Abstract
We have initiated a systematic functional analysis of the MADS box, intervening region, K domain, C domain-type MADS box gene family in petunia. The starting point for this has been a reverse-genetics approach, aiming to select for transposon insertions into any MADS box gene. We have developed and applied a family signature insertion screening protocol that is highly suited for this purpose, resulting in the isolation of 32 insertion mutants in 20 different MADS box genes. In addition, we identified three more MADS box gene insertion mutants using a candidate-gene approach. The defined insertion lines provide a sound foundation for a systematic functional analysis of the MADS box gene family in petunia. Here, we focus on the analysis of Floral Binding Protein2 (FBP2) and FBP5 genes that encode the E-function, which in Arabidopsis has been shown to be required for B and C floral organ identity functions. fbp2 mutants display sepaloid petals and ectopic inflorescences originating from the third floral whorl, whereas fbp5 mutants appear as wild type. In fbp2 fbp5 double mutants, reversion of floral organs to leaf-like organs is increased further. Strikingly, ovules are replaced by leaf-like structures in the carpel, indicating that in addition to the B- and C-functions, the D-function, which specifies ovule development, requires E-function activity. Finally, we compare our data with results obtained using cosuppression approaches and conclude that the latter might be less suited for assigning functions to individual members of the MADS box gene family.
INTRODUCTION
MADS box genes encode a family of transcription factors that are involved in many different developmental processes. More than a decade ago, genetic analysis of floral homeotic mutants in snapdragon and Arabidopsis (Schwarz-Sommer et al., 1990; Yanofsky et al., 1990) led to the discovery of the first MADS box genes in plants and subsequently accelerated the molecular elucidation of the classic ABC model of flower development (Coen and Meyerowitz, 1991). Except for the A-function gene AP2 (Jofuku et al., 1994), all of the major players in the ABC model belong to the MADS box gene family. Much of the initial appeal of this model may be ascribed to its basic simplicity and to the fact that the orthologous genes analyzed in Arabidopsis and snapdragon appeared to be very comparable in number and function, indicating that the model might be a universal model for floral development. Today, MADS box research is revealing a much more detailed and complex picture. Whereas the original ABC model was based largely on the analysis of flower developmental mutants using classic forward-genetics approaches, recent progress using reverse-genetics strategies has revealed redundant functions that were missed before (Liljegren et al., 2000; Pelaz et al., 2000). Likewise, recent studies using protein–protein interactions to elucidate complex formation between the products of different MADS box genes have led to a far more complex interaction scheme than the originally proposed heterodimer formation between the B-function genes. Combining these results has led to extensions of the ABC model toward models with a higher complexity, including a novel class of genes called identity-mediating or E-function genes required for B and C floral organ identity functions (Egea-Cortines et al., 1999; Egea-Cortines and Davies, 2000; Pelaz et al., 2000; Honma and Goto, 2001; Jack, 2001; Theissen and Saedler, 2001).
Furthermore, the MADS box gene family is gaining more and more attention from an evolutionary perspective (Purugganan et al., 1995; Tandre et al., 1995; Theissen and Saedler, 1995; Theissen et al., 1996; Munster et al., 1997; Kramer and Irish, 1999; Winter et al., 1999; Yu et al., 1999; Lamb and Irish, 2003; Vandenbussche et al., 2003). Changes in MADS box gene structure and function during evolution have been suggested to be a major cause for the overwhelming diversity in flower/plant architecture that can be observed in nature today (Theissen et al., 2000). Thus, a comparative functional analysis of MADS box genes in closely and more distantly related species might reveal basic differences in gene activity/function and their specific involvement in floral development. Such studies may include the isolation of a (set of) MADS box gene(s) from a particular species, its sequence, and expression analysis and complementation experiments involving a mutant for the presumed ortholog in Arabidopsis. However, the final determination of the function of any particular gene requires a knockout study in the species of origin.
In petunia, the elucidation of MADS box protein dimer and higher order complex formation is well under way (Ferrario et al., 2003; Immink et al., 2003), and there is an extensive body of research that has used ectopic expression and/or cosuppression studies to identify floral developmental functions. For example, ectopic expression and cosuppression phenotypes of Floral Binding Protein7 (FBP7) and FBP11 led to the proposal of a new organ identity activity encoded by these genes, called the D-function, that is necessary for the determination of ovule identity (Angenent et al., 1995; Colombo et al., 1995, 1997). Indications for the existence of an E-function were presented in 1994 based on the phenotypes of FBP2 and TM5 cosuppression lines in petunia and tomato, respectively (Angenent et al., 1994; Pnueli et al., 1994), although the exact function was not well understood at that time. Nevertheless, although overexpression and cosuppression phenotypes may be considered indicative of the existence of certain functions, it may be difficult to attribute specific functions to individual genes using these approaches.
To address the issues mentioned above (adding to the comparative analysis, further elucidation of additional functions, analyzing mutants in the species of origin, and the limitations of forward and transgenic approaches), we initiated a systematic functional analysis of the petunia MADS box gene family. To this end, we developed an alternative and highly efficient transposon insertion screening strategy that is ideal for the functional analysis of large and homologous gene families, especially in species for which the genome sequence is not yet available. The petunia transposon line used for the insertion screenings is W138, which harbors ∼120 to 200 copies of the endogenous 284-bp nonautonomous dTph1 (defective transposon Petunia hybrida1) element (Gerats et al., 1990; De Keukeleire et al., 2001). The small dTph1 transposon contains stop codons in all three reading frames in both orientations and usually leaves a footprint behind upon excision. Here, we specifically targeted the well-conserved MADS box domain itself (including the first part of the upstream regulatory regions). In parallel, we analyzed six independent mutants selected on a phenotypic basis and displaying homeotic conversions of petals to sepals in various degrees of severity. Three of these mutants could be identified using a candidate-gene approach to represent insertions into specific MADS box genes. Here, we present a first analysis of the two E-function genes FBP2 and FBP5 and compare our overall results with results obtained previously in cosuppression experiments.
RESULTS
General Screening Strategy: Family Signature Insertion Mutagenesis
The selection and analysis of insertion mutants (either T-DNA or transposon insertants) provides a powerful approach for determining the biological functions of specific genes. In theory, such a reverse-genetics approach may sound simple. In practice, however, insertions in single genes (especially members of large and homogeneous gene families) often do not cause a visible phenotype as a result of redundancy (Bouchez and Hofte, 1998; Winkler et al., 1998; Bouche and Bouchez, 2001). Therefore, the functional analysis of such gene families requires the construction of lines carrying mutations in different family members, shifting the emphasis in insertion screenings from single genes to the gene family level.
As a result of background amplification, classic PCR-based insertion screening protocols, including the original petunia dTph1 screening system (Koes et al., 1995), rely on a final hybridization step with a gene-specific probe to unequivocally identify candidate fragments amplified from pooled DNA samples. When screening for insertions into genes that belong to large and highly homologous gene families, cross-hybridization between family members can seriously complicate downstream analysis. This problem might be circumvented by choosing primers and probes in nonconserved regions (Meissner et al., 1999), but then a separate screen for each individual family member is required, making the entire screening process labor intensive, while only the known members of the family can be addressed.
To update the existing screening protocol for the analysis of large gene families and to take advantage of conserved domains present in such families, we optimized PCR conditions, primer design, and product detection. Important improvements were combining hot-start PCR (D'Aquila et al., 1991) with a specific touchdown PCR profile (Don et al., 1991) and the design of screening primers according to strict rules to preferentially recognize members of the gene family to be screened for. We call such primers family signature (FS) primers (see Methods). Using these long screening primers (typically 26- to 30-mers) characterized by high Tm values (70 to 76°C) in combination with a rapidly declining PCR touchdown profile, we achieved PCR conditions under which a number of primer-template mismatches are accepted while still amplifying mainly family-specific amplicons. As a result, insertions into related and yet unknown family members can be recovered using the same FS screening primer.
All of the improvements mentioned above allowed us to use the following setup. A labeled FS primer designed based on a conserved family domain region, and a second PCR primer based on the terminal inverted repeat sequence of the petunia dTph1 transposon, are combined in an optimized PCR procedure. Putative insertants are isolated in a single step from libraries of up to 4000 plants by analyzing only 48 pooled samples. Because this kind of setup usually results in complex gel patterns due to the amplification of multiple insertions into different family members, the resulting amplification products are sized by PAGE. Putative insertion flanking fragments are isolated from the gel, reamplified, and sequenced directly. Because the identification of insertion events is sequence based, insertions into highly similar genes can be distinguished easily, and the exact insertion position also is determined. This approach allows the simultaneous identification of insertion events in different family members and has the additional advantage of identifying unknown family members through their corresponding insertion mutants.
