Abstract
We found that a single week of exercise enhanced cognitive function on the Morris water maze (MWM), such that exercise animals were significantly better than sedentary controls at learning and recalling the location of the platform. In order to elucidate the role that calcium calmodulin protein kinase II (CAMKII) holds in mediating the exercise-induced enhancement in learning and memory, a specific antagonist of CAMKII, KN-62, was used to block CAMKII in the hippocampus during a 1-week voluntary exercise period. Following, a 2-trial-per-day MWM was performed for 5 consecutive days, succeeded by a probe trial 2 days later. Inhibiting CAMKII action during exercise blocked the ability of exercise to enhance memory retention on the MWM; the recall abilities of exercise animals receiving the CAMKII blocker were significantly worse than those of both sedentary and exercise controls. Conversely, CAMKII may not play a significant role in mediating the effects of exercise on learning acquisition as inhibiting CAMKII failed to block the exercise-induced enhancement in learning acquisition. Our results also show that CAMKII activation early during MWM learning may be counterproductive to learning acquisition, as exercising animals given the CAMKII inhibitor performed significantly (p < 0.001) better than exercising control animals and sedentary controls only on day 2 of the MWM. Inhibiting CAMKII also blocked the exercise-induced upregulation of molecules critical for learning and memory, BDNF and the transcription activator CREB, which is regulated by and downstream to BDNF action. These findings indicate that hippocampal CAMKII may have a refined role in mediating the effects of exercise on cognition, selectively functioning to regulate memory retention.
Keywords: BDNF, CREB, Morris water maze, learning and memory, exercise
INTRODUCTION
Experimental evidence underlines the growing consensus that exercise enhances cognitive function, showing its ability to improve learning and memory (Fordyce & Wehner, 1993; Kramer, 1999), facilitate functional recovery following brain injury (Grealy et al., 1999), and counteract the mental decline associated with aging (Laurin et al., 2001). Despite this rigorous confirmation of the beneficial effects of exercise on CNS health, the molecular mechanisms underlying the ability of exercise to benefit neuronal and cognitive functions remain obscure.
Our previous efforts have identified the importance of the serine/threonine kinase calcium/calmodulin protein kinase II (CAMKII) in mediating exercise-induced plasticity in the hippocampus, an area involved in learning and memory formation (Vaynman et al., 2003). Hippocampal CAMKII may likely contribute to perpetuating the effects of exercise on cognitive function. Indeed, CAMKII is highly expressed in postnatal forebrain structures, especially in the hippocampus (Erondu & Kennedy, 1985). Multiple lines of evidence indicate that CAMKII may be critical for learning and memory. Long-term potentiation (LTP), an activity dependent strengthening of synapses believed to be an electrophysiological correlate of learning and memory, induces the activation of CAMKII. Moreover, both pharmacological and genetic manipulations have demonstrated the importance of CAMKII for memory processes (Silva et al., 1992a, 1992b, Giese et al., 1998). A mutation that results in the deletion of the major isoform of CAMKII impairs LTP and produces learning deficits and unstable spatial maps encoded by hippocampal place cells (Cho et al., 1998). Recent evidence shows that the gene encoding hippocampal CAMKII is important for human memory performance (de Quervain & Papassotiropoulos, 2006).
A potential mechanism through which CAMKII may potentiate the effects of exercise on cognitive function is by interacting with brain-derived neurotrophic factor (BDNF). Characteristically, exercise has been found to significantly increase BDNF, in the hippocampal area (Neeper et al., 1995; Vaynman et al., 2003), which has been shown to exhibit an abundant expression of the receptor (TrKB receptor) through which BDNF exerts its action (Maisonpierre et al., 1990; Murer et al., 2001). BDNF was initially consigned as a protein regulating the survival, growth, and differentiation of neurons during development (Barde, 1994), but it has become recognized as a critical modulator of synaptic-plasticity in the adult brain (Lo, 1995).
In this study we tested the possibility that CAMKII may be important for mediating the effects of exercise on learning and memory and synaptic plasticity. Exercise induces synaptic plasticity markers in the hippocampus through a BDNF-mediated mechanism, particularly increasing the mRNA levels of the transcriptional factor involved in memory, and cAMP response-element-binding (CREB) protein (Vaynman et al., 2003; Gomez-Pinilla et al., 2001; Vaynman et al., 2004), in a dose response manner to increasing BDNF mRNA levels (Vaynman et al., 2004). Importantly, CAMKII is able to regulate CREB-dependent transcription (Lonze & Ginty, 2002). As both CAMKII and CREB have been described as molecular memory switches (Lisman et al., 2002, Yin et al., 1995) and have a well-established connection to the BDNF system, it is likely that CAMKII may play a prominent role in supporting learning and memory by affecting both BDNF and CREB expression in the hippocampus.
