment can protect hippocampal neurons against excitotoxic and traumatic injury (Sumanont et al., 2006; Wu et al., 2006).
Our objective is to evaluate the impact of TBI on molecular systems associated with energy metabolism and cognition, and the possibility to reduce energy dysfunction following TBI. The goal of the present study is to determine the influence of curcumin on molecular systems involved with the monitoring, balance, and transduction of cellular energy, in the hippocampus of animals exposed to mild fluid percussion injury (FPI). AMP-activated protein kinase (AMPK) is a serine–threonine kinase, which is described as a “fuel gauge” for cellular metabolism due its ability to sense low energy levels and activate or inhibit the appropriate molecules to reestablish energy balance of the cell. The ubiquitous mitochondrial creatine kinase (uMtCK) is involved in energy maintenance and transduction (Friedman and Roberts, 1994; Chen et al., 1995) and may function to modulate aspects of cognition (Wu et al., 2007; Gomez-Pinilla et al., 2008).
The mitochondrial uncoupling protein 2 (UCP2) regulates energy metabolism via its ability to uncouple mitochondrial electron transport from ATP synthesis by permitting a proton leak across the mitochondrial membrane (Cheng et al., 2003; Kim-Han and Dugan, 2005). Cytochrome c oxidase II (COX-II) is a mitochondrial protein that can provide an index of mitochondrial function/mass. COX-II plays an important role in mitochondrial oxidative phosphorylation (Hüttemann et al., 2008). The silent information regulator 2 (Sir2) has surfaced as an important modulator of genomic stability and cellular homeostasis. The function of Sir2 in cellular metabolic homeostasis (Lin et al., 2000, 2004; Rogina and Helfand, 2004) has been shown to be significant for promoting longevity in yeasts, nematodes and flies. Seven mammalian Sir2 homologs have been identified, named SIRTs 1–7 (Cheng et al., 2003) in which SIRT1, also known as Sir2α, is the closest homolog of yeast Sir2 (Hisahara et al., 2005). Recent studies indicate that the actions of Sir2α on cellular homeostasis can be extended to the maintenance of proper brain function.
Experimental Procedures
The experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were continually monitored and all procedures were approved by the UCLA Chancellor's Animal Research Committee. The suffering and number of animals used were minimized.
Experimental design and tissue preparation
Male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA, USA) approximately 2 months old were housed in cages (two rats per cage) and maintained in environmentally controlled rooms (22–24 °C) with a 12-h light/dark cycle. After acclimatization for 1 week on standard rat chow, one set of rats was randomly assigned to regular diet without or with curcumin (500 ppm) for 4 weeks. This dose of curcumin has been shown to prevent cognitive deficit in animal models of AD (Frautschy et al., 2001). After 4 weeks on the various diets, a mild FPI was performed in approximately half of the rats. After 1 week of being maintained on the same diet post-injury, rats (n=5–8 per group, total 29) were killed by decapitation, and the brain was rapidly dissected, frozen on dry ice, and stored at −70 °C until use for analyses. By the end of the experiment, there were four experimental groups: (I) regular diet plus sham (RD/sham); (II) regular diet plus fluid percussion injury (RD/FPI); (III) curcumin plus sham (Cur/sham); (IV) curcumin plus fluid percussion injury (Cur/FPI). There was no group difference in all groups on body weight and food intake between sham and FPI conditions at the end of experiment. The suffering and number of animals used were minimized.
FPI
The injury was performed as previously described (Wu et al., 2003). In brief, with the aid of a microscope (Wild, Heerburg, Switzerland), a 3.0-mm diameter craniotomy was made 3.0 mm posterior to bregma and 6.0 mm lateral (left) to the midline with a high-speed drill (Dremel, Racine, WI, USA). A plastic injury cap was placed over the craniotomy with silicone-adhesive and dental cement. When the dental cement hardened, the cap was filled with 0.9% saline solution. Anesthesia was discontinued, and the injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a mild fluid percussion pulse (1.5 atm.) was administered. Sham animals underwent an identical preparation with the exception of the lesion. Immediately upon responding to a paw pinch, anesthesia was restored, and the skull was sutured.
Neomycin was applied on the suture, and the rats were placed in a heated recovery chamber for approximately an hour before being returned to their cages.