Screening for MADS Box Gene Insertion Mutants
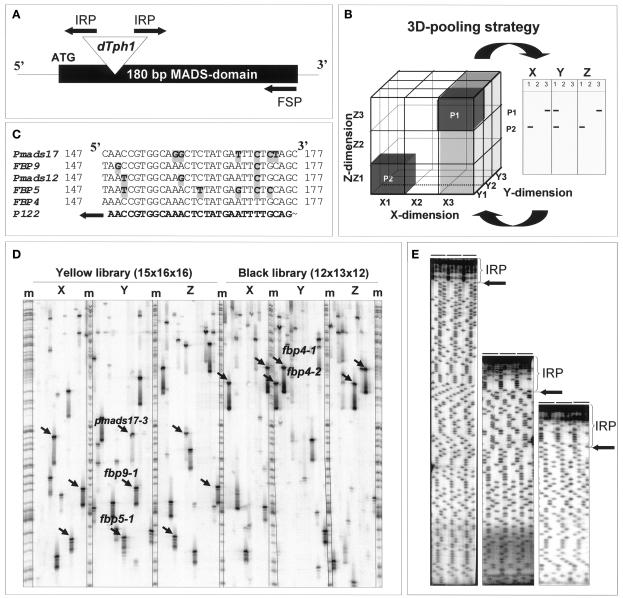
To screen for insertions into any member of the MADS box, intervening region, K domain, C domain (MIKC)-type MADS box gene family, we designed a set of reverse FS primers to anneal at the 3′ end of the highly conserved 180-bp MADS domain (Figure 1A), aiming to identify insertions into the MADS box region or in the 5′ untranslated region and promoter regions of known and unknown family members. An example of a successful screening is shown in Figure 1D. Two insertion libraries were screened with an FS primer derived from the petunia FBP4 gene. Because the insertion libraries are pooled according to a three-dimensional matrix (Figure 1B), insertants are identified by the amplification of a unique set of three fragments of identical size, one for each dimension (indicated by arrows in Figure 1D). All other fragments represent either somatic insertion events or spurious amplicons. Such fragments are ignored. Direct sequencing of the five sets of candidate fragments present on this particular gel revealed insertions in the MADS domain of FBP4 (two independent events), FBP5, FBP9, and PMADS17. Typical results obtained by direct sequencing of reamplified fragments are shown in Figure 1E.
Figure 1.
Overview of the FS Insertion Screening Strategy.
(A) Design of a reverse FS primer (FSP) at the 3′ end of the conserved MADS domain. IRP represents the terminal inverted repeat–based dTph1 transposon primer.
(B) Scheme of the three-dimensional pooling strategy for the insertion libraries. Leaves of each plant are harvested three times (e.g., material from plant 1 [P1] is present in the pools consisting of all individuals in the X3 [gray], Y1, and Z3 planes). If P1 contains an insertion in the gene of interest, three fragments amplified with a gene-specific primer and a transposon primer will appear on the gel, one in each dimension and displaying identical sizes. The positioning of these three fragments on the gel yields a unique address (X, Y, and Z coordinates), allowing the identification of a single positive plant in the original population.
(C) Alignment of part of the MADS domain of the MADS box family members, for which we recovered insertions using an FS primer (reverse and complement of sequence P122) based on the FBP4 gene. Nucleotide mismatches between primer and templates are shaded gray.
(D) Partial gel image from an insertion screening of two insertion libraries with an FS primer based on the FBP4 gene. Amplification products were visualized by labeling the FS primer and sized on a 4.5% polyacrylamide gel. Arrows indicate unique three-dimensional coordinates representing plants that contain insertions in the MADS domain of FBP4, FBP5, FBP9, and PMADS17. M indicates molecular marker lane.
(E) Typical direct sequencing results from reamplified fragments. IRP represents the dTph1 transposon primer sequence, and arrows indicate the precise transposon insertion positions.
To date, we have identified 32 insertions into 20 different family members (Table 1) by screening 12,697 plants with 11 different FS primers. Sixteen of these represent insertions in the MADS domain of 12 different MADS box genes. Approximately half of the insertions in MADS box genes were identified with FS primers based on the sequences of other MADS box genes. These insertions could be identified despite the presence of up to six mismatches between primer and template. As an example, we present the relevant alignment of the MADS box genes for which we found insertions with an FBP4-derived FS primer (Figure 1C).
Table 1.
Overview of Identified Petunia MADS Box Gene Family Members and Corresponding Mutants
| Insertion Position (ATG Start Codon = 1)
|
|||||||
|---|---|---|---|---|---|---|---|
| Subfamily | Subfamily Members | Mutant Allele | Mutagen | 5′ of ATG | In the Open Reading Frame | Progeny Analysis | Reference a |
| SQUA/AP1 | PFG | pfg-1 | dTph1 | +5 bp | Confirmed | This study | |
| FBP26 | fbp26-1 | dTph1 | +74 bp | Confirmed | This study | ||
| FBP29 | |||||||
| DEF/AP3 | GP | gp (PLV) | γ-radiation | Chromosomal deletion | (1) | ||
| PHTM6 | |||||||
| GLO/PI | FBP1 | fbp1-1b | dTph1 | +599 bp | Confirmed | (2) | |
| fbp1-2b | dTph8 | +411 bp | Confirmed | This study | |||
| PMADS2 | pmads2-1 | dTph1 | −171 bp | Confirmed | This study | ||
| pmads2-2 | dTph1 | −84 bp | Confirmed | This study | |||
| pmads2-3 | dTph1 | +49 bp | Confirmed | This study | |||
| Bsister | FBP24 | fbp24-1 | dTph1 | +56 bp | Confirmed | This study | |
| AG | PMADS3 | ||||||
| FBP6 | |||||||
| FBP7/11 | FBP7 | fbp7-1 | dTph1 | +28 bp | Confirmed | This study | |
| fbp7-2 | dTph1 | +64 bp | Confirmed | This study | |||
| FBP11 | fbp11-1 | dTph1 | −2 bp | Confirmed | This study | ||
| AGL2 | FBP2 | fbp2-1b | dTph1 | +332 bp | Confirmed | This study | |
| fbp2-2b | dTph1 | +440 bp | Confirmed | This study | |||
| FBP4 | fbp4-1 | dTph1 | −10 bp | Confirmed | This study | ||
| fbp4-2 | dTph1 | +37 bp | Confirmed | This study | |||
| fbp4-3 | dTph1 | +48 bp | Confirmed | This study | |||
| FBP5 | fbp5-1 | dTph1 | +120 bp | Confirmed | This study | ||
| FBP9 | fbp9-1 | dTph4c | +101 bp | Confirmed | This study | ||
| FBP23 | fbp23-1 | dTph1 | +15 bp | No seeds | This study | ||
| PMADS12 | pmads12-1 | dTph1 | −220 bp | Confirmed d | This study | ||
| pmads12-2 | dTph1 | −181 bp | Confirmed | This study | |||
| AGL6 | PMADS4 | pmads4-1 | dTph1 | −200 bp | Confirmed | This study | |
| TM3 | FBP20 | fbp20-1 | dTph1 | −20 bp | Confirmed | This study | |
| fbp20-2 | dTph1 | +120 bp | Confirmed | This study | |||
| FBP21 | |||||||
| FBP22 | |||||||
| FBP28 | fbp28-1 | dTph1 | −10 bp | Confirmed | This study | ||
| fbp28-2 | dTph1 | +56 bp | Confirmed | This study | |||
| PMADS10 | pmads10-1 | dTph1 | −153 bp | Confirmed | This study | ||
| PMADS16 | pmads16-1 | −43 bp | N.T.e | This study | |||
| STMADS11 | FBP13 | fbp13-1 | dTph1 | −450 bp | Confirmed d | This study | |
| FBP25 | |||||||
| AGL15 | PMADS9 | pmads9-1 | −125 bp | Confirmed | This study | ||
| AGL17 | PMADS14 | pmads14-1 | dTph1 | −250 bp | Confirmed d | This study | |
| pmads14-2 | dTph1 | +74 bp | Confirmed | This study | |||
| PMADS6 | PMADS6 | pmads6-1 | dTph1 | −230 bp | Confirmed | This study | |
| pmads6-2 | dTph1 | −10 bp | Confirmed | This study | |||
| PMADS15 | pmads15-1 | dTph1 | −90 bp | Confirmed | This study | ||
| PMADS17 | pmads17-1 | dTph1 | +4 bp | Confirmed | This study | ||
| pmads17-2 | dTph1 | +48 bp | Confirmed | This study | |||
| pmads17-3 | dTph1 | +76 bp | Confirmed | This study | |||
(1), van der Krol et al., 1993; (2), Van den Broeck et al., 1998; M. Sauer, unpublished results.
