Abstract
Background
White matter microstructural disruptions have been observed in patients with schizophrenia. However, whether changes exist prior to disease onset or in high-risk individuals is unclear. Here we investigated white matter integrity, as assessed by diffusion tensor imaging (DTI), in individuals at ultra high risk for psychosis (UHR), relative to healthy controls (HC), and the relationship between baseline DTI measures and functional outcome over time.
Methods
Thirty-six UHR participants and 25 HC’s completed baseline DTI scans. Subjects also completed clinical follow-up assessments approximately 6 (26 subjects) and 15 months (13 subjects) later. We used a rigorous registration approach (Tract-Based Spatial Statistics (TBSS) to examine fractional anisotropy (FA) in six major white matter tracts.
Results
Relative to the HC group, UHR subjects showed lower baseline FA in the superior longitudinal fasciculus, the major frontal-parietal white matter connection. Cross-sectional analyses demonstrated that UHR youth failed to show the same age-associated increases in FA in the hippocampus and inferior longitudinal fasciculus as HCs. Finally, lower baseline FA in the hippocampus and inferior longitudinal fasciculus predicted deterioration in social and role functioning in UHR participants at 15-month follow-up.
Conclusions
This is the first investigation of white matter microstructural alterations in a clinical high-risk sample. Our findings indicate that white matter development may be altered in youth at risk for psychosis, possibly due to disrupted developmental mechanisms, and further, that white matter integrity may be predictive of functional outcome.
Keywords: Schizophrenia, prodrome, diffusion tensor imaging, DTI, white matter, social functioning
Introduction
Early detection and prevention strategies for schizophrenia have led to investigations of individuals at the highest level of risk, either due to genetic factors (e.g. first degree relatives of patients with schizophrenia) or due to the exhibition of a constellation of clinical symptoms thought to be characteristic of the “prodromal period” of psychosis during the period when onset of schizophrenia would be expected to occur. Such studies seek to characterize the developmental processes that lead to disturbances of brain structure and function associated with onset of psychosis and to find baseline traits that are predictive of later diagnostic conversion or functional decline.
Disruptions in white matter (WM) integrity have been implicated in disease pathogenesis in schizophrenia (1). Evidence includes neuroimaging studies of first-episode and chronic patients that report WM volume reductions and structural abnormalities (2–4) as well as myelin-related gene abnormalities (5; 6). Further, hippocampal and frontal lobe myelination peak during adolescence and early adulthood (7), the time period most associated with psychosis onset. In fact, during normal adolescent brain maturation, gray matter volume decreases, likely due to increased synaptic pruning, while WM volume undergoes a simultaneous increase (8).
How the differences in connectivity observed in schizophrenia develop is of great interest (9). Some behavioral features are observable in patients with schizophrenia years before illness onset (e.g. (10-12)), suggesting that there are neural differences from a very early age that may leave at risk individuals more vulnerable to later insults. One possibility is that this early insult interacts with normal changes that occur later in development (i.e. pruning) thus unveiling the psychotic state in late adolescence (13; 14). It is also possible that the changes observable in patients with schizophrenia result from a disruption in the late developmental process itself (15). However, there are also a number of ways that these models may be combined (i.e. an early insult combined with the second hit of an abnormal developmental process), and the ways in which these different phases of development may interact is of great interest (Keshavan, 1994; Lewis, 2002; Waddington, 1998}.
Diffusion tensor imaging (DTI) is a powerful tool for examining WM microstructure in-vivo, based on patterns of water diffusion in neural tissue. The degree of fractional anisotropy (FA) in a voxel indexes WM integrity, potentially reflecting both myelination and tract organization. Previous DTI studies in schizophrenia have shown decreased FA in many major tracts, including the superior longitudinal fasciculus (16–18), cingulate bundle (19–21), uncinate fasciculus (18; 22–25), inferior longitudinal fasciculus (26–28) and hippocampus (29; 30). Such abnormalities are evident even in the first episode (16; 17; 31; 32). While previous studies of young people at ultra-high risk for developing psychosis (UHR) have utilized structural (e.g. (33; 34) and functional neuroimaging methods (e.g. (35; 36), WM integrity has been less investigated.
