Abstract
Rationale
Previous studies have suggested that chronic food restriction (FR) increases sensitivity of a neural substrate for drug reward. The neuroanatomical site(s) of key neuroadaptations may include nucleus accumbens (NAc) where changes in D-1 dopamine (DA) receptor-mediated cell signaling and gene expression have been documented.
Objectives
The purpose of the present study was to begin bridging the behavioral and tissue studies by microinjecting drugs directly into NAc medial shell and assessing behavioral effects in free-feeding and FR subjects.
Materials and methods
Rats were implanted with microinjection cannulae in NAc medial shell and a subset were implanted with a stimulating electrode in lateral hypothalamus. Reward-potentiating effects of the D-1 DA receptor agonist, SKF-82958, AMPAR antagonist, DNXQ, and polyamine GluR1 antagonist, 1-na spermine, were assessed using the curve-shift method of self-stimulation testing. Motor-activating effects of SKF-82958 were also assessed.
Results
SKF-82958 (2.0 and 5.0 µg) produced greater reward-potentiating and motor-activating effects in FR than ad libitum fed (AL) rats. DNQX (1.0 µg) and 1-na spermine (1.0 and 2.5 µg) selectively decreased the x-axis intercept of rate-frequency curves in FR subjects, reflecting increased responding for previously subthreshold stimulation.
Conclusions
Results suggest that FR may facilitate reward-directed behavior via multiple neuroadaptations in NAc medial shell including upregulation of D-1 DA receptor function involved in the selection and expression of goal-directed behavior, and increased GluR1-mediated activation of cells that inhibit nonreinforced responses.
Keywords: food restriction, nucleus accumbens, reward, self-stimulation, D-1 receptor, GluR1, SKF-82958, 1-naphthylacetyl spermine
INTRODUCTION
Food restriction facilitates the acquisition and maintenance of drug self-administration behavior (Carroll and Meisch 1984) and enhances the reward-potentiating effects of abused drugs as measured in rate-frequency and progressive ratio protocols of intracranial self-stimulation (ICSS) (Cabeza de Vaca and Carr 1998; Cabeza de Vaca et al. 2004). Intracerebral mapping studies have localized reinforcing and reward-potentiating effects of many abused drugs to the nucleus accumbens (NAc) medial shell (e.g., Carlezon and Wise 1996; McBride et al. 1999; Rodd-Henricks et al. 2002; Ikemoto et al. 2005). While numerous pre- and postsynaptic neuroadaptations have been identified in the mesoaccumbens DA pathway of food-restricted (FR) rats – including changes in rate of tyrosine hyroxylation (Pan et al. 2006), DA transporter function (Zhen et al. 2006), and D-1 receptor-mediated cell signaling and transcriptional responses (Carr et al. 2003; Haberny et al. 2004; Haberny and Carr 2005a; Haberny and Carr 2005b) - subregional analyses of NAc have generally not been performed. One exception is the report of higher extracellular DA concentrations in NAc core, but not shell, of FR relative to ad libitum fed (AL) rats injected with amphetamine and cocaine (Cadoni et al. 2003). However, postsynaptic upregulation of D-1 DA receptor function also appears to be a behaviorally important neuroadaptation to FR in so far as systemic and intracerebroventricular injections of D-1 agonists produce reward-potentiating and locomotor-activating effects that are greater in FR than AL rats (Carr et al. 2001; Carr et al. 2003). It is not known whether the NAc locus of upregulated cellular responses to D-1 receptor stimulation also mediates the upregulated behavioral responses. Consequently, the first aim of the present study was to determine whether microinjection of the D-1 DA receptor agonist, SKF-82958, in NAc medial shell produces reward-potentiating and locomotor-activating effects that differ between feeding groups.
The DA innervation of striatum is convergent with glutamatergic inputs from several limbic forebrain regions (Groenwegen et al. 1999; Kalivas et al. 2005). Among the ionotropic glutamate receptor types coexpressed with DA receptors in striatal medium spiny neurons (MSN; Bernard et al. 1997; Wang et al. 2006), AMPA receptors mediate fast excitatory synaptic transmission (Barry and Ziff 2002). DA acting via D-1 receptors modulates excitatory responses of MSNs to glutamatergic input and may play a fundamental role in the selection of neuronal ensembles that mediate specific forms of goal-directed behavior and suppression of competing alternatives (Pennartz et al. 1994; Surmeier et al. 2007). Simultaneous voltammetric and electrophysiological recordings in NAc shell during ICSS behavior revealed anticipatory DA surges and coincident changes in neuronal firing patterns that were predominantly inhibitory (Cheer et al. 2007). Moreover, both the neuronal firing patterns and ICSS behavior were blocked by local infusion of the D-1 DA receptor antagonist, SCH23390. Coordinated electrophysiological and behavioral analyses have suggested that neuronal inhibition in NAc is generally involved in the permissive gating of reward-directed responses (Taha and Fields 2006). Consequently, blockade of AMPA receptors in NAc shell might be expected to potentiate ICSS. This possibility is supported by findings that microinjection of the AMPA/kainate antagonist, DNQX, in NAc shell disinhibited lateral hypothalamic neural activity and induced a robust eating response (Maldonado-Irizarry et al.1995). While feeding and ICSS are not equivalent, a close association is indicated by findings that noncontingent electrical stimulation delivered via lateral hypothalamic ICSS electrodes often elicits feeding behavior (Margules and Olds 1962; Wise 1974). Further, as might be predicted from the DNQX/feeding study and the association between feeding and ICSS, viral vector-mediated overexpression of AMPA GluR1 in NAc shell decreased lateral hypothalamic ICSS (Todtenkopf et al. 2006). The second aim of this study was therefore to determine whether microinjection of the broad spectrum AMPA/kainate antagonist, DNQX, and/or the selective polyamine antagonist of Ca2+-permeable AMPA receptors (i.e., GluR2-lacking), 1-naphthylacetyl spermine (e.g., Takazawa et al. 1996), potentiate lateral hypothalamic ICSS and do so to a greater extent in FR than AL subjects.