Tissue preparation and protein determination
The entire hippocampus on the FPI-treated side of the brain was collected upon decapitation for protein determination. Tissue was collected into 1.5-mL microfuge tubes, immediately frozen on dry ice and stored at −70 °C. Hippocampi ipsilateral to the craniotomy were homogenized in a freshly prepared lysis buffer (NaCl, 137 mm; Tris–HCl pH 8.0, 20 mm; NP-40, 1%; glycerol, 10%; phenylmethylsulphonyl fluoride, 1 mm; aprotinin, 10 μg/mL; leupeptin, 1 μg/mL; and sodium vanadate, 0.5 mm). Homogenates were centrifuged at 12,000×g for 20 min to remove insoluble material. The supernatants were collected into clean 1.5-mL tubes, frozen on dry ice and stored at −70 °C. The total protein concentration of hippocampal homogenates was determined with a MicroBCA kit (Pierce, Rockford, IL, USA), using BSA as a standard.
Western blot
Total proteins from hippocampal tissue were extracted as described above. Equal amounts (25 μg) of protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA). Nonspecific binding sites were blocked with 5% nonfat milk in TBS buffer with 0.1% Tween-20 (pH 7.6). Membranes were incubated with the following primary antibodies: anti-pAMPK (1:1000; Cell Signaling), anti-AMPK (1:1000; Santa Cruz Biotechnology), anti-uMtCK (1:1000; Santa Cruz Biotechnology), anti-UCP-2 (1:1000; Santa Cruz Biotechnology), anti-COX-II (1:1000; Santa Cruz Biotechnology), anti-Sir2 (1:100,000; Upstate, Millipore), and anti-actin (1:2000; Santa Cruz Biotechnology) followed by anti-goat or antirabbit IgG horseradish peroxidase conjugate. After rinsing with buffer (0.1% Tween-20 in TBS), the immunocomplexes were visualized by chemiluminescence using the Amersham ECL Plus Western Blotting Detection kit (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) according to the manufacturer's instructions. The film signals were digitally scanned using an HP Scanner (HP Scanjet 3970) and quantified with NIH Image software, normalized for actin levels.
Statistical analysis
Actin was employed as internal standard for Western blot. The regular diet-fed rats with sham surgery were regarded as experimental controls for comparisons with other experimental groups. For Western blot, the values were expressed as a ratio of actin value and then converted to percent of RD/sham group as presented in bar figures and represented the mean±SEM. All statistical analyses were performed by commercial software SPSS 16.0. A level of 5% probability was considered significant. The data were analyzed by ANOVA and post hoc analyses were conducted using Bonferroni comparisons.
Immunohistochemical localization of metabolic proteins in hippocampus
Additional rats (n=4 from each group) from RD/sham, RD/FPI, and Cur/FPI were anesthetized with isoflurane, then intracardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) and 20% sucrose in 4% paraformaldehyde. Serial coronal brain sections (25 μm) were cut on a cryostat, collected free-floating in PBS. Tissue sections were processed for localization of AMPK according to immunohistochemical procedures described previously (Wu et al., 2003). Negative controls were performed by omission of the primary antibody. The results of immunohistochemistry controls were negative as no staining was observed in cell structures. Analysis was restricted to the hippocampus based on our previous studies showing significant effects of TBI on hippocampal plasticity (Wu et al., 2003; Vaynman et al., 2004). Previous studies from our laboratory have shown that Fluoro-Jade B staining revealed no Fluoro-Jade B-labeled cells in the hippocampus of injured animals (Wu et al., 2003, 2006).
Results
Effect of curcumin on expression of AMPK in the hippocampus
We examined the levels of molecules associated with energy regulation in the hippocampus. AMPK is activated in response to ATP deletion, which has led to the idea that AMPK provides a good estimate of cellular energy (Fryer et al., 2002). The response of AMPK to changes in neuronal energy status and metabolic stress makes the pAMPK/AMPK ratio a reasonable readout of neuronal responsiveness to changes in energy status (Kleman et al., 2008). Curcumin increased the ratio of pAMPK/AMPK in Cur/sham rats to 158% as compared to RD/sham control rats (P<0.01). FPI significantly decreased the levels of pAMPK/AMPK ratio in RD/FPI rats as compared to RD/sham rats, whereas curcumin significantly enhanced the levels of pAMPK/AMPK ratio in Cur/FPI rats as compared to RD/FPI rats (Fig. 1A, B). In the present study we found that immunoreactivity with the AMPK was particularly high in pyramidal neurons in the hippocampus (Fig. 1C, D). Further, immunoreactivity with the AMPK appeared to be localized in the cytoplasm, with faint immunoreactivity around the nucleus as well. This could be due to the fact that we used AMPK antibody that recognizes both α1 and α2 subunits which have been shown to have cytoplasmic and nuclear localization (Culmsee et al., 2001). Immunohistochemical staining for AMPK showed a qualitative decrease in immunoreactivity in pyramidal neurons of CA3 regions of the hippocampus after FPI in RD/FPI rats as compared to RD/Sham rats, whereas curcumin dietary supplementation reduced this decline in immunoreactivity after FPI as shown in Cur/FPI group (Fig. 1D).