Insertion mutants that were identified using a candidate-gene approach.
The low-copy-number 787-bp dTph4 transposable element is another endogenous petunia transposon (Renckens et al., 1996) that shares extensive identity with dTph1 and has identical terminal inverted repeats. Therefore, the dTph1-derived transposon primer also may recognize the more rare dTph4 insertion events.
Transposon insertion was confirmed in the parental line only; progeny were not analyzed.
N.T., insertion was not transmitted to the progeny, or false positive.
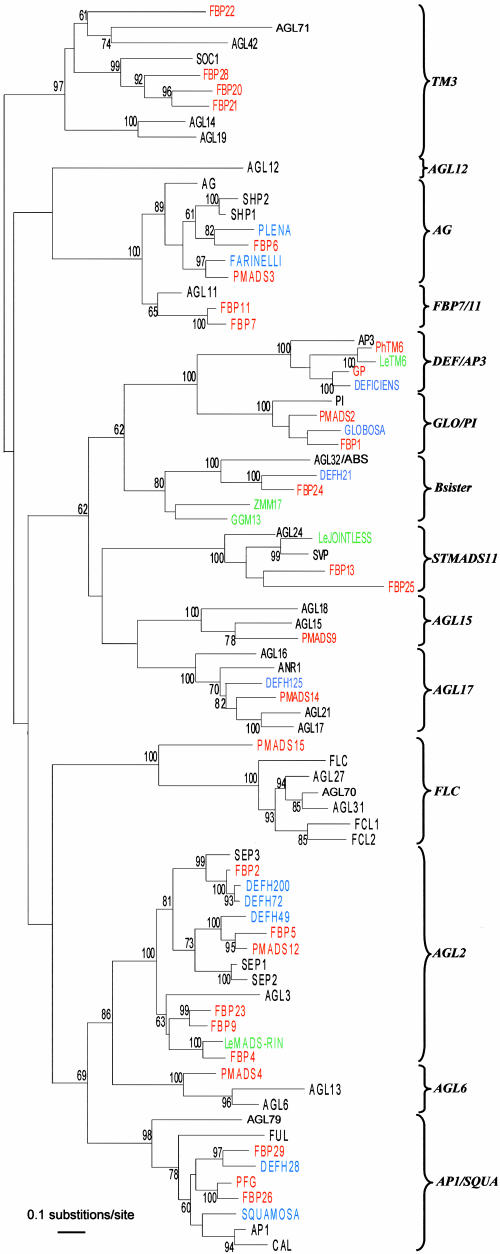
The recovered insertion flanking fragments corresponded partly to cDNA sequences already present in the database or to sequences that had been submitted previously, whereas six family members, called PMADS9, -10, -12, -14, -15, and -16, were encountered for the first time. A comparative phylogenetic analysis of MIKC-type MADS box gene family members from Arabidopsis and petunia and a selection of MADS box genes from other species is shown in Figure 2, suggesting that petunia representatives for all major Arabidopsis subfamilies (Parenicova et al., 2003) have been isolated. In addition to the plant-specific MIKC-type MADS box gene family, another MADS box gene lineage called type I (Alvarez-Buylla et al., 2000) has been identified in plants for which functional data still are largely lacking. The analysis presented here does not include this particular class of MADS box genes.
Figure 2.
Neighbor-Joining Tree of MIKC-Type MADS Box Genes from Petunia, Arabidopsis, Snapdragon, and Selected Members from Other Species.
Genes isolated from Arabidopsis, petunia, and snapdragon are shown in black, red, and blue, respectively. All others (Le-RIN, Le-JOINTLESS, and LeTM6 from tomato, ZMM17 from maize, and GGM13 from Gnetum gnemon) are shown in green. Local bootstrap probabilities are indicated for branches with >60% support. The partial sequences of the petunia MADS box genes PMADS6, -10, -16, and -17 were not included in the phylogenetic analysis because this would have resulted in poorly supported trees. However, PMADS6 and -17 cluster with PMADS15, forming a distinct outgroup of the Arabidopsis AGL27-like genes, and PMADS10 and -16 belong to the TM3 subfamily.
Transmission of Insertion Alleles and Phenotypic Analysis
Transmission of nearly all of the insertion alleles was confirmed in progeny of selfings of the original heterozygous insertants (Table 1) and subjected to a segregation analysis (data not shown) using gene-specific primer pairs flanking the insertion sites. For insertions in the 5′ untranslated region or promoter regions and for insertions into new family members isolated during the screening, initially no flanking sequence from the other side of the transposon insertion site was available. In those cases, we first obtained additional flanking sequence by genome walking, resulting in a data set of partial MADS box gene promoter sequences of members from several subfamilies (see Methods). Surprisingly, from the whole set of MADS box gene insertion mutants selected with our reverse screening approach, only mutants homozygous for the fbp9-1 insertion allele displayed a clear phenotype. These mutants exhibit aberrations in plant architecture during the reproductive phase.
Identification of MADS Box Gene Insertion Mutants Using a Candidate-Gene Approach
To further expand our collection of MADS box insertion mutants, we analyzed six lines in which a recessive phenotype segregated, displaying homeotic conversions of petals to sepaloid organs in various degrees of severity. These lines had been collected on a phenotypic basis and were chosen for further analysis because their phenotypes suggested possible defects in either the B-type MADS box genes and/or the Agamous-like2 (AGL2)/SEPALLATA (SEP)-like MADS box genes, because all of these have been shown to be required for the specification of petal identity. Therefore, we PCR amplified the full coding sequences of the known B-type and AGL2/SEP-like petunia MADS box genes (Figure 2) from the six independent homozygous mutants and analyzed the sequences obtained for the occurrence of a transposon insertion or a transposon-derived footprint. Our results revealed that in three lines, the phenotype was caused by a transposon insertion in a tested MADS box gene family member.
The first line (fbp1-2 allele) harbored a new member of the dTph1 transposon family inserted in the region that encodes the K-domain of the GLO/PI-like protein FBP1 (Table 1). We have named this novel 189-bp transposon dTph8. The fbp1-2 allele probably represents a null mutant because the insertion most likely results in a truncated protein lacking one-third of the complete protein. The phenotype of this line was identical to that of a homozygote for the fbp1-1 insertion allele isolated previously by our group by means of transposon display (Van den Broeck et al., 1998; M. Sauer, unpublished results). Two other lines both contained a dTph1 insertion in the K-domain region of the SEP-like gene FBP2 (Table 1), putatively resulting in truncated proteins lacking 131 and 95 amino acid residues of the 241 residues in the wild type. Both insertion alleles most likely represent null mutants. Homozygotes for both insertion alleles as well as the F1 progeny of an fbp2-1 × fbp2-2 cross displayed an identical phenotype.
Phenotypic Analysis of fbp2 and fbp5 Single Mutants
The fbp2 and fbp5 insertion mutants were selected in a forward- and reverse-genetics manner, respectively. Flowers of plants homozygous for the fbp2-1 or fbp2-2 insertion allele exhibited petals with an overall diffuse green hue, the effect being strongest in the areas surrounding the main veins and at the edges of the petals (Figure 3B). The shape and size of the petals were nearly as in the wild type (Figure 3A). Close inspection of fbp2-1 and fbp2-2 petals using scanning electron microscopy (Figures 3F and 3I) revealed the differentiation of interspersed stomata, the conversion of petal epidermal cells in sepal-like cells, and the development of trichomes on both adaxial and abaxial sides, all of which are characteristic for sepals and leaves (Figure 3G). However, the most striking phenotype of fbp2 mutants was the presence of two to three secondary inflorescences in the third whorl, positioned between the stamens near the nectaries at the base of the pistil (Figures 3C and 3D; compare Kapoor et al., 2002). The presence of these secondary inflorescences suggests that the floral meristem behaves like an inflorescence meristem at these positions. These new structures appear relatively late during development, after the formation of all organs of the primary flower. Further development of the secondary inflorescences resulted in the formation of one inner flower per secondary inflorescence that developed all four floral whorls (Figure 3D). We very rarely found inner flower buds that developed beyond the stage shown in Figure 3D, suggesting that further development normally is arrested at this stage. To date, we have seen only a single secondary flower that grew to maturity.