Materials and methods
Exercise paradigm
Adult male Sprague-Dawley (Charles River) rats (3 months of age) were randomly assigned to generate 4 groups: sedentary with the control injection (Sed; n=7); exercise with the control injection (Exc; n=6); sedentary with KN-62 injection (Sed/KN; n=8); and exercise with KN-62 injection (Exc/KN; n=8). All rats were individually housed in standard polyethylene cages in a 12/12h light/dark cycle at 22–24 °C, with food and water ad libitum. We chose a voluntary exercise paradigm because it simulates aspects of human behavior in which animals choose how much to run. The exercise rats were given access to a wheel (diameter = 31.8 cm, width = 10cm) that freely rotated against a resistance of 100 g, attached to a receiver that monitored revolutions every hour (VitalViewer Data Acquisition System software, Minimitter company, Inc., Sunriver, OR). All groups were allowed to acclimate to their respective environments for 1 week prior to the start of experiments. Animals were exercised for a period of 1 week prior to MWM training, during which respective drug treatments were given to groups. The choice of a short 1-week exercise period was based upon that this period is sufficient to enhance performance on the MWM task and increase the expression of synaptic plasticity markers believed to underlie mechanisms supporting cognitive function (Vaynman et al., 2003; Molteni et al., 2002). The control rats were confined to a cage with no access to a running wheel. Animals continued in their respective experimental conditions for the duration of the experiment. All animals were killed the morning following the last treatment day by decapitation and their hippocampi were rapidly dissected out, immediately placed on dry ice, and stored at −70°C. These studies were performed in accordance with the United State National Institute of Health Guide for the Care and Use of Laboratory Animals, and were approved by UCLA Animal Research Committees.
Drugs
We used the specific CAMKII inhibitor KN-62 (Ito et al., 1991), acquired from Calbiochem Bioscience, La Jolla, CA, USA. KN-62 is a specific inhibitor that interacts with the regulatory domain of CAMKII (Tokomitsu et al., 1990) to block the active form of CAMKII (Kim et al., 2006). KN-62 has been previously used in our lab to effectively block the action of CAMKII on molecular measures of synaptic plasticity in vivo (Vaynman et al., 2003). We used a dose of 7.2 ug/ul DMSO (Vaynman et al., 2003; Wolfman et al., 1999), which has been shown to block CAMKII mediated memory on the water maze task (Wolfman et al., 1999). We used Cytochrome C (cytC), obtained in powder from SIGMA, St. Louis, MO., as the control drug since it has been used as a standard control for microbead injections (Vaynman et al., 2003; Lom & Cohen-Cory, 1999). CytC was dissolved in sterile distilled water, with stock concentration of 100 ng/µl. Fluorescent latex microbeads, used as the vehicle for drug insertion into the hippocampus, were purchased from Lumaflour Corp., Naple, FL.
Preparation of microbeads
Infusion of KN-62 was achieved by coupling them to microbeads, which we have previously used as a reservoir for the successful delivery of viable inhibitors into the hippocampus for 7 days (Vaynman et al., 2003, 2004). We prepared microbeads (Lumaflour, Naples, FL) by the methods described previously (Riddle et al 1997; Lom & Cohen-Cory 1999). Briefly, these consisted of coating the microbeads with each drug via passive absorbency by incubating overnight at 4°C with a 1:5 mix of microbeads to KN-62 or control solution. The morning after coating the microbeads, the solution was centrifuged at 14,000xg for 30 min and the microbeads were resuspended in sterile water at a 10% concentration. These two drugs were administered via injection of fluorescent latex microbeads directly into the right hippocampus, resulting in a consistent and effective blockade of targets as shown in the results. We have previously used this same microbead protocol for a 7-day period (Vaynman et al., 2004). Previous studies by Quattrochi et al. (1989), Riddle et al. (1995, 1997), and Lom & Cohen-Cory (1999) have reported successful delivery by microbeads of bioactive agents such as neurotrophins and neurotransmitter agonists/antagonists into highly localized brain regions.
Injection of drugs into the hippocampus
Exercise and sedentary rats received a single injection of KN-62 or the standard control. We used a unilateral injection to the right hippocampus following the protocol of our previous blocking experiments (Vaynman et al., 2003, 2004). The contralateral hippocampus was not used as a control since, due to connecting fibers, a unilateral injection can cause changes on the contralateral side (Amaral & Witter, 1989). Injections were given to all animals once prior to the onset of the running period, administered early in the morning to provide for an ample recovery time, which permitted all animals to begin running that same evening. All animals were anaesthetized by Isoflurane (2–2.5%) utilizing the Mobile Laboratory Animal Anesthesia System, and positioned in a stereotaxic apparatus that was used to secure the animal and to measure the sight for injection. KN-62 or control solution imbedded in microbeads was injected into the right hippocampus (3.8 mm posterior to Bregma, 1 mm from the midline, and 3.7 mm vertically) using a Hamilton syringe in a volume of 2 µl over 15 min. The location of microbead injection was verified by histological examination of selected brains, as previously described (Quattrochi et al., 1989; Riddle et al., 1995). We visually inspected all the brains at the time of dissection, such that only those showing characteristic markings of microbeads of the right hippocampus were used for mRNA measurements. The microbead injection site was additionally verified by florescence microscopy (Figure 1) using an Olympus BX51 microscope.
Figure 1.
Tissue section in the saggital plane showing the site of microbead injection in the right hippocampus as identified by fluorescence microscopy. The microbead injection is shown concentrated in the stratum lacunosum molecular (slm). For convenience, hippocampal areas CA1, CA3, and the dentate gyrus (DG) have been labeled.