Materials and methods
Subjects and Surgical Procedures
Subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) initially weighing 375–425 g. Food (pelleted Purina rat chow) and water were available ad libitum except when FR conditions applied. Animals were individually housed in clear plastic cages with bedding under a 12 h light:dark photoperiod with lights on at 0700 h. Several days after arrival in the central animal facility, rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and those to be used in Experiments 1, 2 and 4 were stereotaxically implanted with a 0.25 mm diameter monopolar stimulating electrode (Plastics One, Roanoke, VA) in the lateral hypothalamic medial forebrain bundle (skull flat coordinates: 3.0 mm posterior to bregma, 1.6 mm lateral to the sagittal suture, and 8.5 mm ventral to skull surface). An anterior ipsilateral stainless steel skull screw served as ground. All rats were implanted with two chronically indwelling guide cannulae (26 ga) which, in Experiments 1, 3 and 4, were placed bilaterally 2.0 mm dorsal to injection sites in the NAc medial shell (1.6 mm anterior to bregma; 2.1 mm lateral to the sagittal suture, tips angled 8° toward the midline, 5.8 mm ventral to skull surface). In Experiment 2, cannulae were placed bilaterally 2.0 mm dorsal to injection sites in the NAc core (1.6 mm anterior to bregma; 2.9 mm lateral to the sagittal suture, tips angled 8° toward the midline, 5.6 mm ventral to skull surface). The electrode, ground, cannulae, and three additional mounting screws were then permanently secured to the skull by flowing dental acrylic around them. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York Univesity School of Medicine and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85–23).
Feeding regimens
During the second week of training in the ICSS protocol (see below), approximately half of the rats in Experiments 1, 2 and 4 were switched from unlimited access to food to a single 10-g meal each day, representing 40–50% of the ad libitum intake. The daily meal was delivered at approximately 1700 h in the home cage. This food restriction regimen continued until body weights decreased by 20% (approximately two weeks). ICSS training continued throughout this period. For the remainder of each experiment, daily feeding was titrated to maintain body weights at 80% of the initial value, typically requiring an upward adjustment to 12–14 grams. Drug testing did not begin until rats in the FR group had been stabilized for one week at their target body weight (i.e. 80% of pre-restriction body weight). This FR regimen is identical to that used in all prior behavioral and tissue studies of this laboratory (for review see Carr 2007).
Half of the subjects in Experiment 3 (motor activity testing) were similarly food-restricted but were simply habituated to transport and handling during the several week period required to attain and stabilize at the target body weight.
Self-stimulation (ICSS) apparatus
Brain stimulation training and testing were conducted in eight standard test chambers (26 × 26 × 21 cm) placed within sound attenuating cubicles. Each chamber had a retractable lever mounted on one wall and a house light mounted on the opposite wall. Four constant current stimulators (PHM-152B/2; Med-Associates, Georgia, VT), with dual outputs, were used to deliver trains of 0.1 ms cathodal pulses, which were conducted to implanted electrodes by way of commutators and flexible cables. Electrical stimulation, contingencies, and data recording were controlled through Dell XPS R400 computers and interface (Med-Associates). All stimulation parameters were monitored on Tektronix (TAS 455) oscilloscopes.
Self-stimulation procedures
After one week of postsurgical recovery, rats were trained to lever press for 0.5 s trains of electrical stimulation at a frequency of 100 pulses per second (pps). The initial stimulation intensity of 120 µA was systematically varied to locate, for each rat, the lowest intensity that maintained vigorous lever pressing. On subsequent days, rats were trained in a discrete-trials procedure. Each training session consisted of twenty four 60-s trials. Extension of the lever and a 2-s train of ‘priming’ stimulation initiated each trial. Each trial was terminated by retraction of the lever and followed by a 10-s intertrial interval. Each lever press produced a 1-s train of stimulation, except for those presses emitted during the stimulation train, which did not increase reinforcement density. The number of lever presses and reinforcements were recorded for each trial.
Discrete trials training was followed by rate-frequency training, which continued for approximately two weeks. Rate-frequency curves were generated by presenting 12 trials in which the frequency of brain stimulation decreased over successive trials (approximately 0.05 log units each trial) from an initial frequency of 100 pps to a terminal frequency of 28 pps. At least two such series were presented in each training session. During the second week of training, subjects were divided into two groups matched for body weight and M-50 (the brain stimulation frequency that supported 50% of the maximum reinforcement rate) and one of the groups was placed on the FR regimen described above. Training and mock microinjection test sessions (see below) continued, at least twice per week, for all rats, during the ensuing ~3 week period during which the FR group achieved and then stabilized at the target body weight.
Intra-NAc microinjection procedure
For microinjections, solutions were loaded into two 30 cm lengths of PE-50 tubing attached at one end to 25-µl Hamilton syringes filled with distilled water and at the other end to 31-gauge injector cannulae, which extended 2.0 mm beyond the implanted guides. The syringes were mounted on the twin holders of a Harvard 2272 microliter syringe pump which delivered the 0.5 µl injection volumes over a period of 100 sec. One minute following completion of injections, injector cannulae were removed from guides, stylets were replaced, and animals were returned to test chambers where the post-injection test began 5-min after completion of the microinjections.
Experimental testing
Each test session began with a pre-injection test consisting of three rate-frequency series (42 min). The first series in a session was considered a ‘warm-up’ and data were excluded. This was followed by intra-NAc microinjections which were followed, in 5 min, by a post-injection test consisting of two rate-frequency series (28 min). For each rate-frequency series, the number of reinforcements obtained as a function of brain stimulation frequency was recorded. For each rat, the two series from each test were averaged to yield a single rate-frequency function per test.
Data analysis
For each test, the rate-frequency function was used to derive three parameters. The maximum reinforcement rate, described by a line that parallels the x-axis, was defined as the mean of all consecutive values within 10% of the highest rate for the curve. All remaining values comprised the descending portion of the curve, with the lowest point being at the highest frequency to produce fewer than 2.5 reinforcements per minute. Regression analysis of the descending portion of the curve was used to calculate the M-50 and theta-0 reward thresholds which are defined as the log pulse frequency sustaining half the maximum reinforcement rate and x-axis intercept of the regression line, respectively. For threshold parameters, antilog transformations were applied and natural frequencies were used to calculate the percentage change occurring in post-injection tests relative to a pre-injection test. The M-50 is a conventional threshold measure in this protocol, though the theta-0 indicates the lowest frequency at which stimulation becomes rewarding. Changes in the reinforcing efficacy of stimulation produced by drugs of abuse are typically reflected as parallel leftward shifts in the rate-frequency curve with similar effects on the M-50 and theta-0 measures (Wise 1996).