We assessed baseline DTI differences between UHR patients and healthy controls (HC), hypothesizing findings of reduced WM integrity in UHR youth. Additionally, to determine whether WM development is abnormal in UHR individuals, we tested whether age-associated differences in FA differed between UHR youth and HC’s as well as whether baseline WM changes were predictive of change in clinical and psychosocial functioning over time. Finally, in an exploratory analysis, we assessed the relationship of baseline FA with later symptomatology. Longitudinal studies examining conversion to psychosis as the primary outcome can be ambiguous, as conversion may occur subsequent to the final follow up point, and treatment course may ameliorate the natural progression of psychotic-like symptoms. Therefore, our study employed quantitative indices of functional outcome (37; 38) previously associated with cognitive change over time to serve as potential proxies for psychosis-related decline (39). We predicted that UHR youth with subsequent functional decline would have greater WM deficits at baseline, reflecting a more compromised system less amenable to improvement with time and treatment.
Methods
Participants
Thirty-six UHR participants and 25 HCs (see Table 1) were enrolled in an ongoing longitudinal study at the University of California, Los Angeles. HCs were recruited from a community sample via advertising, and were age matched to the UHR sample. Baseline measures included clinical and functional assessments and DTI. Subjects completed follow-up assessments of clinical and functional outcome, on average, at 6.11 ± 2.42 (26 subjects) and 15.96 ± 5.76 months (13 subjects, 92.3% of whom also were assessed at the first follow-up time point). UHR participants met criteria for of one of three prodromal syndrome categories, as assessed by the Structured Interview for Prodromal Syndromes (SIPS(40)): (1) attenuated (sub threshold) psychotic symptoms; (2) transient, recent-onset psychotic symptoms; or (3) a substantial drop in social/role functioning in conjunction with Schizotypal personality disorder diagnosis or a first-degree relative with a psychotic disorder. HC youth did not meet DSM-IV criteria for a psychiatric disorder as determined by SCID-I/P or K-SADS interview, have a first-degree family history of a psychotic disorder, or meet criteria for any of the three prodromal states defined above. Additional exclusion criteria for all participants included the presence of a neurological disorder, drug or alcohol abuse or dependence within the past 6 months, insufficient English fluency, and/or IQ below 70. Detailed information regarding SIPS prodromal criteria, reliability and consensus procedures are described elsewhere (41). All participants completed informed consent or assent (parental consent was also obtained for minors) and were compensated for all participation, as approved by the UCLA Institutional Review Board.
Table 1.
Subject Demographics
| Characteristic | UHR Patients (n = 36) | Healthy Controls (n = 25) |
|---|---|---|
| Age at baseline examination, mean (±SD) | 17.02 (3.37) | 17.96 (3.40) |
| Years of Education, mean (± SD) | 10.66 (2.54) | 11.84 (2.77) |
| Parental Education (years), mean (± SD) | ||
| Estimated WASI IQ, mean (± SD)a | 110.58 (13.52) | 111.82 (11.29) |
| Gender, n (%) | ||
| Males | 27 (75%) | 12 (48%) |
| Females | 9 (25%) | 13 (52%) |
| Race, n (%) | ||
| African American/Black | 5 (14%) | 3 (12%) |
| Asian American/Pacific Islander | 3 (8%) | 3 (12%) |
| Caucasian | 22 (61%) | 13 (52%) |
| Native American | 0 (0%) | 1 (4%) |
| Latino/Hispanic | 4 (11%) | 4 (16%) |
| Other | 2 (6 %) | 1 (4%) |
| Handedness, b n (%) | ||
| Left or Mixed/Right | 3 (9%) | 2 (10%) |