Figure 3.
Phenotype of the Petunia sep-Like Mutants fbp2, fbp5, and fbp2 fbp5.
(A) Wild-type W138 petunia flower.
(B) Flower of fbp2-1 exhibiting a green hue on the corolla.
(C) Longitudinal section through an fbp2-1 flower bud of 3 mm showing the emergence of two secondary inflorescences in the third whorl (arrows). a, anther; ov, ovary; p, petal; s, sepal; sec ifl, secondary inflorescence.
(D) Close-up of a longitudinal section through an fbp2-1 mutant flower at a later stage of development showing an inflorescence and a secondary flower developing all four floral whorls, positioned near the nectary. a, anther; b, bract; c, carpel; nec, nectary; o, ovule; ov, ovary; p, petal; s, sepal; sec ifl, secondary inflorescence.
(E) to (J) Scanning electron microscopy images of wild-type and mutant petunia floral organs.
(E) and (H) Wild-type petals showing the characteristic conical petal epidermis cells (adaxial side).
(F) and (I) fbp2-1 petals with sepal-like epidermis cells, trichomes, and stomata indicated by arrows (adaxial side).
(G) Wild-type sepal with typical sepal epidermis cells, trichomes, and stomata (adaxial side).
(J) Close-up of the epidermal surface of leaf-like organs (abaxial side) inside the ovary of an fbp2-2 fbp5-1 flower (see [M]) showing the presence of trichomes and stomata and the conversion of ovule epidermal cells to sepal- or leaf-like epidermal cells.
(K) Flowers of fbp5-1, fbp2-2, and fbp2-2 fbp5-1 mutants showing enhanced petal-to-sepal conversion in fbp2-2 fbp5-1. From left to right: fbp5-1, fbp2-2, and fbp2-2 fbp5-1.
(L) and (M) Scanning electron microscopy images of the ovaries of fbp2-2 and fbp2-2 fbp5-1 flowers. The carpel wall has been removed to reveal the inner organs.
(L) fbp2-2 ovary showing wild-type-appearing ovules. The inset shows a close-up of the ovules.
(M) fbp2-2 fbp5-1 ovary displaying leaf-like organs replacing the ovules.
(N) to (Q) Comparison of third- and fourth-whorl phenotypes of fbp5-1, fbp2-2, and fbp2-2 fbp5-1.
(N) From left to right: fbp5-1, fbp2-2, and fbp2-2 fbp5-1 showing sepaloid anthers and the development of a huge pistil in fbp2-2 fbp5-1 (sepals and petals have been partially removed).
(O) Ovaries with removed carpel wall. From left to right: side-view of an fbp2-2 fbp5-1 ovary showing densely packed leaf-like organs, an fbp5-1 ovary with normal ovules, and a longitudinal section of an fbp2-2 fbp5-1 ovary showing the leaf-like structures emerging from the positions normally occupied by ovules.
(P) Close-up of anthers showing the partial conversion to sepal-like organs in fbp2-2 fbp5-1 flowers. From left to right: fbp5-1, fbp2-2, and fbp2-2 fbp5-1.
(Q) Close-up of the style and stigma of fbp2-2 fbp5-1 showing trichomes and a stigma composed of two unfused carpels.
Bars = 500 μm in (C) and (D), 100 μm in (E) to (J), and 1 mm in (L) and (M).
Flowers of fbp5-1 mutants displayed no clear morphological aberrations (Figures 3K, 3N, 3O, and 3P), but female fertility was reduced, because many pollinations with either wild-type or fbp5-1 pollen were necessary to obtain a limited number of seeds.
Phenotypic Analysis of fbp2/fbp5 Double Mutants
Previously, the function of FBP2 was analyzed using a cosuppression approach (Angenent et al., 1994). However, FBP2 cosuppression plants displayed a much more severe phenotype than our fbp2 insertion mutants (see Discussion). Molecular analysis of these cosuppression lines revealed that in addition to FBP2, another member of the petunia AGL2 subfamily, FBP5, was silenced (Ferrario et al., 2003).
Together, these findings indicate that FBP2 and FBP5 may be functionally redundant. To investigate this hypothesis, a homozygous fbp2-2 mutant was crossed with a heterozygous fbp5-1 mutant to obtain fbp2-2/fbp5-1. We selected F1 plants for the presence of insertion alleles for both genes and self-fertilized these to obtain F2 progeny. Selection of individuals heterozygous for both insertion alleles in the F1 population and of double mutants in the F2 population was performed based on a PCR segregation analysis with primers flanking the fbp2-2 and fbp5-1 insertion sites. Flowers of fbp2-2 fbp5-1 displayed an enhanced phenotype compared with fbp2 flowers, demonstrating that FBP2 and FBP5 act redundantly. Petals of fbp2-2 fbp5-1 showed an increase of petal-to-sepal conversion compared with fbp2 petals (Figure 3K), and sepal-like structures covered by trichomes developed on top of the anthers (Figures 3N and 3P). On the other hand, development of the small ectopic inflorescences, as found in the third whorl of fbp2 flowers, was not enhanced significantly. The most dramatic phenotype of fbp2-2 fbp5-1 was found in the fourth whorl, where a huge pistil developed (Figure 3N). These pistils were covered with trichomes (Figure 3Q) and frequently encompassed three carpels instead of two. In all cases, the carpels remained unfused at the top of the pistil (Figure 3Q). Inside these pistils, leaf-like organs emerged from the positions normally occupied by ovules in wild-type and in fbp2 or fbp5 flowers (Figure 3O). Analysis of these structures by scanning electron microscopy (Figures 3J and 3M) revealed the presence of sepal-like epidermal cells, stomata, and trichomes on the surface. Longitudinal sections through these ovaries showed that the basic organization, consisting of a placenta covered by a few hundred primordia, was not impaired (Figure 3O).
DISCUSSION
B, C, and D Floral Organ Identity Functions Require FBP2 and FBP5 E-Function Genes in Petunia
In Arabidopsis, the E-function genes SEP1, SEP2, and SEP3 have been shown to be required for B and C function activity, because in the triple sep1 sep2 sep3 mutant, all floral organs are replaced by sepals and the floral meristem becomes indeterminate (Pelaz et al., 2000). Studies in snapdragon and Arabidopsis (Egea-Cortines et al., 1999; Honma and Goto, 2001) suggest how E-function genes act at the molecular level: all current data indicate that SEP proteins participate in the formation of higher order transcription factor complexes that consist of various combinations of MADS box proteins. Our results and studies on protein–protein interactions (Ferrario et al., 2003; Immink et al., 2003) indicate that FBP2 and FBP5 probably act in a similar manner. Ternary complex formation between the FBP2 protein and two putative B-function heterodimers was demonstrated, whereas a four-hybrid assay revealed interactions between FBP2, a B-function heterodimer, and the putative C-function protein PMADS3.
Interestingly, the most obvious phenotype of flowers in which PMADS3 was downregulated was the emergence of secondary inflorescences arising from the third whorl (Kapoor et al., 2002). This finding is striking, because in Arabidopsis, the loss of C-function is associated with indeterminacy in the center of the flower (Yanofsky et al., 1990). In accordance with the proposed higher order complex consisting of at least a B-function heterodimer and the FBP2 and PMADS3 proteins, we observed a very similar phenotype in the fbp2 mutants with regard to the presence of ectopic inflorescences in the third whorl. This result indicates that FBP2, together with PMADS3, is essential for meristem identity. Similarly, petals, stamens, and pistils of fbp2-2 fbp5-1 displayed sepaloid characteristics, indicating that FBP2 and FBP5 also are required for B and C organ identity functions. Finally, the phenotype of fbp2-2 fbp5-1 suggests that SEP proteins also may participate in MADS box protein complexes that specifically direct ovule development. This hypothesis is supported further by the observation that FBP2 and FBP5 are expressed in the developing ovules (Favaro et al., 2002) and that they both are able to interact with the ovule identity MADS box proteins FBP7 and FBP11 (Immink et al., 2002; Ferrario et al., 2003). Future research will be focused on the identification of other components of this hypothetical complex.