Cognitive Testing
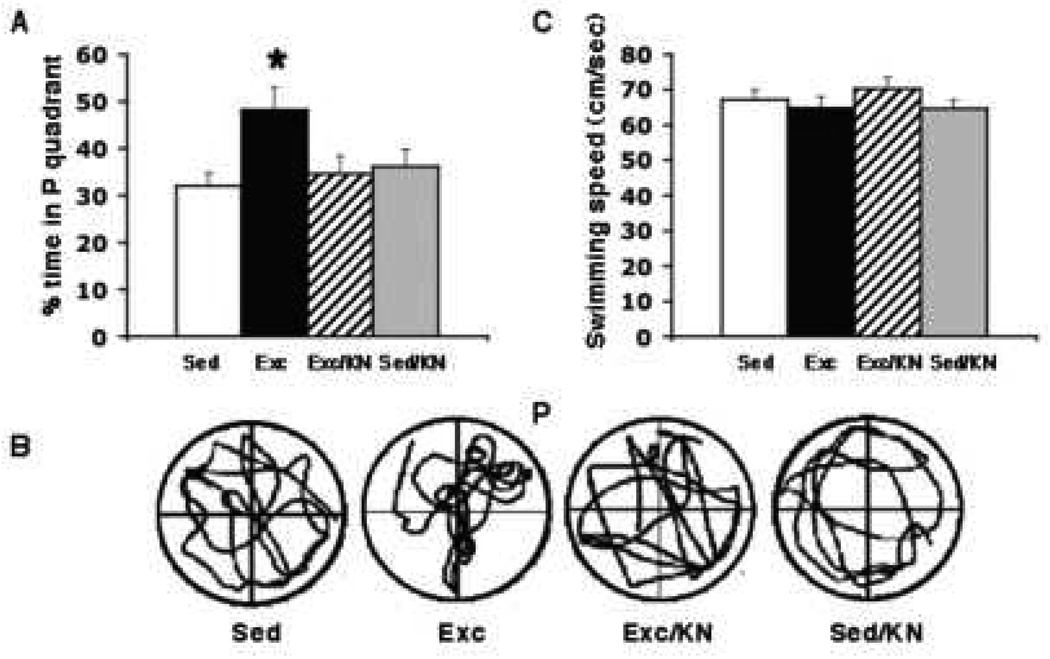
To evaluate the effect of exercise and KN-62 inhibition of CAMKII activity on memory functions, all rats were tested, using the Morris water maze, for spatial memory acquisition and retention (Morris et al., 1982; Sutherland Kolb & Whishaw, 1982). The swimming pool (130 cm diameter, 50 cm height) was divided into four quadrants. As previously discussed in Molteni et al., 2002, the quadrant housing the escape platform (12 cm diameter), was fixed in a permanent position 2cm under the water surface during the course of the MWM training procedure and was defined as the target zone. The water, kept at a steady 22±2°C, was made opaque with white non-toxic biodegradable dye to prevent the rats from seeing the platform. We used a stringent 2-trial-per-day, 5-day MWM training protocol, which we have identified as a good discriminative test for the effect of exercise on learning and memory (Vaynman et al., 2004). The animals were placed into the tank facing the wall from one of the equally spaced start locations, which were randomly altered every trial. Spatial reference cues around the pool were maintained constant through out the duration of the MWM training and probe trials. Each trial lasted until the rat found the platform or for a maximum duration of 60 s. If the rat failed to find the platform, it was gently placed on the platform. At the end of each trial, the rat was allowed to rest on the platform for 10 s. The time to reach the platform (escape latency) was recorded for each animal. To assess spatial memory retention, a probe trial was performed 2 days after the last training trial, during which the platform was removed from the pool, while all other factors remained constant. As previously described (Molteni et al., 2002, 2004), rats were allowed to swim for 60 s, during which the percentage of time spent in each quadrant was calculated and their swim paths were semi-automatically recorded by a video tracking system (SMART: Spontaneous Motor Activity Recording and Tracking. #35E4F-FA9, Pan Lab s.l., Barcelona, Spain). The location of the platform was designated as quadrant P (Fig. 3B).
Figure 3.
Effect of blocking CAMKII action during the exercise period on memory retention using the probe trial on the MWM task. (A) Exercise increased the memory retention on the MWM task as indicated by the finding that exercise animals spent significantly more time in quadrant P than sedentary controls (Exc vs. Sed). Blocking CAMKII action during exercise abolished this exercise-induced preference for the P quadrant (Exc/KN vs. Exc), such that exercise animals receiving the CAMKII blocker spent as much time in the P quadrant as sedentary control animals (Exc/KN vs. Sed). Blocking BDNF action did not have an effect on the preference of sedentary animals for the P quadrant (Sed/KN vs. Sed). (B) Representative samples of trials traveled during the probe test (P =, quadrant which previously housed the platform), illustrating the marked preference of the Exc group for the P quadrant as compared to all other groups. (C) There was no significant difference in swimming speeds between all four groups. Each value represents the mean ± SEM. (ANOVA, Fischer test, * p < 0.05).
Isolation of total RNA and real-time quantitative RT-PCR
Total RNA was isolated using RNA STAT-60 kit (TEL-TEST, Inc., Friendswood, TX) as per manufacturer’s protocol. Quantification was carried out by absorption at 260 nm. The mRNAs for BDNF, CREB, and CAMKII were measured by real-time quantitative RT-PCR using PE Applied Biosystems prism model 7700 sequence detection instrument, which directly detects the reverse transcription polymerase chain reaction (RT-PCR) product without downstream processing. This is achieved by monitoring the increase in fluorescence of a dye labeled DNA probe, one that is specific for the factor of interest plus another specific for glyceraldyehyde-3-phosphate dehydrogenase (GAPDH) gene, which has been previously used as a successful endogenous assay control (Molteni et al., 2002, Greisbach et al., 2002). Total RNA (100 ng) was converted into cDNA using TaqMan EZ RT-PCR Core reagents (Perkin-Elmer, Branchburg, NJ). The sequences of probes, forward and reverse primers, designed by Integrated DNA Technologies (Coralville, IA) were: BDNF: (5I-AGTCATTTGCGCACAACTTTAAAAGTCTGCATT-3I); forward: (5I-GGACATATCCATGACCAGAAAGAAA-3I); reverse: (5I-GCAACAAACCACAACATTATCGAG-3I); CREB: (5I-CATGGCACGTAATGGAGACTACCGCA-3I); forward: (5I-CCGCCAGCATGCCTTC-3I); reverse: (5I-TGCAGCCCAATGACCAAA-3I); CAMKII: (5'-CTC CAC TGT GGC CTC CTG CAT GC-3'); forward: (5'-AGC ACC CCT GGA TCT CGC-3'); reverse: (5'-TTC TTC AGG CAG TCC ACG GT-3'). The endogenous control probe, specific for the GAPDH gene, served to standardize the amount of RNA sample and consisted of the following oligonucleotide sequence (5I-CCGACTCTTGCCCTTCGAAC-3I). The RT-reaction steps consisted of an initial 2 min incubation step at 50 °C to activate uracil glycosylase (UNG) and were followed by 30 min of reverse transcription at 60 °C. A completion step for UNG deactivation was performed for 5 min at 95 °C. The 40 cycles of two-step PCR-reaction consisted of a 20s period at 94 °C and a 1 min period at 62 °C.