Fig. 1.
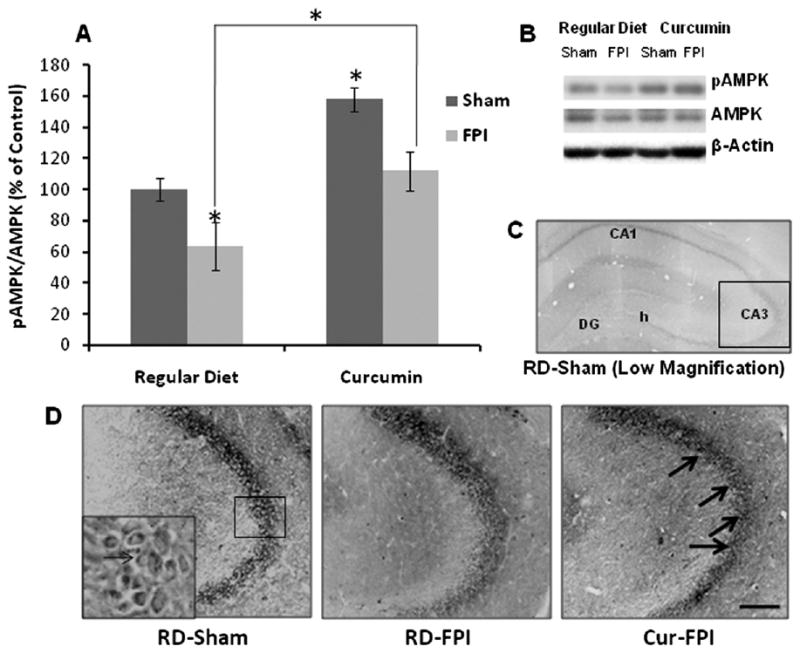
(A) Ratio of pAMPK/AMPK in rat hippocampus revealed by Western blot analysis in all the groups. (B) Representative Western blots for pAMPK, AMPK and actin from different groups in hippocampus. The ratio of pAMPK/AMPK was significantly reduced after FPI, whereas dietary curcumin (Cur) supplementation significantly increased the ratio of pAMPK/AMPK in TBI rats. The values were converted to percent of RD sham group (mean±SEM). * P<0.05, ANOVA followed by post hoc tests with Bonferroni's comparisons. RD, regular diet. (C, D) Immunohistochemical localization of AMPK distribution in the hippocampus. TBI resulted in reduced immunostaining of AMPK in pyramidal neurons of hippocampus, but dietary Cur could counteract the effect of TBI, preserving the expression of these molecules in hippocampus. Scale bars=100 μm.
Effect of curcumin on expression of uMtCK, UCP2, COX-II and SIR2 in the hippocampus
Recent findings indicate that the creatine kinase uMtCK is involved in energy transduction that may support higher order brain functions such as learning and memory (Boero et al., 2003). Curcumin enhanced the levels of uMtCK to 144% in Cur/sham rats as compared to RD/sham control rats (P<0.05; Fig. 2A). Results indicate that FPI decreased the levels of uMtCK to 79% in RD/FPI rats as compared to RD/sham rats (P<0.05). The levels of uMtCK were significantly higher in the Cur/FPI group as compared to the RD/FPI group (P<0.01). Curcumin counteracted the effect of FPI by maintaining the levels of uMtCK to 130% in Cur/FPI as compared to RD/sham control rats (Fig. 2A). Hippocampal pyramidal neurons contain uMtCK-positive mitochondria at birth, but this pattern becomes progressively restricted to interneurons (Boero et al., 2003). FPI seems to downregulate the uMtCK expression in RD/FPI rats, whereas curcumin counteracts the effect of FPI as shown in Cur/FPI rats (data not shown).
Fig. 2.