| Primary SIPS-defined Prodromal Status, n (%) | ||
| Brief Intermittent Psychotic Syndrome | 7 (19%) | |
| Attenuated Positive Symptom Syndrome | 28 (78%) | |
| Genetic Risk & Deterioration Syndrome | 1 (3%) | |
| Diagnosis of Schizotypal Personality Disorder, n | 1 | |
| Family History of Psychotic Disorder (First-degree relative), n | 8 | |
| Clinical Characteristics of Sample | ||
| GAF score, mean (± SD) Baseline | 45.25 (15.02) | |
| Follow-up 1 | 56.15 (13.59) | |
| Follow-up 2 | 60.38 (15.47) | |
| SOPS Positive Symptom Total, mean (± SD) Baseline | 10.69 (4.34) | |
| Follow-up 1 | 7.96 (4.85) | |
| Follow-up 2 | 7.92 (2.3) | |
| SOPS Negative Symptom Total, mean (±SD) Baseline | 10.75 (6.68) | |
| Follow-up 1 | 10.25 (7.15) | |
| Follow-up 2 | 8.76 (9.54) | |
| Global Functioning: Role score, mean (±SD) Baseline | 6.38 (1.72) | |
| Follow-up 1 | 6.37 (1.86) | |
| Follow-up 2 | 6.07 (2.28) | |
| Global Functioning: Social score, mean (±SD) Baseline | 6.22 (1.56) | |
| Follow-up 1 | 6.37 (1.96) | |
| Follow-up 2 | 6.76 (2.12) | |
| Conversion to DSM Diagnosis of Psychotic Disorder n (%) | 6 (17%) | |
| Medication usage at baseline DTI scan, n (%) | 21 (58%) | |
| Atypical Antipsychotics, n (%) | 9 (25%) | |
| SSRIs, n (%) | 12 (33%) | |
IQ and handedness data were missing for 3 control subjects
Gender differed significantly between groups (χ2=5.44, p=.02)
Procedures
Social and role functioning were assessed in UHR subjects with the Global Functioning: Social scale (GFS (42)) and the Global Functioning: Role scale (GFR (38)), measures designed to provide global assessments of psychosocial functioning in adolescent and young adult populations (37). These scales rate social and role function on two separate 10-point Likert scales, independent of symptom severity. Functional change over time was determined by calculating the percent change from baseline (either positive or negative) in these scores over the follow-up period. In addition, subjects were assessed using the Global Assessment of Functioning (GAF) and Brief Psychiatric Rating Scale (BPRS).
Scanning Procedures
Subjects were scanned on a 1.5T Siemens Sonata scanner (Siemens, Erlagen, Germany) at the Ahamanson-Lovelace Brain Mapping Center at UCLA. Head motion was restricted using foam padding. DTI data were acquired using a 6-direction EPI sequence with 75 contiguous 2mm AC-PC aligned interleaved slices with no gap (TR=9.5s, TE=77ms, flip angle =90 deg, matrix=128x96, b-value = 1000, FOV 256 mm x 192 mm resulting in 2mm isotropic voxels). Five repetitions were acquired, with a total scan time of 6 minutes 20 seconds.
Data Analysis
Image Processing
The five image acquisitions for each direction were merged, aligned with McFlirt (FMRIB Software Library; FSL (43)), and averaged to create one file each for the 6 directions and the b0 image. Eddy current correction was done using Flirt (FSL), and images were skull stripped using the Brain Extraction Tool. FA images were calculated using DTIFit (FMRIB’s Diffusion Toolbox), which fits a diffusion tensor model at each voxel, and then were registered to MNI-152 space using a 12-parameter affine registration with a mutual information cost function implemented in Flirt (FSL). A group map was created using Tract-Based Spatial Statistics (TBSS, (44)). An average FA image was created and the tracts were narrowed to generate an FA “skeleton” representing the center of all tracts common to the entire group. The area around the skeleton in each subjects’ aligned FA map was searched and the highest local FA value was assigned to the skeleton. This ensured that each subject’s skeleton was in the group space, yet represented the center of that subject’s own unique fiber tracts.