Statistical analyses
We used GAPDH for RT-PCR as an internal standard as described previously in our other studies (Molteni et al., 2002). Quantification of the TaqMan RT-PCR results was performed by plotting fluorescent signal intensities against the number of PCR cycles on a semilogarithmic scale. A threshold cycle (CT) was designated as the amplification cycle at which the first significant increase in fluorescence occurred. The CT value of each sample was compared to that of the internal standard. These processes were fully automated and carried out using the ABI sequence detector software version 1.6.3 (PE Biosystem). Taqman EZ RT-PCR values for BDNF, CREB, and CAMKII were corrected by subtracting values for GAPDH as previously described (Molteni et al., 2002, Greisbach et al., 2002). These corrected values were used to make cross group comparisons. The mean values for the mRNA levels were computed for all four groups following completion of exercise and MWM training and compared using ANOVA. The analysis of maze data was performed in accordance with techniques described in Molteni et al. (2002), using an ANOVA with repeated measures. The mean values of distances (Km) run over the exercise period were computed for the exercise groups and compared using t-test. A Fischer-test was used for cross group comparisons. Results were expressed as the mean percent of control values for graphic clarity and represent the mean ± standard error of the mean (SEM). We used a regression analysis to evaluate the association between running distances (Avg Km/day) and MWM performance, both the acquisition (escape latency slope) and the consolidation phase (% time spent in P quadrant during probe trial), in each group.
Results
MWM performance; learning acquisition
To assess spatial learning acquisition, we used a challenging 2-trial-per-day, 5-day MWM paradigm, which has proven to be sufficiently sensitive for discerning learning differences between exercise and sedentary animals (Vaynman et al., 2004). Animals were trained every day for 5 consecutive days on the MWM task. Results showed that the escape latencies were similar between all four groups on day 1 of MWM training (Fig. 2). There was a significant “exercise X treatment interaction” found on days 2 [F(3,25) = 8.4, P < 0.001], 3 [F(3,25) = 9.5, P < 0.0005], 4 [F(3,25) = 8.2, P < 0.001], and 5 [F(3,25) = 5.5, P < 0.005] of the MWM task. We found that exercise significantly (p < 0.05) decreased the latency to locate the platform by day 3 as compared to sedentary groups (Sed and Sed/KN) and remained significant on days 4 and 5. Specifically, we found that Exc rats had significantly shorter escape latencies on day 3 (16.8 ± 3.3 s, p<0.05), 4 (11.6 ± 0.7 s, p<0.05), and 5 (7.3 ± 1.8 s, p<0.05) of MWM training as compared to Sed control rats (36.7 ± 5.3 s, 26.2 ± 3.8 s, and 18.2 ± 2.0 s, respectively; Fig. 2). Application of the CAMKII inhibitor failed to significantly alter the escape latencies of sedentary control animals from Sed controls; the Sed/KN group had similar escape latencies on days 3 (34.3 ± 2.2 s), 4 (20.6 ± 1.7 s), and 5 (18.3 ± 3.4 s) to those of Sed controls (Fig. 2). Injection of KN-62 into the hippocampus of exercising animals failed to significantly alter the exercise-induced effect on learning acquisition on days 3, 4, and 5 of MWM training; the Exc/KN animals had similar escape latencies on days 3 (14.6 ± 3.8 s), 4 (11.5 ± 2.5 s), and 5 (9.3 ± 1.7 s) to Exc animals (16.8 ± 3.3 s, 11.6 ± 0.7 s, and 7.3 ± 1.8, respectively). Application of the CAMKII inhibitor significantly (p<0.05) enhanced the learning acquisition of exercising animals on the MWM task on day 2; the Exc/KN animals had significantly faster escape latencies than exercising animals on day 2 (Exc/KN 28.4 ± 2.9 s vs. Exc 48.5 ± 3.8 s) as well as Sed control (51.7 ± 3.6 s) and Sed/KN animals (46.4 ± 4.4 s; Fig. 2).
Figure 2.
Effect of blocking CAMKII action during the exercise period on learning using the MWM task. Exercise effectively improved learning ability, as exercise animals took significantly less time to find the platform (shorter escape latencies in the Exc group). Blocking CAMKII action during exercise did not abolish the exercise-induced enhancement in learning ability, as the escape latencies of exercise animals given the CAMKII blocker (KN-62) were comparable on days 1, 3, 4, and 5 to exercise control animals (Exc/KN vs. Exc). Surprisingly, blocking CAMKII action in exercise animals enabled animals to acquire the faster learning curve by day 2 of MWM acquisition as opposed to day 3 seen in exercise control animals. *; indicates significance for the Exc group as compared to the Sed control group for that day. ‡; indicates significance for the Exc/KN group as compared to the Sed control group for that day Data are expressed as mean ± SEM. (ANOVA, Fischer test, *, ‡ p < 0.05).