Genetic Redundancy and Screening Technology
Surprisingly, from the set of insertion mutants selected using the reverse-genetics approach, only the fbp9 insertion mutant displayed a clearly visible phenotype, even though 16 of 32 MADS box insertion mutants were disrupted in the first exon, encoding the essential DNA binding MADS domain (Table 1). Comparable results were described for a reverse-genetics screen of the R2R3 Myb transcription factor family (Meissner et al., 1999). Those authors reported the isolation of 32 lines homozygous for insertions in any of 26 R2R3 Myb family members; none of those lines displayed an obvious phenotype when grown under standard conditions. Genetic redundancy of eukaryotic genomes may be one of the major causes of this general lack of phenotype. Second, 16 of the 32 insertion alleles isolated in our study represent insertions into the 5′ untranslated region/promoter region of different MADS box genes. It is not unlikely that some of these insertions will have no influence on gene expression. Third, we may have overlooked subtle phenotypes that may become apparent upon careful microscopic analysis. Finally, we cannot exclude the possibility that some MADS box genes might have a function only under specific environmental conditions or might contribute to general fitness, so the lack of a phenotype could result simply from the fact that the plants were not challenged in the appropriate way (cf. the involvement of Arabidopsis ANR1 in nitrate signaling and root architecture; Zhang and Forde, 1998). This is a problem inherent to reverse-genetics approaches; on the other hand, by definition, forward-genetics approaches usually miss redundant functions.
Nevertheless, the high sequence similarity, overlapping expression domains, and similarities in dimerization partners (Immink et al., 2003) of a number of petunia MADS box genes, especially among members of the same subfamily, suggest that genetic redundancy may be the major cause of the lack of phenotypes. Here, we demonstrate that FBP2 and FBP5 are at least partly redundant. Full or partial genetic redundancy among different MADS box genes in Arabidopsis already has been demonstrated for SEP1, SEP2, and SEP3 (Pelaz et al., 2000), SHATTERPROOF1 (SHP1) and SHP2 (Liljegren et al., 2000), CAL and AP1 (Kempin et al., 1995), CAL, AP1, and FUL (Ferrandiz et al., 2000), and AGL11, AG, SHP1, and SHP2 (Pinyopich et al., 2003).
To perform successfully in this struggle against redundancy, two important criteria should be fulfilled. First, it is necessary to identify all family members that may share redundant functions, based on sequence homology and overlapping expression domains. Second, one should have access to insertion libraries that have a high probability of containing insertions in any or all genes screened for. In Arabidopsis, for which the complete genome sequence and large insertion libraries are now publicly available, the analysis of complete gene families has become in principle quite straightforward.
To analyze gene families in petunia, a species for which the genome sequence is not yet available, we have developed an alternative and highly efficient FS screening strategy to allow screening for insertions into known genes combined with the isolation of insertions into related but yet unknown family members. This approach has proven to be efficient in analyzing the MADS box gene family in petunia, resulting in the isolation of 32 insertions into 20 different family members. Moreover, six new family members were identified during the screening, and approximately half of the insertions were identified with FS primers based on the sequence of other related family members.
Library Efficiency and Saturation
To evaluate the overall observed efficiency of the petunia insertion libraries in the screening presented here, we counted the number of genes (12) for which we found insertions within an interval of 150 bp upstream of the screening primers (our primary target, the MADS domain) against the total number of genes (24) that we intentionally screened for. These data indicate an observed 50% probability for finding an insertion in a 150-bp target sequence in a population of ∼12,700 plants (insertions into new family members identified during the screening were not included in these calculations because they could be found only because these insertions were present). Extrapolating these data for a 750-bp target (approximately the size of the open reading frame of an average MADS box gene) results in an expected insertion frequency as high as 96%, indicating that a petunia transposon population of ∼12,700 individuals represents a fairly saturated insertion library. Thus, we would have a good chance of finding insertions in the remaining coding regions outside of the MADS domain for the family members for which we did not find insertions in these screens.
In screenings for insertions into several other unrelated genes (van Houwelingen et al., 1998; our unpublished results), comparable insertion frequencies were observed, distributed equally along the full coding sequences of these genes. This finding indicates that there is no bias for insertions into regions surrounding transcription initiation sites or into specific genes. However, our data suggest that there may be a strong bias for the insertion of dTph1 elements into genic or gene-rich regions of the genome. Using the observed insertion frequencies, we calculated that the theoretical number of unique transposon insertion sites per individual has to be >400 to reach these numbers, assuming that transposon insertions are distributed randomly over the complete genome. This theoretical number is significantly higher than that observed in reality. The insertion populations are composed of small families of ∼20 to 25 plants, of which each individual contains ∼120 to 200 dTph1 elements, but the majority of the transposon insertion sites within families are common to most members. Based on our own observations in various transposon display experiments (Van den Broeck et al., 1998; De Keukeleire et al., 2001; unpublished results), we estimated that only 10 to 20% (12 to 40) of the transposon insertion sites are unique within these families. This minimal 10-fold discrepancy between theory and practice might be explained by a preference for transposon insertions into actively transcribed regions of the genome. Similar observations have been described for the Mu1 element of maize and various other plant transposable element systems (Capel et al., 1993; Cresse et al., 1995) and for the heterologous En/Spm system in Arabidopsis (Meissner et al., 1999).
Insertion Mutagenesis Versus Antisense/Sense Suppression Approaches
Gene silencing by antisense or sense suppression (the latter also is referred to as cosuppression) also is a commonly applied approach to obtain loss-of-function phenotypes. However, the specificity and extent of function loss from such methods in many cases have not been tested extensively. Moreover, transgenes have been shown to suppress endogenous genes that share only 80% DNA identity in limited regions (Napoli et al., 1990; van der Krol et al., 1990a, 1990b), which can seriously complicate the functional analysis of genes that share conserved domains. In contrast to antisense/cosuppression approaches, transposon insertion mutagenesis acts in a completely gene-specific manner. In this study, we isolated several transposon insertion alleles for genes that have been analyzed previously using cosuppression approaches. It is interesting to compare the results. We have summarized these data in Table 2. All phenotypes obtained by cosuppression were systematically more severe than those of the insertion alleles isolated in this study or, alternatively, displayed drastic phenotypes, whereas the corresponding insertion mutants appeared as seemingly wild type. To determine whether differences in genetic background might explain the observed differences in phenotypes between the two approaches, we crossed several insertion alleles (e.g., fbp1-2, petunia flowering gene [pfg-1], and fbp26-1) into the same genetic background (W115) in which the transgenic lines were obtained. However, no enhancement of phenotypes was observed for these lines.
Table 2.
Comparison of Phenotypes Obtained by Cosuppression and Transposon Insertion Mutagenesis
| Gene | Cosuppression Phenotype and References | Alleles from This Study | Phenotype |
|---|---|---|---|
| FBP1 | Homeotic conversion of petals to sepals and stamens to carpels (Angenent et al., 1993) |
fbp1-1, fbp1-2 | Sepal-like tissue at outer edge of corolla midribs, stamen filaments not fused with petal tube |
| FBP2 FBP6a | Green corolla, strongly reduced in size, stamens replaced by green sepaloid structures, reduced or absent development of gynoecial whorl; indeterminacy in the axils of the carpels; development of a new inflorescence originating from the fourth whorl (Angenent et al., 1994; Ferrario et al., 2003) |
fbp2-1, fbp2-2 | Light green corolla with sepal-like epidermal cells, indeterminacy in the third whorl; development of two to three secondary inflorescences arrested early in development |
| FBP5a | fbp5-1 | No phenotype | |
| FBP11 | Ovules replaced by carpeloid organs (Angenent et al., 1995) |
fbp11-1 | No phenotype |
| FBP7a | fbp7-1, fbp7-2 | No phenotype | |
| PFG | Plants are nonflowering, remaining in the vegetative state (Immink et al., 1999) |
pfg-1 | No phenotype |
| FBP26a | fbp26-1 | No phenotype |
Additional genes that were shown to be downregulated in the cosuppression transgenic lines.