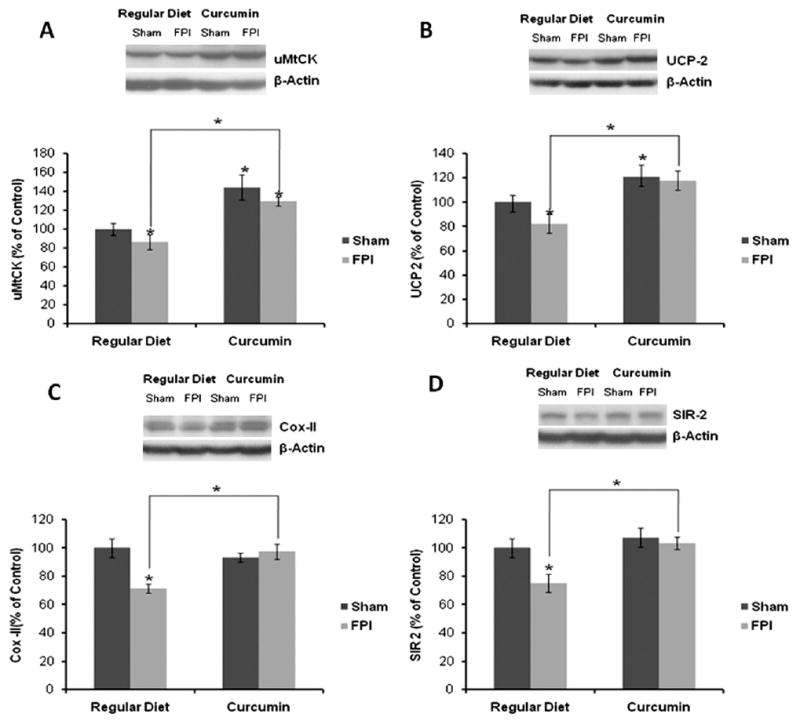
(A–D) Expression of (A) uMtCK, (B) UCP2, (C) COX-II and (D) Sir 2 in rat hippocampus revealed by Western blot analysis. Expression of these molecular systems associated with energy homeostasis was significantly reduced after FPI, whereas dietary curcumin (Cur) supplementation significantly increased their levels in TBI rats. The values were converted to percent of RD sham group (mean±SEM). * P<0.05, ANOVA followed by post hoc tests with Bonferroni's comparisons. RD, regular diet.
Further, UCP2 has been shown to play a role in energy metabolism and may interact with the substrates for hippocampal synaptic plasticity (Vaynman et al., 2006). Curcumin increased the levels of UCP2 to 121% in Cur/sham rats, whereas FPI decreased the levels of UCP2 to 76% in RD/FPI rats as compared to RD/sham rats (P<0.01; Fig. 2B). The levels of UCP2 were significantly higher in the Cur/FPI group as compared to the RD/FPI group. Curcumin counteracted the effects of FPI and upregulated the levels of UCP2 to 119% in Cur/FPI as compared to RD/FPI control rats (P<0.05; Fig. 2B).
COX-II is a mitochondrial protein that can provide an index of mitochondrial function/mass and alterations in its levels have been shown to affect synaptic plasticity (Vaynman et al., 2006). In the present study, we found that FPI resulted in decrease in COX-II levels to 69% in RD/FPI rats as compared to RD/sham rats (Fig. 2C). The levels of COX-II were significantly higher in the Cur/FPI group as compared to the RD/FPI group. Curcumin normalized the levels of COX-II to 96% in Cur/sham rats as compared to RD/FPI control rats (P<0.01; Fig. 2C). A similar increase in immunostaining of COX-II is observed in Cur/FPI rats in hippocampus as compared to RD/FPI rats (data not shown). In addition, results showing a significant effect of curcumin on cognitive status of rats as revealed by the Morris water maze have been published previously from our laboratory (Wu et al., 2006).
Given the association of Sir2 with energy metabolism, we also examined the Sir2 levels in the present paradigm. We found no difference in levels of Sir2 in Cur/sham rats as compared to RD/sham control rats (Fig. 2D). FPI significantly decreased the levels of Sir2 to 71% in RD/FPI rats as compared to RD/sham rats (P<0.01; Fig. 2D). Curcumin efficiently counteracted the effects of FPI by normalizing the levels of Sir2 to 105% in Cur/FPI rats as compared to RD/FPI rats (P<0.01; Fig. 2D).
Discussion
The objective of the present study was to evaluate the impact of TBI on molecular systems associated with energy metabolism and the possibility to reduce energy dysfunction following TBI. The results show the potential of curcumin to restore the levels of proteins involved in energy homeostasis following TBI. Dietary curcumin is a non-invasive strategy that can be used during the management of TBI patients with the potential to promote functional restoration. These studies may foster a new line of therapeutic treatments for TBI patients by endogenous upregulation of molecules important for functional recovery.