Motor activity testing
Activity was monitored in a Lucite test chamber (25.4 × 30.5 × 30.5 cm) with a textured plastic floor. Automated data collection, including distance travelled and vertical (rearing) activity, was accomplished by 12 infrared photo beam detectors along the length of the test chamber plus 12 beams on the vertical axis (Accuscan, Columbus, OH). Rats were microinjected with SKF-82958 or vehicle immediately prior to placement, for 30 min, in the test chamber. The chamber used was designed for training and testing of conditioned place preference; thus, half of the subjects in each feeding condition received SKF-82958 in a chamber containing walls with horizontal white stripes while the remaining half received SKF-82958 in a chamber containing walls with vertical white stripes. The motor activity data recorded and reported here are from the first pair of training sessions of a conditioned place preference pilot study. That study is in progress and place conditioning results are not reported.
Histology
Upon completion of all behavioral testing, rats were euthanized with CO2 and decapitated. Brains were removed and fixed in 10% buffered formalin for at least 48 hr. Frozen coronal sections, 40 µm thick, were cut on a Reichert-Jung Cryostat, thaw-mounted on gelatin-coated glass slides and stained with cresyl violet. Electrode placements and injection sites were determined by visual inspection of sections under an Olympus SZ40 microscope. In order for a rat’s behavioral data to be included in the analysis, cannula placements for subjects in Experiments 1, 3 and 4 were required to be judged as bilaterally accurate within the NAc shell (with several placements on the shell/core and shell/olfactory tubercle borders included). Placements in Experiment 2 were required to be bilaterally placed within the mid to lateral NAc core.
Drugs
SKF-82958 (Sigma-Aldrich) was dissolved in a small volume of DMSO and diluted with sterile 0.9% saline (final DMSO concentration 5%) and microinjected bilaterally in doses ranging from 0.25 to 5.0 µg in 0.5 µl in Experiments 1–3. D-amphetamine (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline and microinjected bilaterally in doses of 5.0 µg in 0.5 µl in Experiment 1, and 2.0 µg in 0.5 µl in Experiment 2. SCH-23390 (Sigma-Aldrich) was dissolved in 0.9% saline and injected systemically at a dose of 0.01 mg/kg (i.p.) in Experiments 1 and 2. DNQX (Tocris, Ellsville, MO) was dissolved in DMSO and diluted with sterile 0.9% saline (final DMSO concentration 20%) and microinjected bilaterally in a dose of 1.0 µg in 0.5 µl in Experiment 4. 1-naphthylacetyl spermine (Sigma-Aldrich) was dissolved in sterile 0.9% saline and microinjected bilaterally in doses of 1.0 and 2.5 µg in 0.5 µl in Experiment 4.
Experiment 1 ICSS: Intra-NAc shell microinjection of SKF-82958
7 AL and 8 FR rats with cannulae in the NAc medial shell were tested in four sessions over a 10 day period to determine the effects of SKF-82958 on ICSS. Test sessions were separated by at least 3 days and the two SKF-82958 treatment sessions were preceded and followed by vehicle test sessions. Results of the two vehicle treatment sessions were averaged for purposes of data analysis.
Five days after completion of “a” above, subjects were tested in two additional sessions, three days apart. In both sessions all animals received intra-NAc microinjection of d-amphetamine (5.0 µg) 5 min prior to the post-injection test. In one session this was preceded by injection of the D-1 DA receptor antagonist, SCH-23390 (0.01mg/kg, i.p.; 10 min prior), and in the other session by injection of saline vehicle. The order of the two treatments was counterbalanced and matched between groups.
Experiment 2 ICSS: Intra-NAc core microinjection of SKF-82958
6 AL and 6 FR rats with cannulae in the NAc core were tested in five sessions over a 13 day period to determine the effects of SKF-82958 on ICSS. SKF-82958 testing began with the lower dose used in Experiment 1 (i.e., 2.0 µg). Upon observing a strong response and possible ceiling effect in the two feeding groups, two lower doses were subsequently tested in counterbalanced order. Vehicle treatment sessions preceded and followed the series of SKF-82958 sessions and results were averaged for purposes of data analysis.
Five days after completion of “a” above, subjects were tested in two additional sessions as in Experiment 1 except that the intra-NAc dose of d-amphetamine was decreased to 2.0 µg
Experiment 3 Motor activity: Intra-NAc shell microinjection of SKF-82958
To further elucidate effects observed in Experiment 1, two new groups of 7 AL and 7 FR rats with cannulae in NAc medial shell were tested for effects of the 2.0 µg dose of SKF-82958 and vehicle on distance moved and vertical activity.
Experiment 4 ICSS: Intra-NAc shell microinjection of AMPAR antagonists
12 AL and 12 FR rats with cannulae in NAc shell were tested in two sessions at least 3 days apart. In one session animals received intra-NAc shell microinjection of DNQX (1.0 µg) 5 min prior to the post-injection test and in the other session received microinjection of vehicle. The order of microinjection treatments was counterbalanced and matched between feeding groups.
At least 5 days after “a” above, the 12 AL rats and 11 FR rats were tested in three sessions spaced at least 3 days apart to test effects of intra-NAc shell microinjection of 1-na spermine at doses of 1.0 and 2.5 µg. The 1.0 µg dose was tested first in all subjects and the two 1-na spermine test sessions were preceded and followed by vehicle test sessions, the results of which were averaged for purposes of data analysis.
Results
Experiment 1 ICSS: Intra-NAc shell microinjection of SKF-82958
For illustrative purposes average rate-frequency curves, based on M-50, theta-0, and maximum response rate values, are displayed in Figure 1. As suggested by these curves, SKF-82958 decreased the M-50 reward threshold (F2,26=18.3, p<.001) with a greater effect in FR than AL subjects (F1,13=5.8, p=.03; Fig. 2 top). The significant interaction between drug treatment and feeding condition (F2,26=3.4, p=.05), followed by Fisher LSD tests, indicated that both the 2.0 µg (p<.01) and 5.0 µg (p<.001) doses lowered threshold in FR rats while only the 5.0 µg (p<.01) dose lowered threshold in AL rats. Moreover, the 5.0 µg dose had a greater threshold-lowering effect in FR than AL rats (p<.02). Similar results were obtained for the theta-0 measure, with SKF-82958 decreasing the threshold (F2,26=11.8, p<.001) to a greater extent in FR than AL subjects (F1,13=6.7, p=.02; Fig. 2 bottom). Post-SKF-82858 rate-frequency curves displayed parallel leftward shifts as indicated by the absence of effect of SKF-82958 (F2,26=1.0), feeding condition (F1,13=0.7), or their interaction (F2,26=1.1) on slopes. In four FR subjects whose cannulae were deliberately placed 2.0 mm dorsal to the NAc shell placement, in medial caudate-putamen adjacent to the lateral ventricle, a 2.0 µg dose of SKF-82958 was tested and produced the relatively small mean changes of −1.85% and −7.4% in the M-50 and theta-0 measures, respectively.