For all of the cosuppression experiments summarized in Table 2, the full-length coding sequence (including the highly conserved MADS domain) was incorporated in the cosuppression constructs. The combined data strongly suggest that in all of these experiments, one or more additional MADS box gene besides the intended target gene was downregulated. In a number of cases, the downregulation of additional closely related genes was demonstrated, but this test obviously was limited to the family members known at that time. Thus, it remains to be proven whether the observed phenotypes of the different cosuppression lines are attributable to inactivation of only the suggested genes. Recently, it was proposed that the dramatic phenotype of the fbp2 cosuppression mutant was caused by the inactivation of both FBP2 and FBP5 (Ferrario et al., 2003). Here, we have shown that fbp2 fbp5 double mutants display a phenotype that is still less severe than that of the strongest FBP2 cosuppression lines. Although the size of the sepaloid petals in fbp2 fbp5 was reduced further compared with that in fbp2, they remained considerably larger than the small sepaloid petals of FBP2 cosuppression lines. Also, the development of ectopic inflorescences in fbp2 fbp5 was not enhanced further, and although the ovules were replaced by leaf-like structures, the structural organization inside the ovary remained intact.
In severe FBP2 cosuppression lines, the placenta and ovules were fully absent and replaced by a new inflorescence. Similarly, cosuppression of PFG (Immink et al., 1999) resulted in plants that exhibited a drastic nonflowering phenotype. Besides the downregulation of transgenic and endogenous PFG expression, the closely related FBP26 gene was shown to be downregulated as well. In our insertion screening, we isolated knockout alleles for both PFG and FBP26, and none of the single insertion mutants exhibited a phenotype when homozygous. Recently, we obtained homozygous pfg fbp26 double mutants. In contrast to the single insertion lines, these double mutants exhibited a subtle phenotype, but the drastic nonflowering phenotype of the transgenic lines was not obtained, indicating that at least three genes must have been downregulated.
Unless special precautions are taken to obtain truly gene-specific downregulation, gene silencing by antisense/sense approaches cannot be applied easily to assign functions to individual members of gene families. Moreover, specific functional data could be missed using such an approach (e.g., the direct involvement of FBP2 and FBP5 in ovule development could not be deduced from the FBP2 cosuppression experiments). On the other hand, antisense/sense approaches may provide a fast and efficient way to obtain phenotypes indicative of the function of a set of redundant paralogs. In species for which no insertion libraries are available, this might be the only option to obtain loss-of-function phenotypes. Thus, an insertion mutagenesis approach is the preferred strategy for assigning functions to individual members of a gene family, although in this case, the burden is to identify all members that share a particular function.
Toward a Fuller Functional Characterization of the Petunia MADS Box Gene Family
Here, we have presented our FS insertion screening strategy and the selection and first analysis of 36 insertion mutants in 22 different MADS box genes (including those identified using a forward candidate-gene approach), covering the diversity of MADS box genes found in Arabidopsis. Only a few of the single mutants exhibited a visible phenotype under the growth conditions used. We argue that genetic redundancy might be one of the major causes of this general lack of phenotype. To begin an analysis of the expected functional redundancy between the different subfamily members, we are currently creating specific sets of double, triple, and quadruple mutant combinations based on phylogenetic relationships, known expression patterns, and two-hybrid interactions. The set of insertion mutants presented here, combined with the identification of higher order protein complexes (Ferrario et al., 2003), provides a strong foundation for a fuller characterization of the MADS box gene family in petunia. Such a family-wide functional analysis will increase our understanding of how variations in expression patterns and changes in coding sequences at the level of a homeotic gene family may have contributed to the morphological diversity present within the plant kingdom.
METHODS
Insertion Libraries
Petunia hybrida insertion libraries have been set up in Amsterdam and Wageningen (The Netherlands), Verona (Italy), and Ghent (Belgium) according to the methodology described by Koes et al. (1995). An overview of the screened libraries is given in Table 3.
Table 3.
Overview of the Insertion Libraries Screened in This Study
| Origin | Library | Dimensions | Size (No. of Plants) |
|---|---|---|---|
| Ronald Koes, Amsterdam, The Netherlands | Yellow library | 15 × 16 × 16 | 3840 |
| Black library | 12 × 13 × 12 | 1872 | |
| Tom Gerats, Gent, Belgium | Library V | 10 × 10 × 10 | 1000 |
| Mario Pezzotti, Verona, Italy | Juliet library | 16 × 16 × 16 | 4096 |
| Gerco Angenent, Wageningen, The Netherlands | Library D | 9 × 9 × 9 | 729 |
| Library E | 9 × 10 × 12 | 1080 | |
| Library G | 9 × 10 × 12 | 1080 |
Family Signature Primer Design
We designed family signature (FS) primers as follows. In a first step, an aligned nucleotide sequence data set is assembled containing all known family members to be screened for, and candidate screening primers (typically 26- to 30-mers with high Tm values [70 to 76°C]) are chosen in the highest conserved region of the gene family. Because in our screening setup no hybridization step is involved and thus the specificity of the detection depends solely on the performance of the screening primer, the specificity of the candidate screening primers is first tested in a computer model. Using the Findpatterns function of the GCG software package (Genetics Computer Group, Madison, WI), we search the nonredundant nucleotide database for the occurrence of the candidate primer sequence in any gene, allowing up to seven mismatches between primer and nucleotide sequence. If the majority of the identified sequences belong to the gene (sub)family to be screened for, we consider the candidate primer a good FS primer. The number of screening primers to be designed to cover all family members depends on the degree of conservation within the family-specific domains. Primer sequences of our MADS box gene screening set are available upon request.
Primer Labeling
For 50 PCR reactions, the 5′ ends of 50 pmol of FS primers were labeled with 1 unit of T4 polynucleotide kinase and 5 μL of γ-33P-ATP (50 μCi, 10 mCi/mL) in a final volume of 50 μL in 1× T4 PNK A buffer (50 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 5 mM DTT, 0.1 mM spermidine-HCL, and 0.1 mM EDTA) (Eurogentec, Seraing, Belgium) and incubated at 37°C for 30 min. Finally, the kinase was inactivated by heating at 80°C for 10 min.
PCR Screening of the Insertion Libraries
For one PCR reaction, the following components were mixed together: 5 μL of template DNA (10 to 20 ng/μL), 2.5 μL of 10× PCR buffer (with 15 mM MgCl2), 1 μL of labeled FS primer (1 pmol/μL), 0.5 μL of deoxynucleotide triphosphates (10 mM), 1 μL of inverted repeat primer (5′-GAATTCGCTCCGCCCCTG-3′) complementary to the terminal inverted repeats of dTph1 (1 pmol/μL), 0.08 μL of platinum Taq DNA polymerase (Gibco BRL), and deionized water to 25 μL. The mixture was amplified in a Perkin-Elmer 9600 thermocycler with the following PCR profile: 14 cycles of 94°C for 15 s, 71°C for 30 s minus 1°C/cycle, and 72°C for 30 to 60 s, followed by 40 cycles of 94°C for 15 s, 56°C for 30 s, and 72°C for 30 to 60 s. Twenty microliters of formamide loading dye (98% formamide, 10 mM EDTA, 0.06% bromphenol blue, and 0.06% xylen cyanol) was added to the PCR and denatured for 5 min at 94°C before loading on a 4.5% denaturing polyacrylamide gel. After electrophoresis, gels were dried onto Whatman 3MM paper on a standard slab gel drier. Gels were exposed to Molecular Dynamics PhosphorImager screens (Sunnyvale, CA) for 2 to 3 h and visualized using the Molecular Dynamics 445 SI PhosphorImager analysis system followed by an overnight exposure to Kodak BioMax films to allow fragment isolation.
Fragment Isolation and Sequencing
Fragments of interest were cut from the gel, soaked in 200 μL of water for 45 min to 1 h at room temperature, and vortexed briefly four to five times. Five microliters of the eluate was reamplified in a volume of 30 μL under the PCR conditions described above, with the exception that the cycling number after the touchdown step was reduced to 35 cycles. After reamplification, products were checked on a 1% agarose gel. Sequence analysis of the reamplification products was performed with the Thermo Sequenase cycle sequencing kit (Amersham Life Science) using 2 to 3 μL of nonpurified reamplification product as a template and 1 pmol of labeled inverted repeat primer or FS primer as a sequencing primer. Note that below the fragments of interest (e.g., the fragments indicated with arrows in Figure 1), additional fragments and a smear of smaller size and lower intensity often were present. Because these extra fragments originate from PCR and running artifacts, we analyzed only the main upper fragment for sequencing.