We found that FPI markedly decreased the expression of molecular systems such as AMPK, uMtCK, UCP2 and COX-II which are associated with energy regulation. It is well established that a TBI results in an increased energy demand due to disturbance of ion homeostasis (Nilsson et al., 1993; Osteen et al., 2001), and activation of energy consuming repair processes (Laplaca et al., 2001). This is reflected by increased glucose utilization in the immediate post-injury phase observed in both experimental and clinical TBI (Ginsberg et al., 1997; Marklund et al., 2001). In addition, seizure activity (Nelson and Silverstein, 1994), release of ATP from presynaptic terminals (North and Verkhratsky, 2006), and mitochondrial dysfunction (Xiong et al., 1997; Marklund et al., 2001; Lifshitz et al., 2004) may contribute to a disturbed balance between energy demand and supply. Previous studies from our laboratory and others have indicated beneficial effect of curcumin in various brain diseases including TBI (Wu et al., 2006) and AD (Frautschy et al., 2001; Begum et al., 2008). These studies led us to hypothesize that the beneficial effect of curcumin in these brain injuries could be due to the capacity of curcumin to regulate brain energy homeostasis.
In the present study TBI resulted in a decrease in AMPK levels, consistent with a disruption of energy homeostasis. The rise in AMPK levels with curcumin observed in the present study suggests that curcumin may activate mechanisms to conserve ATP levels in the hippocampus, with subsequent effects on the modulation of intracellular processes. In addition to the AMPK control of energy through phosphorylation of metabolic enzymes, AMPK activation regulates numerous transcription factors (Bronner et al., 2004; Hardie, 2004) that may link intracellular energy levels with protein synthesis (Jones et al., 2005). During food deprivation, an increase in the cellular AMP/ATP ratio results in activation of AMPK that initiates a signaling process that recruits mediators of fatty acid-dependent oxidative metabolism and mitochondrial biogenesis including PGC-1α, PPARδ and others (de Lange et al., 2006). It has been shown that in conditions of mild energy restriction, AMPK activity in the hippocampus works to improve cognitive function (Dagon et al., 2005). Accordingly, it is possible that our dietary curcumin regimen may engage AMPK to protect learning and memory performance following the mild energy depletion state associated with TBI.
Under normal conditions, the brain, which constitutes only about 2% of the body mass, may spend up to 20% of the body's energy consumption. UMtCK is highly expressed in hippocampal granule and pyramidal cells (Eppenberger et al., 1967), and its regulation by neural activity may provide a mechanism to protect neurons during periods of increased energy demand (Boero et al., 2003). UMtCK is functionally coupled to oxidative phosphorylation, as mitochondrial-derived ATP is preferentially used by uMtCK to transfer high energy phosphates to creatine (Jacobus and Lehninger, 1973; Rojo et al., 1991). The resultant phosphocreatine acts as the cytosolic transport and storage form of energy; it is used to regenerate ATP from cytosolic ADP, which is essential for maintaining the ATP supply during high synaptic transmission (Whittingham and Lipton, 1981). In our study dietary curcumin restored the decreased levels of uMtCK after TBI, thus providing a mechanism of energy transduction in which ATP synthesized in the mitochondrion is transferred to sites of ATP utilization.
Uncoupling proteins (UCPs; thermogenins) are encoded by nuclear DNA and are located in the inner membrane of the mitochondria. Under normal circumstances, UCP2 is expressed predominantly in neurons in several brain regions in both rodents and primates (Horvath et al., 1999; Arsenijevic et al., 2007), although microglia, resident monocytes of the brain (Bechmann et al., 2002), invading monocytes and neutrophils (Arsenijevic et al., 2007), and cells of the choroid plexus (Richard et al., 1999) also express this mitochondrial uncoupling protein. High levels of UCP2 expression have been shown to protect neonatal neurons from excitotoxic cell death by inhibiting reactive oxygen species production and preventing mitochondrial dysfunction (Sullivan et al., 2003). Our study indicated a decline in UCP2 levels in hippocampus after TBI, and dietary curcumin significantly enhanced the levels of UCP2 after TBI. The primary physiologic and pathophysiologic functions of UCP2 are probably related to regulation of ATP synthesis and ATP/ADP ratio (Brand et al., 1994), the regulation of glucose and fatty acid metabolism and fatty acid anion export (Garlid et al., 1996), and ROS production (Negre-Salvayre et al., 1997; Arsenijevic et al., 2000; Sullivan et al., 2003). Recent studies indicate that UCP2 is neuroprotective after excitotoxic neuronal death (Sullivan et al., 2003), TBI (Mattiasson et al., 2003), and Parkinson's disease (Andrews et al., 2005).