MWM performance; memory retention
To evaluate memory retention, we performed a probe trial 2 days after the last MWM training day. Rats were allowed to swim for 60 seconds in the pool in which they received their training, but with the escape platform removed. The percentage of time spent in the probe quadrant, which previously housed the platform (quadrant P), was calculated for each animal. We found that the Exc group showed a clear preference for the P quadrant over Sed rats, as they spent a significantly (p< 0.05) greater percentage of time in quadrant P (48.2 ± 4.9%) than Sed controls (32.1 ± 2.7%). Administration of KN-62 fully prevented the exercise-induced preference for the target quadrant, such that there was no difference between the amount of time spent by Exc/KN rats (34.6 ± 3.9%) and Sed controls in quadrant P (Fig. 3A). KN-62 administration did not significantly affect the preference of Sed/KN rats for quadrant P, as there was no significant difference in the percentage of time spent in quadrant P between Sed/KN (36.2 ± 3.6%) and sedentary control rats (Fig. 3A). To control for differences in MWM performance, we recorded each animal’s swimming speed. We found no difference in swimming speeds between all four groups; Exc (64.8 ± 3.2 cm/sec), Sed (67.2 ± 27 cm/sec), Exc/KN (70.2 ± 3.3 cm/sec), and Sed/KN (64.6 ± 2.6 cm/sec;Fig. 3C). We recorded the running distances for each exercise group and found that the average distance (Km) run per day did not significantly differ between the exercise groups, Exc (2.42 ± .86) and Exc/KN-62 (2.70 ± .96).
Effect of Exercise and CAMKII blockage on CAMKII expression
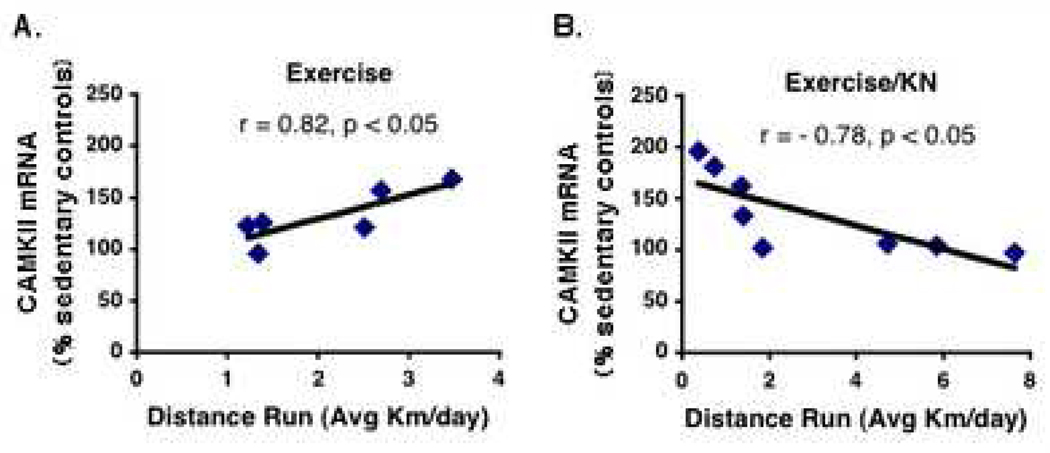
We found that exercise increased CAMKII expression (131.8 ± 10.7%) above sedentary controls (100 ± 5.1%). Blocking CAMKII did not significantly affect CAMKII mRNA levels in sedentary animals (92 ± 4.6%). Although the administration of the CAMKII inhibitor into the hippocampi of exercising animals did not inhibit the exercise induced increase in CAMKII expression (135.1 ± 14.0%), the CAMKII inhibitor did block the association between exercise and CAMKII expression. We found a significant (p < 0.05) positive correlation in individual animals, between running distance (Km/day) and CAMKII expression in exercising animals (r = 0.82, n=6; Fig.4A). Conversely, application of the CAMKII inhibitor disrupted this association and resulted in a negative correlation between running distance and CAMKII expression (r = −0.78, n=8; Fig. 4B).
Figure 4.
A significant positive correlation was found between running distance (Avg Km/day) and CAMKII expression in the Exc group (Excise; r = 0.82, n=6, p < 0.05). Administration of the CAMKII blocker disrupted this positive association and resulted in a negative correlation between running distance and CAMKII expression (Exercise/KN; r = −0.78, n=8, p < 0.05) in individual animals. The association is illustrated by the computer generated best-fit line.