Region of Interest Definition
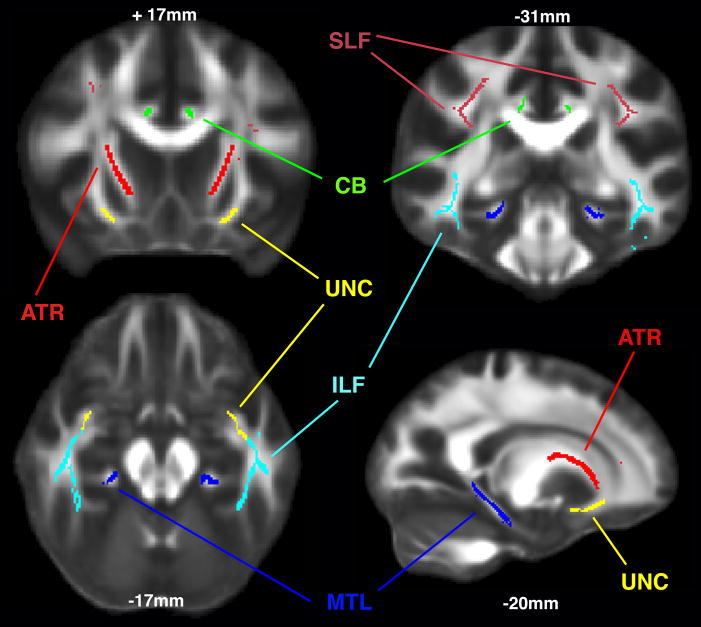
Regions of interest (ROIs) were defined in the anterior thalamic radiation (ATR), cingulate bundle (CB), medial temporal lobe structures (MTL), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF) and uncinate fasciculus (UF). Regions were created by overlying the TBSS generated skeleton (Figure 1) with the John Hopkins University DTI-based probabilistic tractography atlas for the tracts of interest. (45-47). To ensure the validity of the tractography-based ROIs for our TBSS skeleton, in any instance where the skeleton and JHU tract differed, the ROI was edited to incorporate contiguous and inclusive sections of skeleton. Each subjects’ FA skeleton was masked using each of the ROIs, and the average FA was calculated for that segment of the skeleton in each region (see Figure 2).
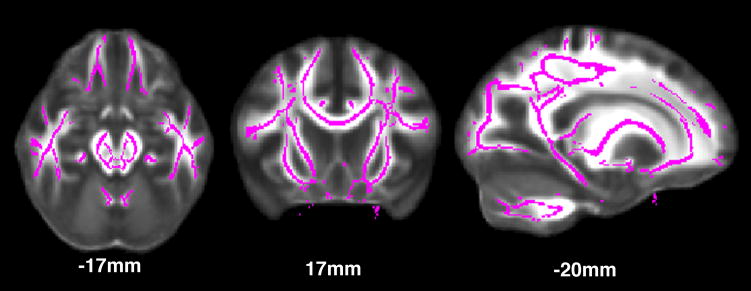
Figure 1.
Tract-based spatial statistics (TBSS) skeleton.
Figure 2.
Regions of Interest; SLF = superior longitudinal fasciculus; ILF = inferior longitudinal fasciculus; ATR = anterior thalamic radiation; MTL = medial temporal lobe; CB = cingulate bundle; UNC = uncinate fasciculus
Statistical Analyses
Analysis I, Group-wise Differences at Baseline
Mean FA data were extracted from each ROI. To reduce the number of statistical comparisons, data were collapsed across both hemispheres. A repeated measures within-subjects ANOVA was performed with region (for the 6 ROIs) as a repeated measure, with age and sex as covariates. To decompose the significant effects, an analysis of covariance (ANCOVA), assessed FA by group, covarying for age and sex.
Analysis II, Age Analysis
For the cross-sectional age analysis, a robust regression was performed in Stata (v8) with age predicting FA (for each ROI), covarying for gender. As in the previous analysis, ROI data were collapsed across hemispheres. Data for each individual analysis were tested for outliers, and subjects with high studentized residuals (>2) were excluded from that regression. Group differences in the age-FA relationship were tested using the interaction between the slopes of the regression lines, and tested for significance using a Wald test. To correct for the number of ROIs (six) in this analysis, the p-threshold was set at .05/6=.0083.
Analysis III, Functional Changes
In this robust regression analysis, FA (for each ROI in each hemisphere) was included as a predictor of functional outcome (social and role functioning) in UHR patients, while controlling for age and gender. Twenty-eight UHR subjects completed 6-month follow-up assessments, while 13 UHR subjects completed follow-up at 15-months. The data for each individual regression were again tested for outliers and subjects with high studentized residuals (>2) were excluded. For this analysis, to correct for the number of ROIs (twelve), the p-threshold was .05/12=.0042.
Analysis IV, Exploratory Analysis of Clinical Changes
In a post-hoc exploratory analysis of clinical symptoms we assessed the relationship between baseline FA and negative symptoms, positive symptoms, GAF scores, and indices of dysphoric mood (from the SIPS) and depression (from the BPRS) at 15 months follow up. A robust regression was performed of the clinical measure in question with FA for each of the 12 regions of interest at baseline, covaried for age and gender. Due to the post-hoc nature of the analysis, a p value of .05 was used for all comparisons.