Figure 1.
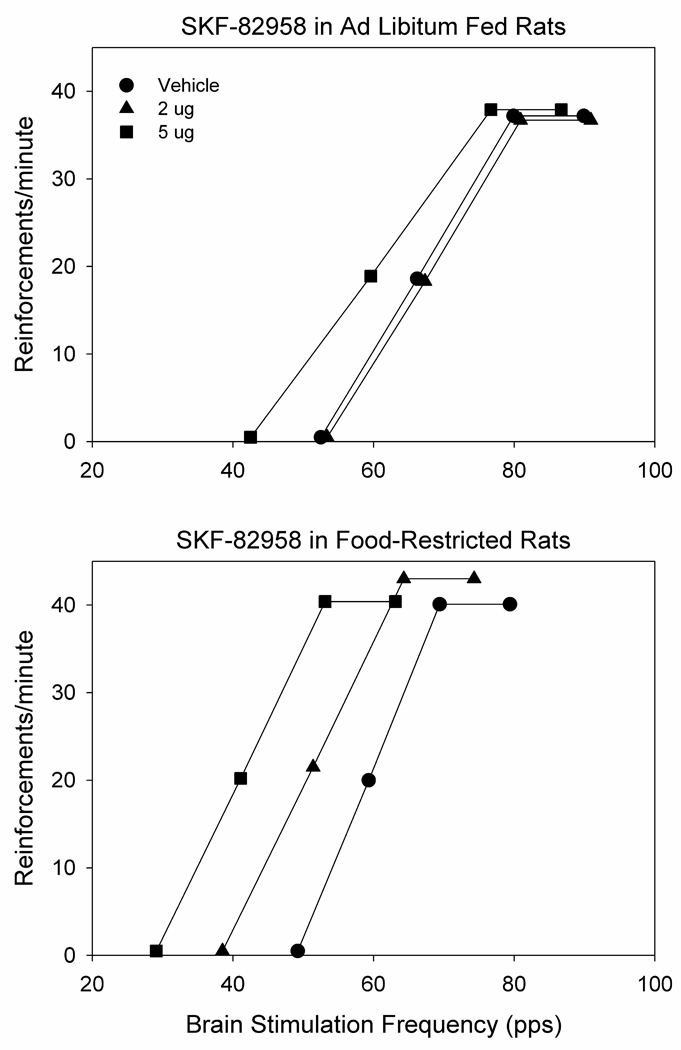
Average post-injection ICSS rate-frequency curves of ad libitum fed (n=7; top) and food-restricted (n=8; bottom) rats. The average pre-injection rate-frequency curves (not shown) are essentially superimposable on the post-vehicle curves (see Fig. 2 for % change in key parameters following vehicle injection). Bilateral microinjection of SKF-82958 in nucleus accumbens medial shell produced greater leftward-shifts in curves of food-restricted relative to ad libitum fed subjects (see text for details). Points indicated by symbols correspond to mean theta-0 (x-axis intercept), M-50 (frequency supporting 50% of maximum reinforcement rate) and maximum/asymptotic reinforcement rate.
Figure 2.
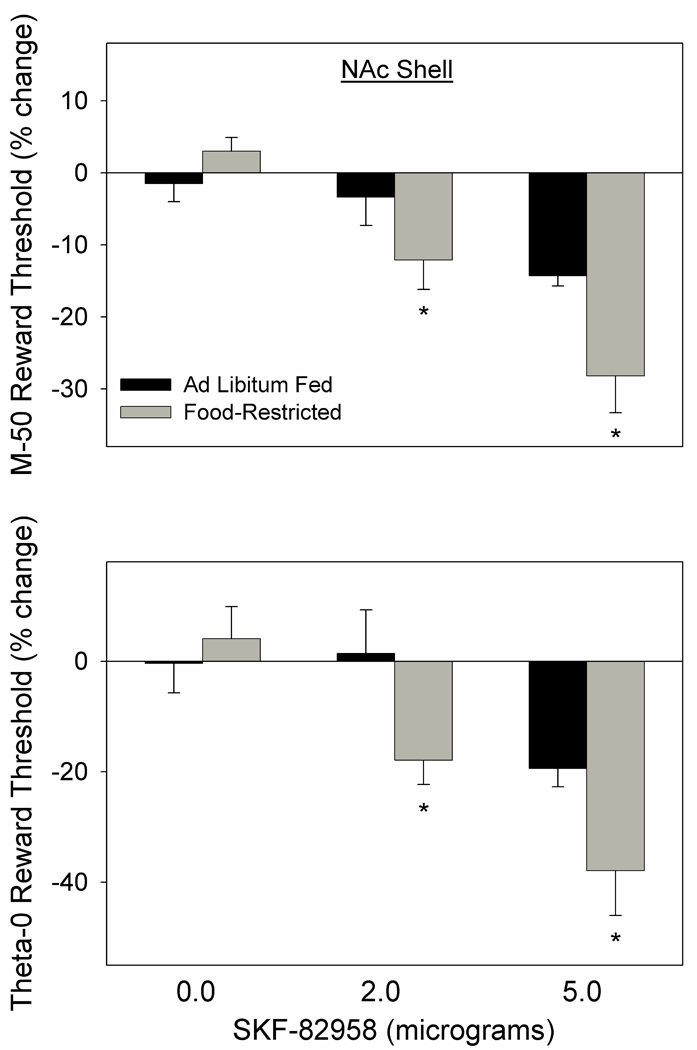
Mean (± s.e.m.) percentage changes in the M-50 (top) and theta-0 (bottom) measures of reward threshold, derived from ICSS rate-frequency curves, following microinjection of 0.0, 2.0, and 5.0 µg SKF-82958, bilaterally, in NAc medial shell of ad libitum fed (black fill) and food-restricted (gray fill) rats. *p at least <.05 as compared to the ad libitum fed group (see text for details).