In the present study we observed a significant decline in COX-II levels after TBI and dietary supplementation maintained the levels of COX-II after TBI. Studies have provided evidence for reduced activity of the cytochrome oxidase/complex IV of the electron transport chain, not only following TBI (Hovda et al., 1991), but also with such injuries as stroke (Nelson and Silverstein, 1994). COX-II mRNA has been demonstrated to be selectively vulnerable to TBI (Harris et al., 2001; Dai et al., 2009). Sir2 is a NAD+ dependent deacetylase that can act to deacetylate and thereby increase its activity (Nisoli and Carruba, 2006). A previous study from our laboratory has provided novel evidence showing that mild TBI reduces the expression of Sir2 in the hippocampus, in proportion to increased levels of protein oxidation (Wu et al., 2007). Sir2 has been implicated in the regulation of transcription factors including FOXO and PGC-1α that control energy homeostasis. Our current findings indicate that TBI reduces the expression of Sir2, in conjunction with levels of energy metabolic markers in the hippocampus. Dietary supplementation of curcumin counteracted the effects of TBI on reducing the levels of Sir2 and energy metabolic markers.
Our previous findings that supplementation of curcumin normalizes the protein levels of BDNF after TBI suggest that BDNF mediates the beneficial effects of curcumin on cognitive function (Wu et al., 2006). We have recently shown that TBI impairs cognitive function and that this seems to be associated with low levels of BDNF and of its downstream effectors in synaptic plasticity synapsin I and CREB (Wu et al., 2003). Here we provided evidence supporting the harmful impact of TBI on molecular systems involved in energy homeostasis. Possible mechanisms underlying the beneficial effects of curcumin dietary supplementation on the injured brain could be due to its ability to regulate the mitochondrial proteins involved in the energy homeostasis as indicated by levels of molecules like AMPK, uMtCK, UCP2, and COX-II (Fig. 3). Our findings suggest that curcumin supplementation might be an effective therapy to counteract the deleterious effects of TBI on energy homeostasis and cognitive function. Dietary curcumin is a non-invasive strategy that can be used during the management of TBI patients with the potential to promote functional restoration. There is a need to do more mechanistic and interventional studies to evaluate the role of curcumin in clinical brain disorders and injuries.
Fig. 3.
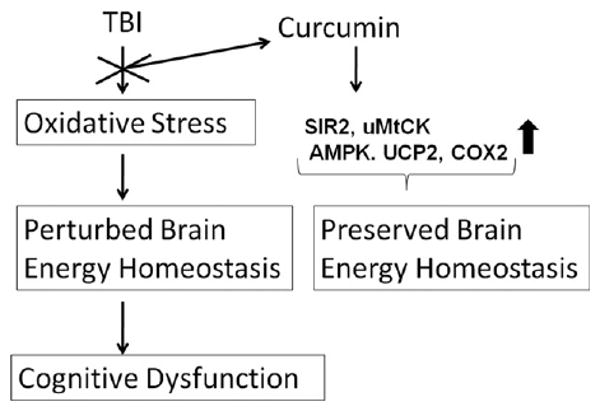
Possible mechanisms underlying the beneficial effects of curcumin dietary supplementation on the injured brain. TBI may lead to disturbances in energy homeostasis and mitochondrial dysfunction resulting in neurodegeneration and altered synaptic plasticity, which could further lead to cognitive dysfunction. On the other hand, the beneficial effect of curcumin dietary supplementation could be due to its ability to restore the energy homeostasis as indicated by levels of molecules like AMPK, uMtCK, UCP2, COX-II and SIR2.
Acknowledgments
Studies were supported by funds related to awards covered under National Institutes of Health, NS50465. We are also thankful to the Brain Injury Research Center, UCLA for their cooperation.