Effect of exercise and CAMKII blockage on BDNF and CREB
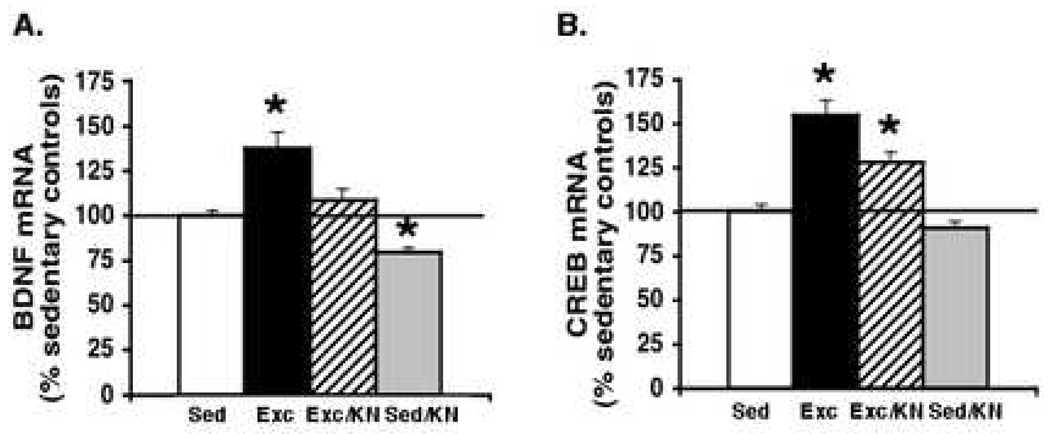
Exercise significantly (p < 0.05) increased the mRNA levels of BDNF (137.5 ± 8.95%) and CREB (154 ± 8.47%) in the Exc groups above those of Sed controls (Fig. 5A–B). Blocking CAMKII with KN-62 was sufficient to completely abrogate the exercise-induced increase in BDNF (137 ± 9% to 108.5 ± 7%), effectively reducing BDNF expression to Sed control levels (Fig. 5A, 5B: Exc/KN vs. Sed). In contrast, KN-62 significantly (p < 0.05) but only partially knocks out the exercise-induced increase in CREB (154.57 ± 8.47% to 128.25 ± 5.79%; Fig. 5B). KN-62 application seemed to have no effect during the sedentary condition on CREB mRNA levels (91.13 ± 3.30%), but was effective at reducing BDNF levels (79.38 ± 3.06%) slightly below Sed control levels.
Figure 5.
Effect of blocking CAMKII action during the exercise period on the mRNA levels of BDNF and CREB, as measured after the probe trial. Results are displayed as percentages of Sed control levels (represented by the 100% line). Exercise significantly increased the mRNA levels of BDNF (A) and CREB (B) above those of sedentary controls (p < 0.05). Blocking CAMKII action abolished the exercise-induced increase in BDNF expression (A) and significantly (p < 0.05) but partially reduced the exercise-induced increase in CREB mRNA levels (B). Blocking CAMKII action had no significant effect on the expression of CREB in sedentary animals but significantly reduced BDNF mRNA levels below sedentary controls. Each value represents the mean ± SEM. (ANOVA, Fischer test, * p < 0.05).
DISCUSSION
This study provides novel evidence that CAMKII serves a refined role in mediating the effects of exercise on cognitive function, selectively functioning to mediate the exercise-induced enhancement in memory retention. Application of the CAMKII inhibitor KN-62 during the exercise period was sufficient to prevent the exercise-induced enhancement in recall and abrogate the exercise-induced increase in the mRNA levels of BDNF and a specific consummate end-product of BDNF action, CREB. We found that running distance in exercising animals was significantly associated with the amount of CAMKII expression in the hippocampus. The CAMKII blocker not only eliminated this association during exercise but also resulted in inversing the relationship between the amount of exercise and CAMKII expression. Interestingly, application of the CAMKII inhibitor during the exercise period only partially blunted the exercise-induced increase in CREB expression but fully abolished the exercise-induced increase in BDNF.
The role of CAMKII in mediating the exercise-induced enhancement in learning and memory
The hippocampus is a critical brain area involved in learning and memory, whose plasticity is prominently affected by exercise (Vaynman et al., 2004). Blocking experiments revealed that hippocampal CAMKII is involved in mediating the ability of exercise to enhance learning and memory. Exercise enhanced learning acquisition; exercise rats receiving control injections had shorter escape latencies to find the platform than their sedentary counterparts (Fig. 2). Application of the CAMKII inhibitor during exercise did not significantly alter the latency to acquire the task on days 1, 3, 4, 5 from that of exercising controls, although it managed to significantly decrease the latency of acquisition on day 2 of the MWM task (Exc/KN vs. Exc; Fig. 2). We found that exercise enhanced the recall ability of animals above sedentary control levels; exercise animals spent significantly more time in quadrant P than their sedentary counterparts (Exc vs. Sed; Fig. 3A–B). Presence of the CAMKII inhibitor during the exercise period effectively abolished this exercise-induced recall effect (Exc/KN vs. Exc; Fig. 3A). Importantly, we found that the CAMKII inhibitor was selective for mediating the effects of exercise in the hippocampus as it did not noticeably affect the acquisition or recall abilities of sedentary animals (Sed/KN vs. Sed; Fig. 2, Fig. 3A). Although, we found that the CAMKII inhibitor did not abolish the exercise-induced increase in CAMKII expression, we found that the amount of running was associated with the levels of CAMKII, such that animals that exercised more had greater levels of CAMKII expression in their hippocampi (Fig. 4). As KN-62 is a specific inhibitor that interacts with the regulatory domain of CAMKII (Tokomitsu et al., 1990) to block the active form of CAMKII (Kim et al., 2006), the effect of the CAMKII inhibitor on the CAMKII system may be more conspicuous from our finding that KN-62 administration in exercising animals disrupted this positive association between exercise and CAMKII. CAMKII inhibition resulted in a negative association between CAMKII expression and running distance, such that CAMKII expression incrementally decreased with more exercise in individual animals (Fig. 4; Exc vs. Exc/KN).