In the second part of this experiment, microinjection of d-amphetamine produced a greater threshold-lowering effect in FR than AL rats as reflected in both the M-50 (t14=2.7, p<.02) and theta-0 (t14=2.3, p<.05) measures. However, when preceded by systemic administration of SCH-23390, the effects of d-amphetamine were diminished and feeding groups did not differ on either measure (Fig. 3).
Figure 3.
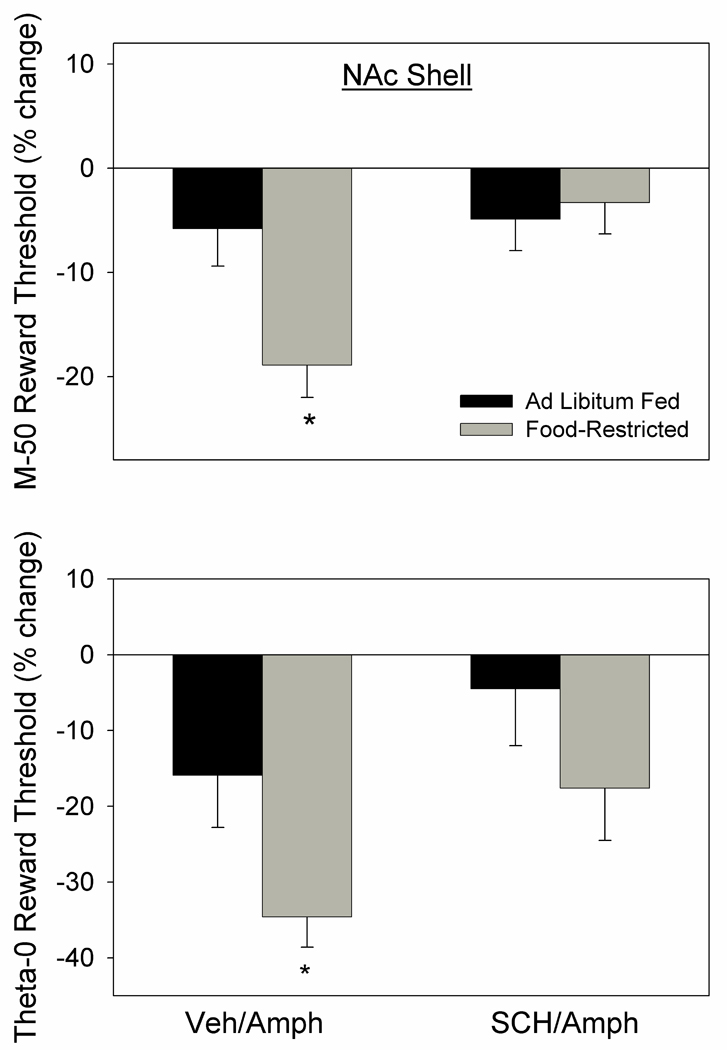
Mean (± s.e.m.) percentage changes in M-50 (top) and theta-0 (bottom) measures of reward threshold following microinjection of d-amphetamine (5.0 µg) bilaterally in NAc medial shell of ad libitum fed (black fill) and food-restricted (gray fill) rats. D-amphetamine microinjections were preceded (10 min) by systemic injection of SCH-23390 (0.01 mg/kg, i.p.) or vehicle. *p at least <.05 relative to the ad libitum fed group.
Representative histology and a schematic diagram indicating microinjection sites are provided in Fig. 4 & Fig 5).
Figure 4.
A representative stained coronal section displaying bilateral microinjection sites in NAc shell.
Figure 5.
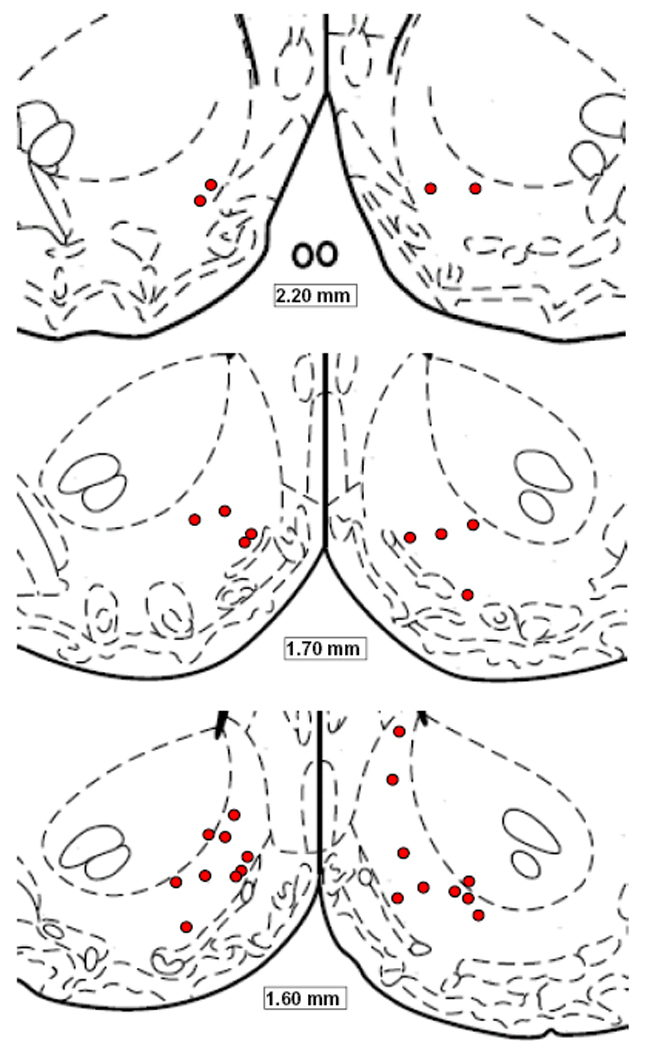
Schematic diagrams adapted from Paxinos and Watson (1998), indicating microinjection sites in NAc shell of subjects tested in Experiment 1.
Experiment 2 ICSS: Intra-NAc core microinjection of SKF-82958
SKF-82958 decreased the M-50 reward threshold (F3,30=32.4, p<.001) with no difference between FR and AL subjects (Fig. 6 top) and no interaction between drug treatment and feeding condition. Similar results were obtained for the theta-0 measure, with SKF-82958 decreasing the threshold (F3,30=15.3, p<.001) with no difference between FR and AL subjects (Fig. 6 bottom) and no interaction between drug treatment and feeding condition.