Results
There was no significant difference between UHR participants and controls in age (t(59)=−.86, p=.29)), although there was a significant difference in gender distribution (χ2=5.44, p=.02), so gender was included as a covariate in all analyses (see Table 1).
Analysis I
In the UHR vs. control FA repeated measures ROI analysis, there was a significant effect of region [F(5,285)=6.801, p<.001], no effect of group [F(1,57)=.100, p=.753], and a significant group by region interaction [F(1,285)=2.452, p=.034]. When this latter effect was decomposed using ANCOVA covarying for age and sex, there was a significant effect of group in the SLF [F(3,57)=4.41, p=.040], with UHR subjects showing reduced FA relative to HCs at baseline. There were no significant differences in the other ROIs examined.
Analysis II
There was a significant group x age interaction in the hippocampus (F(4,53)=6.47, p=.006), and a trend towards an interaction in the ILF (F(4,52)=3.49, p=.022), such that as age increased, FA increased in the control group, but not in the UHR group (see Figure 3). The other ROIs (CB, UF, SLF, and ATR) did not show group x age interactions, although there was a significant main effect of age in the CB (F(4,54)=12.17, p<.001) in the full sample.
Figure 3.

Robust regression of age and FA in hippocampus (F(4,53)=6.47, p=.006) (top) and ILF (F(4,52)=3.49, p=.022) (bottom)
Analysis III
The subjects who remained in the study for follow up assessments did not differ from those who were lost to follow up in terms of age [t(34)=1.2070, p=.2358], gender [χ2=.3612, p=.548], family history [χ2=.8600, p=.354], baseline GAF [t(34)=1.011, p=.3193], or the SIPS criteria on which their recruitment was based [χ2=1.273, p=.736]. At 6 month follow-up, no significant associations between baseline FA and change in functioning were observed for UHR individuals, although there were trend associations between lower values of FA in the left ILF (F(3,21)=2.86, p=.033) and right ILF (F(3,21)=2.46, p=.071) and declines in social functioning at 6-months.
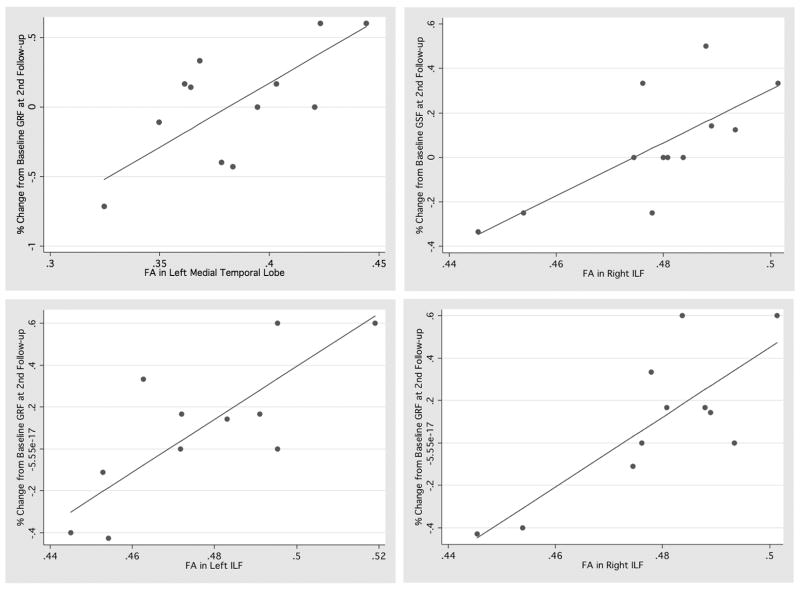
However, at the 15-month follow up, lower FA in the right ILF significantly predicted deterioration in role (F(3.7)=14.26, p=.002) and social functioning (F(3.8)=12.39, p<.0001) in the UHR sample. Deterioration in role functioning was also significantly predicted by lower FA in the left hippocampus (F(3.8)=7.78, p=.003) and left ILF (F(3.7)=13.01, p=.004). Furthermore, there were trends towards a relationship of deterioration in social functioning with lower FA in the right hippocampus (F(3,8)=5.83, p=.074) and right ATR (F(3,8)=6.33, p=.073) as well as a trend towards a relationship of deterioration in role functioning with lower FA in the right UF (F(3,8)=1.45, p=.09; See figure 4).