A possible explanation for the enhancement in learning seen on day 2 under the CAMKII inhibitor may be educed from experiments showing that CAMKII serves as an inhibitory constraint on hippocampal neurotransmitter release during high frequency presynaptic activity (Hinds et al., 2003). As KN-62 did not have any effect in sedentary controls, the effect of exercise on the synapse may be specifically related to the CAMKII system. Indeed, previous work in our lab has demonstrated a specific effect of exercise on vesicular release proteins in the hippocampus (Vaynman et al., 2005), of which some have been found to be under the regulatory control of the CAMKII inhibitor KN-62 (Vaynman et al., 2004). Thus, it is possible that blocking CAMKII may have disinhibited its constraint on synaptic release to result in an enhancement in learning acquisition only in exercising animals.
A recent study has reported the capacity of CAMKII activation to disrupt the formation of spatial memory using the MWM task (Yasuda & Mayford, 2006). Moreover, earlier research has demonstrated that blocking CAMKII with KN-62 has a facilitative effect on learning using the conditioned taste aversion paradigm in rats (Saccetti et al., 2001). This hypothesis has recently been reinforced by the finding that an upregulation of endogenous CAMKII inhibitors in the hippocampus, occurring shortly after an associative learning task, may serve a prominent facilitative role in the learning and memory consolidation processes (Lepicard et al., 2006). Given the complexity of learning and memory mechanisms, it may be postulated that spatial and temporal constraints may dictate the functionality of CAMKII action. Thus, CAMKII may function both as an inhibitory constraint on vesicular release at certain presynaptic sites and as a promoter of synaptic strengthening at the postsynaptic membrane. The potential physiological significance of this may be applied to information processing in the hippocampus. A recent study has found that CAMKII is central to spike timing-dependent plasticity in the hippocampus, such that the perseverance of potentiation or depression is dependent on the precise timing of pre- and post-synaptic spikes (Wang et al., 2005). These findings demonstrating the dual nature of CAMKII action may explain why we found that the CAMKII inhibitor produced an enhancement in learning acquisition (as seen on days 2 in Exc/KN animals; Fig. 2), but in the long-term, it precluded memory recall (as seen by the behavior of Exc/KN animals on the probe trial; Fig. 3). Importantly, the action of the CAMKII blocker appears to occur during exercise as supported by the finding that sedentary animals exposed to the inhibitor had similar acquisition latencies and memory retention to those sedentary animals that received the control injection. If the action of CAMKII was blocked during the MWM task, sedentary animals receiving KN-62 would have performed below sedentary control levels. Thus, if the effects of KN-62 on learning acquisition and retention are related to CAMKII’s mediation of spike-timing dependent plasticity, these effects act as preparatory mechanisms to strengthen or weaken synaptic properties that ultimately mediate exercise-induced cognitive enhancement.
CAMKII modulates BDNF and CREB expression under the direction of exercise; implications for learning and memory formation
The results of this study demonstrate that the CAMKII blocker during exercise may be highly successful at paring down the exercise-induced increase in BDNF and its subsequent effect on synaptic plasticity underlying exercise-induced cognitive enhancement (Fig. 4A–B). The CAMKII inhibitor significantly but only partially prevented the exercise-induced increase in CREB mRNA levels (Fig. 4B), although it fully eliminated the exercise-induced enhancement in memory retention (Fig. 3). This was surprising given the long procession of studies asserting the importance of CREB in activity-dependent long-term neuronal plasticity, especially as an evolutionarily conserved molecule necessary for the formation of long-term memory (Dash et al., 1990; Bourtchouladze et al., 1994; Yin et al., 1995). It has postulated that the increase in CREB expression during learning facilitates long-term memory formation by reducing the usual requirements for repetition and rest during training (Yin et al., 1995). The exercise-induced enhancement in CREB expression, which we have also found to be significantly increased immediately after exercise (Vaynman et al., 2003), may enable exercise animals to both learn and recall the location of the platform with less training.
The partial reduction in CREB expression (Fig. 5B) seen under the CAMKII blocker may explain why under the exercise-enhanced learning acquisition persists (Fig. 3). It is possible that this lingering CREB expression is due to the action of additional alternate signaling pathways that act in concert with BDNF to mediate the effects of exercise on synaptic plasticity (Vaynman et al., 2003). One such alternative signaling pathway may be mitogen-activated protein kinase (MAPK), which we previously found to be a selective pathway in regulating CREB expression (as it did not modulate BDNF and TrkB mRNA levels) during exercise (Vaynman et al., 2003). CAMKII may be under the dominion of other upstream elements, such as the NMDA receptor during exercise (Vaynman al., 2003), which has been found to regulate the activity, synaptic level, and localization of CAMKII (Lisman et al., 2002; Thiagarajan et al., 2002). Regulation of CAMKII by the NMDA receptor may also contribute to the discriminative effects of CAMKII action as it would provide for spatial specificity, localizing it to certain regions of the hippocampus, such as CA1, which fall under NMDA receptor mediated synaptic plasticity (Tsien et al., 1996; Geddes et al., 1986). A recent study found that the phosphorylation of CAMKII and CREB are NMDA-R-dependent in CA1 during late LTP, a period which is proposed to coincide with the consolidation phase of memory (Ahmed & Frey, 2005). Moreover, we previously found that exercise-induced increases in BDNF levels did not occur in CA1 but were localized to CA3 and DG regions of the hippocampus (Vaynman et al, 2004a). Taken together, these findings suggest differential regulation of CAMKII by the NMDA receptor and BDNF as dictated by its spatial hippocampal designation.