Figure 6.
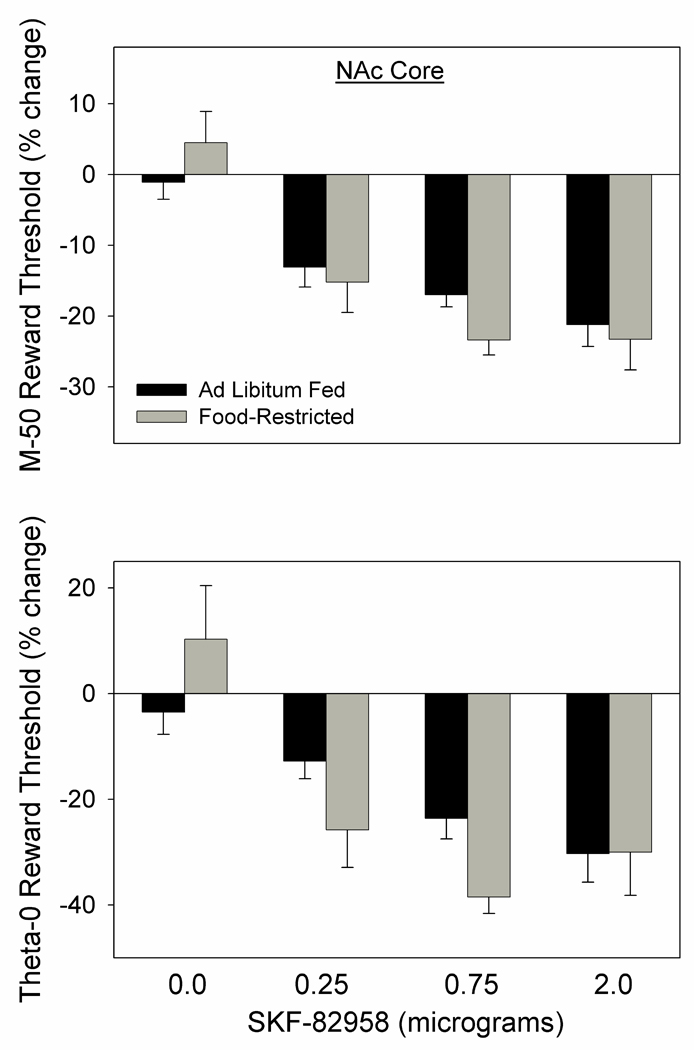
Mean (± s.e.m.) percentage changes in the M-50 (top) and theta-0 (bottom) measures of reward threshold, derived from ICSS rate-frequency curves, following microinjection of 0.0, 0.25, 0.75, and 2.0 µg SKF-82958, bilaterally, in NAc core of ad libitum fed (black fill) and food-restricted (gray fill) rats.
Because testing in this experiment began with the 2.0 µg dose which produced a greater than expected, and possible ceiling, effect in the two groups, a second analysis of results was performed that was limited to the two subsequent lower doses. Nevertheless, the effect of SKF-82958 on the M-50 (F2,20=38.6, p<.001) and theta-0 (F2,20=17.0, p<.001) measures remained unaccompanied by a difference between feeding groups.
In the second part of this experiment, microinjection of d-amphetamine produced a greater threshold-lowering effect in FR than AL rats as reflected in theta-0 (t10=2.3, p<.05) with the difference between groups on the M-50 measure failing to meet criteria for statistical significance (t10=1.86, p<.10). When preceded by systemic administration of SCH-23390, the effects of d-amphetamine were not diminished and feeding groups differed on both the theta-0 measure (t10=2.3, p<.05) and M-50 measure (t10=2.66, p<.05) of reward threshold (Fig. 7).
Figure 7.
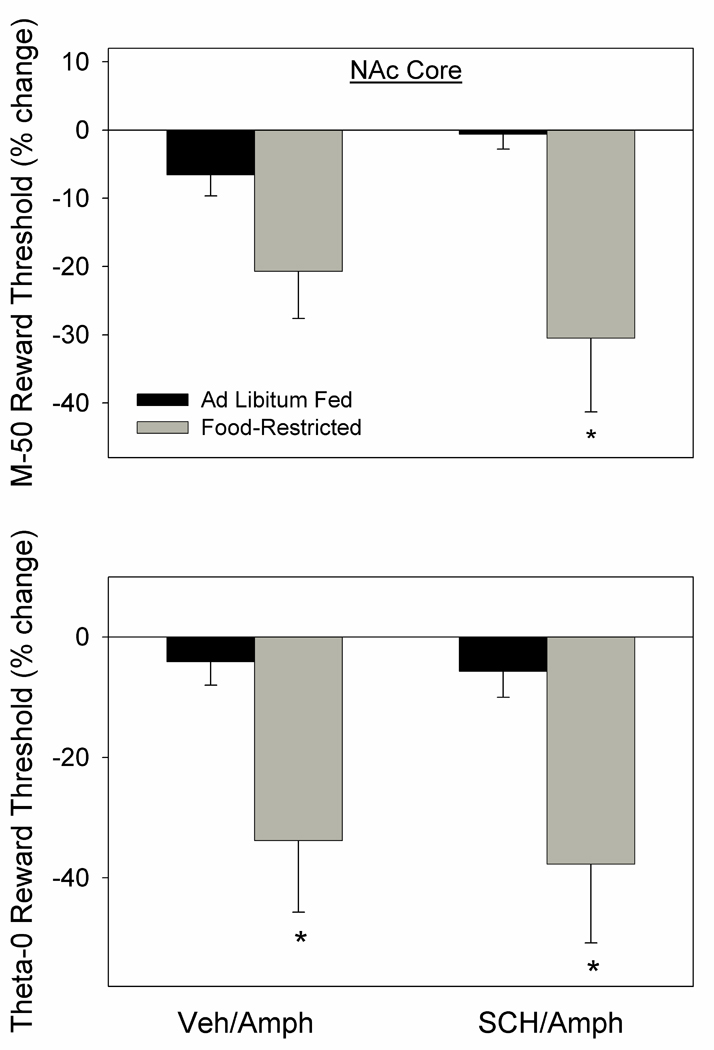
Mean (± s.e.m.) percentage changes in M-50 (top) and theta-0 (bottom) measures of reward threshold following microinjection of d-amphetamine (2.0 µg) bilaterally in NAc core of ad libitum fed (black fill) and food-restricted (gray fill) rats. D-amphetamine microinjections were preceded (10 min) by systemic injection of SCH-23390 (0.01 mg/kg, i.p.) or vehicle. *p at least <.05 relative to the ad libitum fed group.