Figure 4.
Robust regression of functional outcome and FA: a. GFR and left hippocampus (F(3.8)=7.78, p=.003); b. GFS and right ILF (F(3.8)=12.39, p<.0001); c. GFR and left ILF F(3.7)=13.01, p=.004); d. GFR and right ILF (F(3.7)=14.26, p=.002)
Analysis IV
At 15 months follow up, there were significant relationships between GAF scores and left SLF (p=.029) and right anterior thalamic radiation (p=.040) with trends in the left anterior thalamic radiation and right hippocampal region. Negative symptoms were significantly associated with the right ILF (p=.045) and with the right hippocampal region (p=.024) with trends in the left SLF and left ILF. Dysphoria was associated with the left SLF (p=.010) with a trend in the right ILF. Depressed mood was associated with right hippocampal region (p=.049). There were no significant relationships between any regions and positive symptoms or conversion status, although there was a trend towards a relationship of conversion and hippocampal FA (p=.0697), and conversion was highly related to functional outcome, particularly social functioning at long-term follow-up (p=.003). (Supplemental Table 1).
Conclusions
Regional FA differed in the UHR and HC groups in the SLF, and cross-sectional analyses indicated differences in the pattern of age-associated change, suggesting an altered developmental trajectory of WM in UHR youth. Further, WM alterations at baseline in the UHR sample were predictive of changes in social and role functioning over the ensuing 15 months, indicating that structural brain changes are detectable before illness onset and may differentiate patients who will and will not deteriorate functionally over time.
Overall, UHR patients had lower baseline FA than controls in the SLF, a fronto-parietal connection, which is consistent with previous findings in first-episode schizophrenia patients (16-18). Previous structural neuroimaging work in UHR subjects has identified gray matter reduction in frontal and temporal lobe structures and the cerebellum (34; 48-50). However, findings regarding gray matter abnormalities prior to illness onset in medial temporal structures such as the hippocampus are inconsistent (50; 51). A prior study of individuals at clinical high risk revealed differences in callosal shape, supporting the notion that WM alterations may exist prior to illness onset (52). However, UHR samples are highly heterogeneous with regards to age and clinical outcome, which may sometimes obscure group differences and contribute to trend level differences, for instance, a study examining fiber tracking in a smaller sized UHR group also did not find simple group differences when compared to controls (53). Exploration of factors that contribute to such variance, and how they relate to WM alterations, may elucidate differences that are relevant within the UHR samples but that make comparisons between UHR and control samples problematic.
Our results suggest different developmental patterns in temporal lobe WM tracts (MTL and ILF) between UHR youth and healthy controls, such that UHR youth fail to show the expected age-associated increase in FA. This result is consistent with previous longitudinal findings in adolescents with schizophrenia, in which significantly greater gray matter loss over time was seen in patients than controls, suggesting abnormal frontal and temporal development (54). Additionally, hippocampal volume decreases were observed across adolescence in schizophrenia patients but not controls (55), with specific hippocampal subregions showing distinct developmental trajectories, which differed between adolescent schizophrenia patients and healthy controls (56). Moreover, in late adolescence and early adulthood schizophrenia patients show abnormal developmental trajectories in both gray and WM (57), with greater tissue loss seen in patients with poor outcome. While schizophrenia is considered to be a developmental disorder with both early and late developmental influences (58), it is of interest whether the changes seen in adulthood arise as the result of a normal process exerted on a compromised structure or an abnormal developmental process (9; 15; 59). Our findings indicating that the slope of developmental change differs UHR cases and controls are consistent with the theory that there is a disruption in the developmental process itself.