Phylogenetic Analysis
For the phylogenetic inference of the MADS box gene family, we aligned 79 protein sequences using CLUSTAL W (Thompson et al., 1994). This alignment was checked manually and edited using BioEdit (Hall, 1999), keeping only the conserved and phylogenetic informative residues of the sequences (174 residues from the MADS, the I, and the K domains). Neighbor-joining trees (Saitou and Nei, 1987) were computed using PHYLIP-based (Felsenstein, 1993) point accepted mutation-corrected distances. To assess support for the inferred relationships, 500 bootstrap samples were generated.
Calculations of Probability
The probability of finding an insertion into a 0.75-kb sequence in a population of 12,700 plants was calculated using the formula P = 1 − e[n × ln(1−f)], with P representing the probability of finding an insertion, n the number of independent insertion events present in a population, and f the inverse number of potential targets in the complete genome. For an observed insertion frequency of 50% in a 0.15-kb target, we found an n value of 5,545,177, assuming a petunia haploid genome size of 1.2 × 106 kb (Arumuganathan and Earle, 1991), with the f value being 1.25 × 10−7 = 1/(1.2 × 106 kb/0.15 kb). The n value then was reintroduced in the formula to calculate the probability for a 0.75-kb target.
Isolation of Upstream Transposon Flanking Sequences and cDNA Isolation
Fragments encoding petunia W138 partial promoter sequences of the MADS box genes PMADS2, PMADS9, PMADS10, PMADS12, PMADS14, PMADS15, and PMADS16 were obtained by genome walking using the Universal Genome Walker kit (Clontech, Palo Alto, CA). W138 genomic fragments containing partial promoter sequences of the petunia MADS box genes GP (=PMADS1), PMADS4, PMADS6, PMADS17, PFG, FBP6, FBP24, FBP26, and FBP28 were isolated using a different approach (Vandenbussche and Gerats, 2002). The full open reading frames of PMADS9, PMADS14, and PMADS15 were isolated by rapid amplification of cDNA ends PCR (Marathon kit; Clontech) with cDNA derived from total RNA isolated from young petunia floral buds as a template. PMADS12 clones were obtained as described previously (Ferrario et al., 2003). The new dTph8 transposon was isolated from the fbp1-2 allele.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Tom Gerats, tgerats@sci.kun.nl.
Accession Numbers
GenBank accession numbers for all sequences are as follows: PMADS2 (AY370521), PMADS9 (AY370510), PMADS10 (AY370522), PMADS12 (AY370515), PMADS14 (AY370513), PMADS15 (AY370516), PMADS16 (AY370520), PMADS1 (AY370519), PMADS4 (AY370517), PMADS6 (AY370524), PMADS17 (AY370523), PFG (AY370518), FBP6 (AY370525), FBP24 (AY370514), FBP26 (AY370512), FBP28 (AY370511), the full open reading frames of PMADS9 (AY370526), PMADS14 (AY370528), PMADS15 (AY370529), PMADS12 clones (AY370527), and dTph8 (AY283799).
Acknowledgments
We thank Brendan Davies for his efforts in coordinating the program, Nico Smet for assistance with the plant work, Stefan Royaert for help with the analysis of the fbp2 fbp5 double mutant, Stefanie Debodt for help with Figure 2, and Suzanne Rodrigues Bento for her patience and support. This work was supported by a grant from the European Community BIOTECH program Bio4-CT97-2217.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017376.
References
- Alvarez-Buylla, E.R., Pelaz, S., Liljegren, S.J., Gold, S.E., Burgeff, C., Ditta, G.S., Ribas de Pouplana, L., Martinez-Castilla, L., and Yanofsky, M.F. (2000). An ancestral MADS box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 97, 5328–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent, G.C., Franken, J., Busscher, M., Colombo, L., and van Tunen, A.J. (1993). Petal and stamen formation in petunia is regulated by the homeotic gene FBP1. Plant J. 4, 101–112. [DOI] [PubMed] [Google Scholar]
- Angenent, G.C., Franken, J., Busscher, M., van Dijken, A., van Went, J.L., Dons, H.J., and van Tunen, A.J. (1995). A novel class of MADS box genes is involved in ovule development in petunia. Plant Cell 7, 1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenent, G.C., Franken, J., Busscher, M., Weiss, D., and van Tunen, A.J. (1994). Co-suppression of the petunia homeotic gene FBP2 affects the identity of the generative meristem. Plant J. 5, 33–44. [DOI] [PubMed] [Google Scholar]
- Arumuganathan, K., and Earle, E.D. (1991). Nuclear DNA content of some important plant species. Plant Mol. Biol. Rep. 9, 208–218. [Google Scholar]
- Bouche, N., and Bouchez, D. (2001). Arabidopsis gene knockout: Phenotypes wanted. Curr. Opin. Plant Biol. 4, 111–117. [DOI] [PubMed] [Google Scholar]
- Bouchez, D., and Hofte, H. (1998). Functional genomics in plants. Plant Physiol. 118, 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel, J., Montero, L.M., Martinez-Zapater, J.M., and Salinas, J. (1993). Non-random distribution of transposable elements in the nuclear genome of plants. Nucleic Acids Res. 21, 2369–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen, E.S., and Meyerowitz, E.M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353, 31–37. [DOI] [PubMed] [Google Scholar]
- Colombo, L., Franken, J., Koetje, E., van Went, J., Dons, H.J., Angenent, G.C., and van Tunen, A.J. (1995). The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7, 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, L., Franken, J., Van der Krol, A.R., Wittich, P.E., Dons, H.J., and Angenent, G.C. (1997). Downregulation of ovule-specific MADS box genes from petunia results in maternally controlled defects in seed development. Plant Cell 9, 703–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresse, A.D., Hulbert, S.H., Brown, W.E., Lucas, J.R., and Bennetzen, J.L. (1995). Mu1-related transposable elements of maize preferentially insert into low copy number DNA. Genetics 140, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila, R.T., Bechtel, L.J., Viteler, J.A., Eron, J.J., Gorczyca, P., and Kaplan, J.C. (1991). Maximizing sensitivity and specificity of PCR by pre-amplification heating. Nucleic Acids Res. 19, 3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keukeleire, P., Maes, T., Sauer, M., Zethof, J., Van Montagu, M., and Gerats, T. (2001). Analysis by transposon display of the behavior of the dTph1 element family during ontogeny and inbreeding of Petunia hybrida. Mol. Genet. Genomics 265, 72–81. [DOI] [PubMed] [Google Scholar]
- Don, R.H., Cox, P.T., Wainwright, B.J., Baker, K., and Mattick, J.S. (1991). ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 19, 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Cortines, M., and Davies, B. (2000). Beyond the ABCs: Ternary complex formation in the control of floral organ identity. Trends Plant Sci. 5, 471–476. [DOI] [PubMed] [Google Scholar]
- Egea-Cortines, M., Saedler, H., and Sommer, H. (1999). Ternary complex formation between the MADS box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 18, 5370–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro, R., Immink, R.G., Ferioli, V., Bernasconi, B., Byzova, M., Angenent, G.C., Kater, M., and Colombo, L. (2002). Ovule-specific MADS box proteins have conserved protein-protein interactions in monocot and dicot plants. Mol. Genet. Genomics 268, 152–159. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1993). PHYLIP (Phylogeny Inference Package) Version 3.5c. (Seattle: Department of Genetics, University of Washington).