A schematic diagram indicating microinjection sites is provided in Fig. 8.
Figure 8.
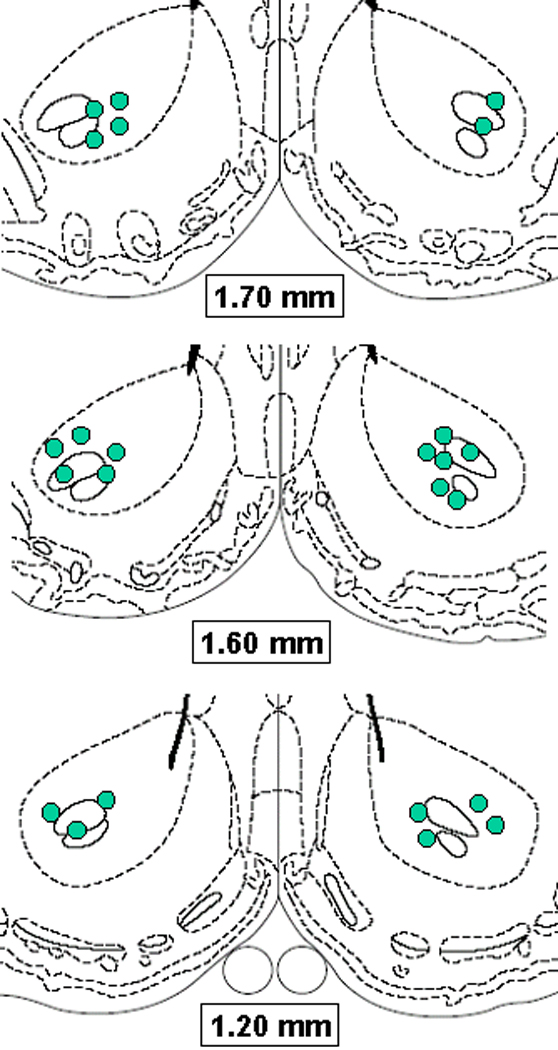
Schematic diagrams adapted from Paxinos and Watson (1998), indicating microinjection sites in NAc core of subjects tested in Experiment 2.
Experiment 3 Motor activity: Intra-NAc shell microinjection of SKF-82958
A significant interaction between drug treatment and feeding condition (F1,12=15.2, p<.01) followed by Fisher LSD tests indicated that SKF-82958 induced greater vertical activity in the FR than AL group (p<.001; Fig. 9 top). Similarly, a significant interaction (F1,12=6.67, p<.025) followed by Fisher LSD tests indicated that SKF-82958 induced a greater increase in distance moved in FR than AL rats (p<.01; Fig. 9 bottom).
Figure 9.
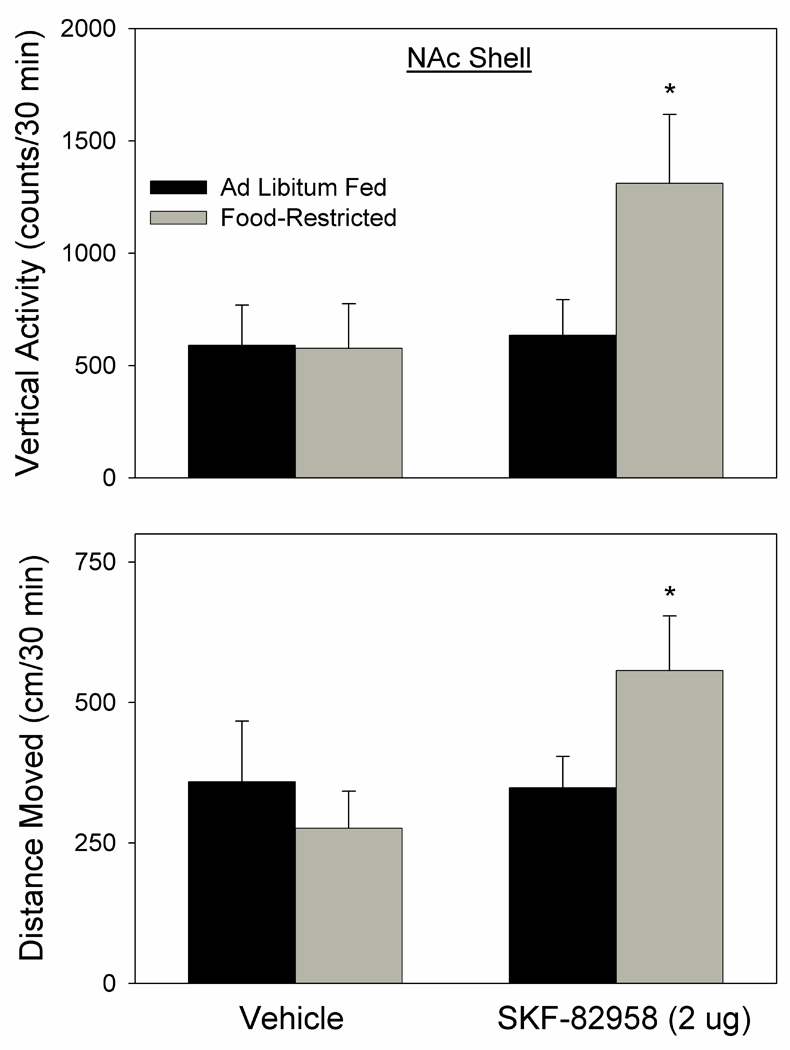
Vertical activity (top; mean ± s.e.m. counts/30 min) and distance moved (bottom; mean ± s.e.m. cm/30 min) induced by of NAc medial shell microinjection of SKF-82958 (2.0 µg) versus vehicle in ad libitum fed (black fill) and food-restricted (gray fill) rats. Food-restricted subjects were more active than ad libitum fed subjects following SKF-82958, but not vehicle, microinjection. *p at least <.01.
Experiment 4 ICSS: Intra-NAc shell microinjection of AMPAR antagonists
The 1.0 µg dose of DNQX did not affect the M-50 measure of reward threshold in either feeding group but did decrease the theta-0 measure (F1,22=9.7, p<.01; Fig. 10). The significant interaction between factors (F1,22=4.1, p=.05), followed by Fisher LSD tests indicated that the effect of DNQX was only significant in the FR group (p<.01). As implied by the selective effect on theta-0, DNQX produced a significant decrease in slope of rate-frequency curves (F1,22=17.7, p<.001).