Finally, lower baseline FA in temporal regions was predictive of a course of declining social and role function in UHR youth. Thus, early microstructural changes may indicate a pervasive neurologically-based deficit, as opposed to a transient symptomatically-based deficit. Although FA in these regions did not predict conversion to a categorical psychosis diagnosis, we had limited power to examine this question as only 6 subjects converted over the follow-up period. This is a common obstacle for UHR studies examining conversion to psychosis as the primary outcome variable (60). However, conversion was highly correlated with functional outcome, particularly social functioning at long-term follow-up (p=.003). This analysis of social and role functioning, rather than conversion, in relationship to structural brain changes provides a unique contribute to the literature. The results indicate that, while functional outcome and DSM-IV diagnosis are closely related, additional power and information can be gained through use of a continuous variable (i.e., functioning). Our findings further suggest that DTI measures of WM integrity may be a powerful prognostic indicator in at-risk youth. Previous findings in FA and other high risk groups (17; 23; 26; 27; 31; 32; 61–63) support the idea that neural changes are present early in the progression of the illness. However, the use of a longitudinal design in which FA predicts later outcome is novel. That these effects are largely in temporal lobe regions is interesting and consistent with what we know about normal adolescent development- that the temporal lobes are expected to be myelinating during this time and thus have the potential to show changes quite proximal to disease onset. This pattern is also consistent with cognitive findings in the prodrome, in which functions thought to rely on the temporal lobes such as verbal learning and memory are impaired (38).
Our study is limited in part by sample size and heterogeneity. By their nature, UHR groups contain subjects who are experiencing transient changes and patients who are prodromal for other non-psychotic psychiatric disorders in addition to those truly prodromal schizophrenia subjects. Therefore, larger follow-up cohorts may provide additional insight. Additionally, while some UHR participants had been medicated for a relatively brief period of time, none of the FA measures were significantly correlated with antipsychotic use (all p>.05), so there is no evidence that medication use influenced the direction of the results. Although gender distribution differed between groups, gender was included a covariate in all analyses to account for its potential relationship with DTI measures. With only 6 directions in the DTI sequence, our analysis was limited to FA, but future studies in which more directions are measured and in which analyses such as tractography can be performed, will be very informative. Further, due to susceptibility artifacts in the orbitofrontal regions that occur with echo planar imaging scans, the signal for the uncinate should be interpreted with caution. Finally, given the low conversion rate we also must consider other potential explanations, for instance, that WM integrity is predictive of decline across a variety of psychopathologies and not just schizophrenia. However, these subjects did have symptoms consistent with those associated with the prodromal phase of schizophrenia.
In conclusion, our cross-sectional findings suggest a different pattern of WM development in UHR youth relative to healthy controls, particularly in temporal lobe regions, such that UHR youth fail to show the normal progressive increase in FA with age. Further, lower baseline FA in temporal WM tracts predicted deterioration in social and role functioning over follow up. These data thus provide the first evidence that structural brain changes in temporal lobe WM may predict outcome in young people at ultra high risk for psychosis.
Supplementary Material
Acknowledgments
We would like to acknowledge the help of Dr. Russell Poldrack, Molly Hardt, Lara Zimmerman, Sabrina Lux, and Malin McKinley as well as the participants. This research was supported by NIH Grants MH65079, MH066286, GM072978 and RR021992 to T.D.C, 5-F31-MH068111-02 to K.H.K., MH14584 to T.A.N., the National Alliance for Research on Schizophrenia and Affective Disorders (C.E.B.), and a gift to the UCLA Foundation by Garen and Shari Staglin.