Abbreviations
- AD
Alzheimer's disease
- AMPK
AMP-activated protein kinase
- COX-II
cytochrome c oxidase II
- Cur/FPI
curcumin plus fluid percussion injury
- Cur/sham
curcumin plus sham
- FPI
fluid percussion injury
- RD/FPI
regular diet plus fluid percussion injury
- RD/sham
regular diet plus sham
- Sir2
silent information regulator 2
- TBI
traumatic brain injury
- UCP2
uncoupling protein 2
- uMtCK
ubiquitous mitochondrial creatine kinase
References
- Andrews ZB, Horvath B, Barnstable CJ, Elseworth J, Yang L, Beal MF, Roth RH, Matthews RT, Horvath TL. Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson's disease. J Neurosci. 2005;25:184–191. doi: 10.1523/JNEUROSCI.4269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic D, Clavel S, Sanchis D, Plamondon J, Huang Q, Ricquier D, Rouger L, Richard D. Induction of Ucp2 expression in brain phagocytes and neurons following murine toxoplasmosis: an essential role of IFN-[gamma] and an association with negative energy balance. J Neuroimmunol. 2007;186:121–132. doi: 10.1016/j.jneuroim.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Diano S, Warden CH, Bartfai T, Nitsch R, Horvath TL. Brain mitochondrial uncoupling protein 2 (UCP2): a protective stress signal in neuronal injury. Biochem Pharmacol. 2002;64:363–367. doi: 10.1016/s0006-2952(02)01166-8. [DOI] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A. Cannabinoids as neuroprotective agents in traumatic brain injury. Curr Pharm Des. 2004;10:2177–2183. doi: 10.2174/1381612043384196. [DOI] [PubMed] [Google Scholar]
- Boero J, Qin W, Cheng J, Woolsey TA, Strauss AW, Khuchua Z. Restricted neuronal expression of ubiquitous mitochondrial creatine kinase: changing patterns in development and with increased activity. Mol Cell Biochem. 2003;244:69–76. [PubMed] [Google Scholar]
- Brand M, Chien L, Ainscow E, Rolfe D, Porter R. The causes and functions of mitochondrial proton leak. Biochim Biophys Acta. 1994;1187:132–139. doi: 10.1016/0005-2728(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Bronner M, Hertz R, Bar-Tana J. Kinase-independent transcriptional co-activation of peroxisome proliferator-activated receptor alpha by AMP-activated protein kinase. Biochem J. 2004;384:295–305. doi: 10.1042/BJ20040955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Roberts R, Friedman D. Expression of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase in the fetal rat brain: evidence for a nuclear energy shuttle. J Comp Neurol. 1995;363:389–401. doi: 10.1002/cne.903630305. [DOI] [PubMed] [Google Scholar]
- Cheng G, Polito CC, Haines JK, Shafizadeh SF, Fiorini RN, Zhou X, Schmidt MG, Chavin KD. Decrease of intracellular ATP content downregulated UCP2 expression in mouse hepatocytes. Biochem Biophys Res Commun. 2003;308:573–580. doi: 10.1016/s0006-291x(03)01409-8. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp B, Mattson M. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM. Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem. 2005;280:42142–42148. doi: 10.1074/jbc.M507607200. [DOI] [PubMed] [Google Scholar]
- Dai W, Cheng HL, Huang RQ, Zhuang Z, Shi JX. Quantitative detection of the expression of mitochondrial cytochrome c oxidase subunits mRNA in the cerebral cortex after experimental traumatic brain injury. Brain Res. 2009;1251:287–295. doi: 10.1016/j.brainres.2008.11.034. [DOI] [PubMed] [Google Scholar]
- de Lange P, Farina P, Moreno M, Ragni M, Lombardi A, Silvestri E, Burrone L, Lanni A, Goglia F. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. FASEB J. 2006;20:2579–2581. doi: 10.1096/fj.06-6025fje. [DOI] [PubMed] [Google Scholar]
- Eppenberger M, Eppenberger H, Kaplan N. Evolution of creatine kinase. Nature. 1967;214:239–241. doi: 10.1038/214239a0. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of A[beta]-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Friedman D, Roberts R. Compartmentation of brain-type creatine kinase and ubiquitous mitochondrial creatine kinase in neurons: evidence for a creatine phosphate energy shuttle in adult rat brain. J Comp Neurol. 1994;343:500–511. doi: 10.1002/cne.903430311. [DOI] [PubMed] [Google Scholar]