- Ferrandiz, C., Gu, Q., Martienssen, R., and Yanofsky, M.F. (2000). Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127, 725–734. [DOI] [PubMed] [Google Scholar]
- Ferrario, S., Immink, R.G., Shchennikova, A., Busscher-Lange, J., and Angenent, G.C. (2003). The MADS box gene FBP2 is required for SEPALLATA function in petunia. Plant Cell 15, 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerats, A.G.M., Huits, H., Vrijlandt, E., Marana, C., Souer, E., and Beld, M. (1990). Molecular characterization of a non-autonomous transposable element (dTph1) of petunia. Plant Cell 2, 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T.A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. [Google Scholar]
- Honma, T., and Goto, K. (2001). Complexes of MADS box proteins are sufficient to convert leaves into floral organs. Nature 409, 525–529. [DOI] [PubMed] [Google Scholar]
- Immink, R.G., Ferrario, S., Busscher-Lange, J., Kooiker, M., Busscher, M., and Angenent, G.C. (2003). Analysis of the petunia MADS box transcription factor family. Mol. Genet. Genomics 268, 598–606. [DOI] [PubMed] [Google Scholar]
- Immink, R.G., Gadella, T.W., Jr., Ferrario, S., Busscher, M., and Angenent, G.C. (2002). Analysis of MADS box protein-protein interactions in living plant cells. Proc. Natl. Acad. Sci. USA 99, 2416–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immink, R.G., Hannapel, D.J., Ferrario, S., Busscher, M., Franken, J., Lookeren Campagne, M.M., and Angenent, G.C. (1999). A petunia MADS box gene involved in the transition from vegetative to reproductive development. Development 126, 5117–5126. [DOI] [PubMed] [Google Scholar]
- Jack, T. (2001). Relearning our ABCs: New twists on an old model. Trends Plant Sci. 6, 310–316. [DOI] [PubMed] [Google Scholar]
- Jofuku, K.D., den Boer, B.G., Van Montagu, M., and Okamuro, J.K. (1994). Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, M., Tsuda, S., Tanaka, Y., Mayama, T., Okuyama, Y., Tsuchimoto, S., and Takatsuji, H. (2002). Role of petunia pMADS3 in determination of floral organ and meristem identity, as revealed by its loss of function. Plant J. 32, 115–127. [DOI] [PubMed] [Google Scholar]
- Kempin, S.A., Savidge, B., and Yanofsky, M.F. (1995). Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267, 522–525. [DOI] [PubMed] [Google Scholar]
- Koes, R., et al. (1995). Targeted gene inactivation in petunia by PCR-based selection of transposon insertion mutants. Proc. Natl. Acad. Sci. USA 92, 8149–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, E.M., and Irish, V.F. (1999). Evolution of genetic mechanisms controlling petal development. Nature 399, 144–148. [DOI] [PubMed] [Google Scholar]
- Lamb, R.S., and Irish, V.F. (2003). Functional divergence within the APETALA3/PISTILLATA floral homeotic gene lineages. Proc. Natl. Acad. Sci. USA 100, 6558–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren, S.J., Ditta, G.S., Eshed, Y., Savidge, B., Bowman, J.L., and Yanofsky, M.F. (2000). SHATTERPROOF MADS box genes control seed dispersal in Arabidopsis. Nature 404, 766–770. [DOI] [PubMed] [Google Scholar]
- Meissner, R.C., et al. (1999). Function search in a large transcription factor gene family in Arabidopsis: Assessing the potential of reverse genetics to identify insertional mutations in R2R3 MYB genes. Plant Cell 11, 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster, T., Pahnke, J., Di Rosa, A., Kim, J.T., Martin, W., Saedler, H., and Theissen, G. (1997). Floral homeotic genes were recruited from homologous MADS box genes preexisting in the common ancestor of ferns and seed plants. Proc. Natl. Acad. Sci. USA 94, 2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli, C., Lemieux, C., and Jorgensen, R. (1990). Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenicova, L., de Folter, S., Kieffer, M., Horner, D.S., Favalli, C., Busscher, J., Cook, H.E., Ingram, R.M., Kater, M.M., Davies, B., Angenent, G.C., and Colombo, L. (2003). Molecular and phylogenetic analyses of the complete MADS box transcription factor family in Arabidopsis: New openings to the MADS world. Plant Cell 15, 1538–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz, S., Ditta, G.S., Baumann, E., Wisman, E., and Yanofsky, M.F. (2000). B and C floral organ identity functions require SEPALLATA MADS box genes. Nature 405, 200–203. [DOI] [PubMed] [Google Scholar]
- Pinyopich, A., Ditta, G.S., Savidge, B., Liljegren, S.J., Baumann, E., Wisman, E., and Yanofsky, M.F. (2003). Assessing the redundancy of MADS box genes during carpel and ovule development. Nature 424, 85–88. [DOI] [PubMed] [Google Scholar]
- Pnueli, L., Hareven, D., Broday, L., Hurwitz, C., and Lifschitz, E. (1994). The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan, M.D., Rounsley, S.D., Schmidt, R.J., and Yanofsky, M.F. (1995). Molecular evolution of flower development: Diversification of the plant MADS box regulatory gene family. Genetics 140, 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renckens, S., De Greve, H., Beltran-Herrera, J., Toong, L.T., Deboeck, F., De Rycke, R., Van Montagu, M., and Hernalsteens, J.P. (1996). Insertion mutagenesis and study of transposable elements using a new unstable virescent seedling allele for isolation of haploid petunia lines. Plant J. 10, 533–544. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and Nei, M. (1987). The neighbour-joining method: A new method for constructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer, Z., Huijser, P., Nacken, W., Saedler, H., and Sommer, H. (1990). Genetic control of flower development by homeotic genes in Antirrhinum majus. Science 250, 931–936. [DOI] [PubMed] [Google Scholar]
- Tandre, K., Albert, V.A., Sundas, A., and Engstrom, P. (1995). Conifer homologues to genes that control floral development in angiosperms. Plant Mol. Biol. 27, 69–78. [DOI] [PubMed] [Google Scholar]
- Theissen, G., Becker, A., Di Rosa, A., Kanno, A., Kim, J.T., Munster, T., Winter, K.U., and Saedler, H. (2000). A short history of MADS box genes in plants. Plant Mol. Biol. 42, 115–149. [PubMed] [Google Scholar]
- Theissen, G., Kim, J.T., and Saedler, H. (1996). Classification and phylogeny of the MADS box multigene family suggest defined roles of MADS box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol. 43, 484–516. [DOI] [PubMed] [Google Scholar]
- Theissen, G., and Saedler, H. (1995). MADS box genes in plant ontogeny and phylogeny: Haeckel's ‘biogenetic law’ revisited. Curr. Opin. Genet. Dev. 5, 628–639. [DOI] [PubMed] [Google Scholar]
- Theissen, G., and Saedler, H. (2001). Plant biology: Floral quartets. Nature 409, 469–471. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck, D., Maes, T., Sauer, M., Zethof, J., De Keukeleire, P., D'Hauw, M., Van Montagu, M., and Gerats, T. (1998). Transposon display identifies individual transposable elements in high copy number lines. Plant J. 13, 121–129. [DOI] [PubMed] [Google Scholar]
- Vandenbussche, M., and Gerats, T. (2002). PCR cloning approaches. In Molecular Plant Biology, Vol. 1 (Practical Approach Series), P.M. Gilmartin and C. Bowler, eds (Oxford, UK: Oxford University Press), pp. 179–204.
- Vandenbussche, M., Theissen, G., Van de Peer, Y., and Gerats, T. (2003). Structural diversification and neo-functionalization during floral MADS box gene evolution by C-terminal frameshift mutations. Nucleic Acids Res. 31, 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol, A.R., Brunelle, A., Tsuchimoto, S., and Chua, N.H. (1993). Functional analysis of petunia floral homeotic MADS box gene pMADS1. Genes Dev. 7, 1214–1228. [DOI] [PubMed] [Google Scholar]
- van der Krol, A.R., Mur, L.A., Beld, M., Mol, J.N., and Stuitje, A.R. (1990. a). Flavonoid genes in petunia: Addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2, 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol, A.R., Mur, L.A., de Lange, P., Mol, J.N., and Stuitje, A.R. (1990. b). Inhibition of flower pigmentation by antisense CHS genes: Promoter and minimal sequence requirements for the antisense effect. Plant Mol. Biol. 14, 457–466. [DOI] [PubMed] [Google Scholar]
- van Houwelingen, A., Souer, E., Spelt, K., Kloos, D., Mol, J., and Koes, R. (1998). Analysis of flower pigmentation mutants generated by random transposon mutagenesis in Petunia hybrida. Plant J. 13, 39–50. [DOI] [PubMed] [Google Scholar]
- Winkler, R.G., Frank, M.R., Galbraith, D.W., Feyereisen, R., and Feldmann, K.A. (1998). Systematic reverse genetics of transfer-DNA-tagged lines of Arabidopsis: Isolation of mutations in the cytochrome P450 gene superfamily. Plant Physiol. 118, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, K.U., Becker, A., Munster, T., Kim, J.T., Saedler, H., and Theissen, G. (1999). MADS box genes reveal that genetophytes are more closely related to conifers than to flowering plants. Proc. Natl. Acad. Sci. USA 96, 7342–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Yu, D., Kotilainen, M., Pollanen, E., Mehto, M., Elomaa, P., Helariutta, Y., Albert, V.A., and Teeri, T.H. (1999). Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae). Plant J. 17, 51–62. [DOI] [PubMed] [Google Scholar]
- Zhang, H.M., and Forde, B.G. (1998). An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409. [DOI] [PubMed] [Google Scholar]