Conclusions
The benefits gained by voluntary exercise on synaptic and cognitive plasticity have elicited the fundamental question of what molecular mechanisms serve to impart these effects. Central to answering this question was the finding that exercise employs a BDNF-mediated mechanism to promote synaptic plasticity and learning and memory in the hippocampus (Vaynman et al., 2003. 2004b). In an effort to elaborate on the downstream mechanisms of BDNF action, this study tested the importance of CAMKII in mediating the exercise-induced enhancement in hippocampal dependent learning and memory. Our results provide evidence that exercise may employ CAMKII to selectively mediate the facilitative effects of exercise on memory formation. Finally, our results illustrate that, while CAMKII may have a modulatory impact on BDNF and CREB expression it may not supersede the importance of BDNF action in mediating the effects of exercise on neural and cognitive function in the hippocampus.
Acknowledgments
This study was supported by NIH awards NS 045804 and NS 39522
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neurosci. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 3.Barde YA. Neurotrophins: A family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 1994;390:45–56. [PubMed] [Google Scholar]
- 4.Blanquet PR, Lamour Y. Brain-derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J Biol Chem. 1997;272:24133–24136. doi: 10.1074/jbc.272.39.24133. [DOI] [PubMed] [Google Scholar]
- 5.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 6.Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta- mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- 7.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 8.de Quervain DJ, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci U S A. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 11.Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 12.Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 13.Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 14.Geddes JW, Chang-Chui H, Cooper SM, Lott IT, Cotman CW. Density and distribution of NMDA receptors in the human hippocampus in Alzheimer's disease. Brain Res. 1986;399:156–161. doi: 10.1016/0006-8993(86)90611-6. [DOI] [PubMed] [Google Scholar]
- 15.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Research. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- 17.Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch. Phys. Med. Rehabil. 1999;80:661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 18.Griesbach GS, Hovda DA, Molteni R, Gomez-Pinilla F. Alterations in BDNF and synapsin I within the occipital cortex and hippocampus after mild traumatic brain injury in the developing rat: reflections of injury-induced neuro-plasticity. Neurotrauma. 2002;19:803–814. doi: 10.1089/08977150260190401. [DOI] [PubMed] [Google Scholar]
- 19.Hinds HL, Goussakov I, Nakazawa K, Tonegawa S, Bolshakov VY. Essential function of alpha-calcium/calmodulin-dependent protein kinase II in neurotransmitter release at a glutamatergic central synapse. Proc Natl Acad Sci U S A. 2003;100:4275–4280. doi: 10.1073/pnas.0530202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito I, Hidaka H, Sugiyama H. Effects of KN-62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, on long-term potentiation in the rat hippocampus. Neurosci Lett. 1991;121:119–121. doi: 10.1016/0304-3940(91)90663-e. [DOI] [PubMed] [Google Scholar]
- 21.Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Lee J-A, Song YM, Park C-H, Hwang S-J, Kim Y-S, Kaang B-K, Son H. Overexpression of calbindin-D28K in hippocampal progenitor cells increases neuronal differentiation and neurite outgrowth. The Faseb Journal. 2006;20:109–111. doi: 10.1096/fj.05-4826fje. [DOI] [PubMed] [Google Scholar]
- 23.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 24.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 25.Lepicard EM, Mizuno K, Antunes-Martins A, von Hertzen LS, Giese KP. An endogenous inhibitor of calcium/calmodulin-dependent kinase II is up regulated during consolidation of fear memory. Eur J Neurosci. 2006;23:3063–3070. doi: 10.1111/j.1460-9568.2006.04830.x. [DOI] [PubMed] [Google Scholar]
- 26.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 27.Lo DC. neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 28.Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J. Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 30.Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 31.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 32.Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of Brain-derived neurotrophic factor. Neurosci. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 34.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 35.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 36.Quattrochi JJ, Mamelak AN, Madison RD, Macklis JD, Hobson JA. Mapping neuronal inputs to REM sleep induction sites with carbachol-fluorescent microspheres. Science. 1989;245:984–986. doi: 10.1126/science.2475910. [DOI] [PubMed] [Google Scholar]
- 37.Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques. 1997;23:928–937. doi: 10.2144/97235rr02. [DOI] [PubMed] [Google Scholar]
- 38.Riddle DR, Lo DC, Katz LC. NT-4-mediated rescue of lateral geniculate neurons from effects of monocular deprivation. Nature. 1995;378:189–191. doi: 10.1038/378189a0. [DOI] [PubMed] [Google Scholar]
- 39.Saccetti B, Baldi E, tassoni G, Bielavski E. CAMKII inhibition in the parabrachial nuclei elicits conditioned taste averion in rats. Neurob of Learning and Memory. 2001;75:253–261. doi: 10.1006/nlme.2000.3978. [DOI] [PubMed] [Google Scholar]
- 40.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 41.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 42.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 44.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 45.Tokomitsu H, Chijiwa T, Hagiwara M, Mizitani A, Terasawa M, Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4 phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- 46.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–3138. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 47.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 48.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between BDNF and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neurosci. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004a;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 50.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004b;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 51.Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 52.Wang HX, Gerkin RC, Nauen DW, Bi GQ. Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat Neurosci. 2005;8:187–189. doi: 10.1038/nn1387. [DOI] [PubMed] [Google Scholar]
- 53.Wolfman C, Izquierdo LA, Schroder N, Izquierdo I. Intra-hippocampal KN-62 hinders the memory of habituation acquired alone, but not simultaneously with a water-finding task. Behav Pharmacol. 1999;10:99–104. doi: 10.1097/00008877-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda M, Mayford MR. CAMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron. 2006;50:309–318. doi: 10.1016/j.neuron.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 55.Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 56.Yin JC, Tully T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]