- Fryer LGD, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, Juyal RC, Pandav R, Belle SH, DeKosky ST. Apolipoprotein E polymorphism and Alzheimer's disease: the Indo-US Cross-National Dementia Study. Arch Neurol. 2000;57:824–830. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Orosz DE, Modrianskýy M, Vassanelli S, Jezek P. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J Biol Chem. 1996;271:2615–2620. doi: 10.1074/jbc.271.5.2615. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Zhao W, Alonso OF, Loor-Estades JY, Dietrich WD, Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol Heart Circ Physiol. 1997;272:H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Harris LK, Black RT, Golden KM, Reeves TM, Povlishock JT, Phillips LL. Traumatic brain injury-induced changes in gene expression and functional activity of mitochondrial cytochrome c oxidase. J Neurotrauma. 2001;18:993–1009. doi: 10.1089/08977150152693692. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Chiba S, Matsumoto H, Horio Y. Transcriptional regulation of neuronal genes and its effect on neural functions: NAD-dependent histone deacetylase SIRT1 (Sir2alpha) J Pharmacol Sci. 2005;98:200–204. doi: 10.1254/jphs.fmj05001x2. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Warden CH, Hajos M, Lombardi A, Goglia F, Diano S. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J Neurosci. 1999;19:10417–10427. doi: 10.1523/JNEUROSCI.19-23-10417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- Hüttemann M, Lee I, Kreipke CW, Petrov T. Suppression of the inducible form of nitric oxide synthase prior to traumatic brain injury improves cytochrome c oxidase activity and normalizes cellular energy levels. Neuroscience. 2008;151:148–154. doi: 10.1016/j.neuroscience.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Jacobus WE, Lehninger AL. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973;248:4803–4810. [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han J, Dugan L. Mitochondrial uncoupling proteins in the central nervous system. Antioxid Redox Signal. 2005;7:1173–1181. doi: 10.1089/ars.2005.7.1173. [DOI] [PubMed] [Google Scholar]
- Kleman AM, Yuan JY, Aja S, Ronnett GV, Landree LE. Physiological glucose is critical for optimized neuronal viability and AMPK responsiveness in vitro. J Neurosci Methods. 2008;167:292–301. doi: 10.1016/j.jneumeth.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaca M, Zhang J, Raghupathi R, Li JH, Smith F, Bareyre F, Snyder S, Graham D, McIntosh T. Pharmacologic inhibition of poly(ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. J Neurotrauma. 2001;18:369–376. doi: 10.1089/089771501750170912. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin S, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund N, Clausen F, McIntosh T, Hillered L. Free radical scavenger posttreatment improves functional and morphological outcome after fluid percussion injury in the rat. J Neurotrauma. 2001;18:821–832. doi: 10.1089/089771501316919184. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- Nelson C, Silverstein F. Acute disruption of cytochrome oxidase activity in brain in a perinatal rat stroke model. Pediatr Rev. 1994;36:9–12. doi: 10.1203/00006450-199407001-00003. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Olsson Y, Sheardown M, Hansen A. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- North R, Verkhratsky A. Purinergic transmission in the central nervous system. Pflügers Arch Eur J Physiol. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Osteen C, Moore A, Prins M, Hovda D. Age-dependency of 45Calcium accumulation following lateral fluid percussion: acute and delayed patterns. J Neurotrauma. 2001;18:141–162. doi: 10.1089/08977150150502587. [DOI] [PubMed] [Google Scholar]
- Richard D, Huang Q, Sanchis D, Ricquier D. Brain distribution of UCP2 mRNA: in situ hybridization histochemistry studies. Int J Obes Relat Metab Disord. 1999;23:S53–S55. doi: 10.1038/sj.ijo.0800947. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo M, Hovius R, Demel RA, Nicolay K, Wallimann T. Mitochondrial creatine kinase mediates contact formation between mitochondrial membranes. J Biol Chem. 1991;266:20290–20295. [PubMed] [Google Scholar]
- Sullivan P, Dubée S, Dorenbos K, Steward O, Baram T. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanont Y, Murakami Y, Tohda M, Vajragupta O, Watanabe H, Matsumoto K. Prevention of kainic acid-induced changes in nitric oxide level and neuronal cell damage in the rat hippocampus by manganese complexes of curcumin and diacetylcurcumin. Life Sci. 2006;78:1884–1891. doi: 10.1016/j.lfs.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-derived neurotrophic factor-mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Whittingham T, Lipton P. Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem. 1981;37:1618–1621. doi: 10.1111/j.1471-4159.1981.tb06337.x. [DOI] [PubMed] [Google Scholar]
- Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–375. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wu A, Ying Z, Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, Peterson P, Muizelaar J, Lee C. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid {beta} oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhao Y, Zheng W, Lu Y, Feng G, Yu S. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–232. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]