Footnotes
Financial Disclosure
All authors reported no known biomedical financial interests or other potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 2.Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 3.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev P, Brodaty H. Quantitative study of signal hyperintensities on T2-weighted magnetic resonance imaging in late-onset schizophrenia. Am J Psychiatry. 1999;156:1958–1967. doi: 10.1176/ajp.156.12.1958. [DOI] [PubMed] [Google Scholar]
- 5.Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- 6.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold SE, Rioux L. Challenges, status, and opportunities for studying developmental neuropathology in adult schizophrenia. Schizophr Bull. 2001;27:395–416. doi: 10.1093/oxfordjournals.schbul.a006883. [DOI] [PubMed] [Google Scholar]
- 8.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 9.Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, Cannon TD. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- 10.Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- 11.Cannon M, Huttunen MO, Tanskanen AJ, Arseneault L, Jones PB, Murray RM. Perinatal and childhood risk factors for later criminality and violence in schizophrenia. Longitudinal, population-based study. Br J Psychiatry. 2002;180:496–501. doi: 10.1192/bjp.180.6.496. [DOI] [PubMed] [Google Scholar]
- 12.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 14.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg I. Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence. J Psychiatr Res. 1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 16.Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Federspiel A, Begre S, Kiefer C, Schroth G, Strik WK, Dierks T. Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis. 2006;22:702–709. doi: 10.1016/j.nbd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, et al. Clinical and Neuropsychological Correlates of White Matter Abnormalities in Recent Onset Schizophrenia. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, Fukuyama H, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215–222. doi: 10.1016/j.schres.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Kubicki M, Westin C, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasiculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003 doi: 10.1016/s0006-3223(03)00419-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, et al. Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry. 2004;161:573–575. doi: 10.1176/appi.ajp.161.3.573. [DOI] [PubMed] [Google Scholar]
- 22.Burns JJDBMEWHMTJECLSM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 23.Price G, Cercignani M, Parker GJ, Altmann DR, Barnes TR, Barker GJ, et al. White matter tracts in first-episode psychosis: A DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39:949–955. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubicki M, Westin C, Maier S, Frumin M, Nestor PG, Salisbury D, et al. Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 27.Cheung V, Cheung C, McAlonan GM, Deng Y, Wong JG, Yip L, et al. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 28.O’Daly OG, Frangou S, Chitnis X, Shergill SS. Brain structural changes in schizophrenia patients with persistent hallucinations. Psychiatry Res. 2007;156:15–21. doi: 10.1016/j.pscychresns.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 29.White T, Kendi AT, Lehericy S, Kendi M, Karatekin C, Guimaraes A, et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia--a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Schlosser RG, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S, et al. White matter abnormalities and brain activation in schizophrenia: a combined DTI and fMRI study. Schizophr Res. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Hao Y, Liu Z, Jiang T, Gong G, Liu H, Tan L, et al. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport. 2006;17:23–26. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- 32.Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 33.Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Whalley HC, Simonotto E, Moorhead W, McIntosh A, Marshall I, Ebmeier KP, et al. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60:454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62:254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornblatt BA, Auther AM, Niendam T, Smith CW, Zinberg J, Bearden CE, Cannon TD. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O’Brien M, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Niendam TA, Bearden CE, Zinberg J, Johnson JK, O’Brien M, Cannon TD. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophr Bull. 2007;33:772–781. doi: 10.1093/schbul/sbm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Clinical Interview for Prodromal Syndromes v3.1. Yale School of Medicine: PRIME Research Clinic 2001 [Google Scholar]
- 41.Meyer SE, Bearden CE, Lux SR, Gordon JL, Johnson JK, O’Brien MP, et al. The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adolesc Psychopharmacol. 2005;15:434–451. doi: 10.1089/cap.2005.15.434. [DOI] [PubMed] [Google Scholar]
- 42.Auther A, Smith C, Cornblatt B. Global Functioning: Social Scale (GF: Social) Glen Oaks, NY: Zucker Hillside Hospital; 2006. [Google Scholar]
- 43.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PC. MRI Atlas of Human White Matter. Amsterdam: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 46.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 49.Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 50.Meisenzahl EM, Koutsouleris N, Gaser C, Bottlender R, Schmitt GJ, McGuire P, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 51.Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 52.Walterfang M, McGuire PK, Yung AR, Phillips LJ, Velakoulis D, Wood SJ, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- 53.Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- 54.Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- 55.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 56.Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Lenane M, et al. Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr Res. 2007;90:62–70. doi: 10.1016/j.schres.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 57.van Haren NE, Pol HE, Schnack HG, Cahn W, Brans R, Carati I, et al. Progressive Brain Volume Loss in Schizophrenia Over the Course of the Illness: Evidence of Maturational Abnormalities in Early Adulthood. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 59.Waddington JL, Lane A, Scully PJ, Larkin C, O’Callaghan E. Neurodevelopmental and neuroprogressive processes in schizophrenia. Antithetical or complementary, over a lifetime trajectory of disease? Psychiatr Clin North Am. 1998;21:123–149. doi: 10.1016/s0193-953x(05)70364-6. [DOI] [PubMed] [Google Scholar]
- 60.Cannon TD, Cornblatt B, McGorry P. The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull. 2007;33:661–664. doi: 10.1093/schbul/sbm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2005;76:585–587. doi: 10.1136/jnnp.2004.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.