Figure 10.
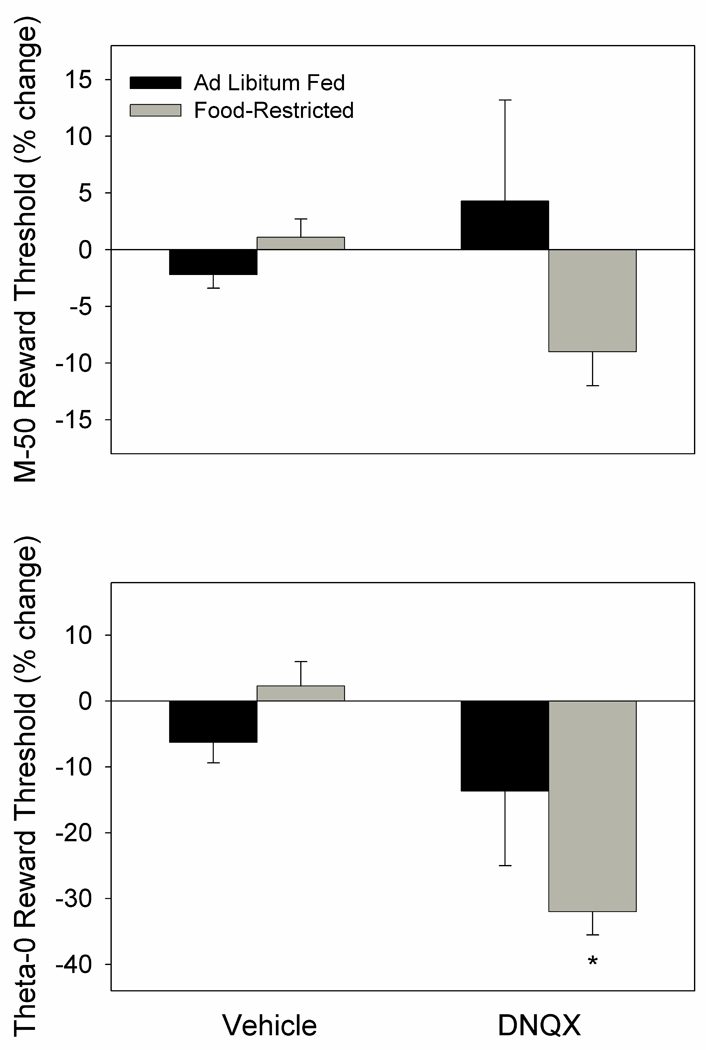
Mean (± s.e.m.) percentage changes in M-50 (top) and theta-0 (bottom) measures of reward threshold following microinjection of DNQX (1.0 µg) or vehicle bilaterally in NAc medial shell of ad libitum fed (black fill) and food-restricted (gray fill) rats. *p <.01 relative to vehicle treatment.
In the second part of this experiment 1-na spermine was found to have no effect on M-50. While the main effect of drug treatment on the theta-0 measure was not significant, there was a main effect of feeding condition (F1,21= 9.2, p<.01), with FR subjects showing a greater threshold-lowering effect (theta-0) than AL subjects in response to both the 1.0 (p<.01) and 2.5 µg (p<.01) doses (Fig. 11). This difference was supported by a significant effect of feeding condition on slope of the rate-frequency curves (F1,21=4.8, p<.05).
Figure 11.
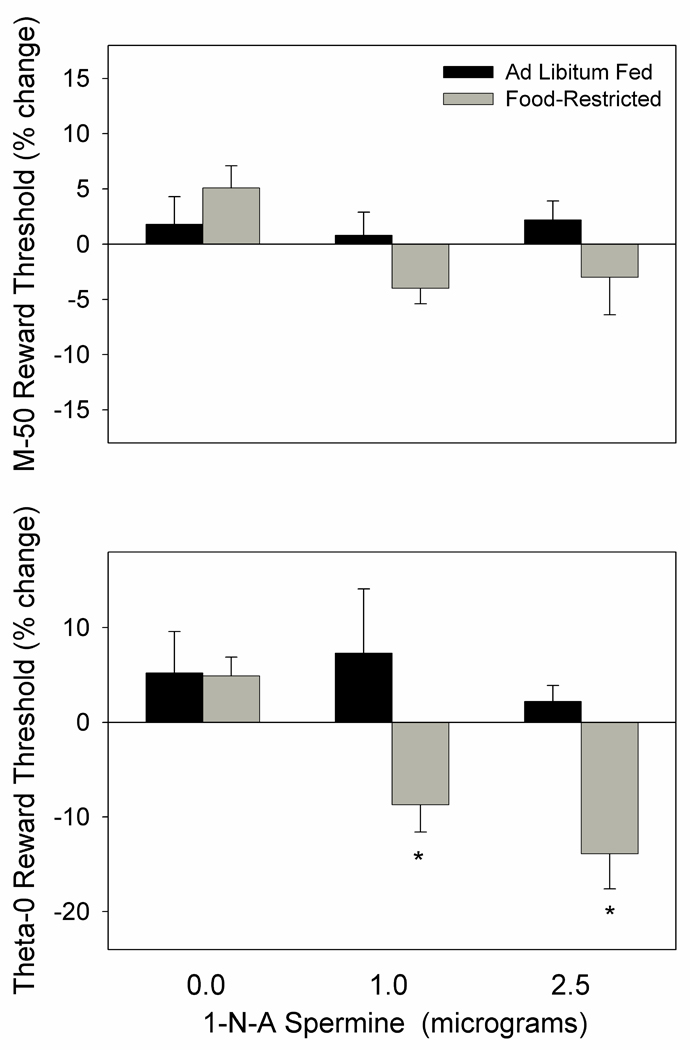
Mean (± s.e.m.) percentage changes in the M-50 (top) and theta-0 (bottom) measures of reward threshold following microinjection of 0.0, 1.0, and 2.5 µg 1-naphthylacetyl spermine, bilaterally, in NAc medial shell of ad libitum fed (black fill) and food-restricted (gray fill) rats. *p at least <.01 as compared to the ad libitum fed group.
A schematic diagram indicating microinjection sites is provided in Figure 12.
Figure 12.
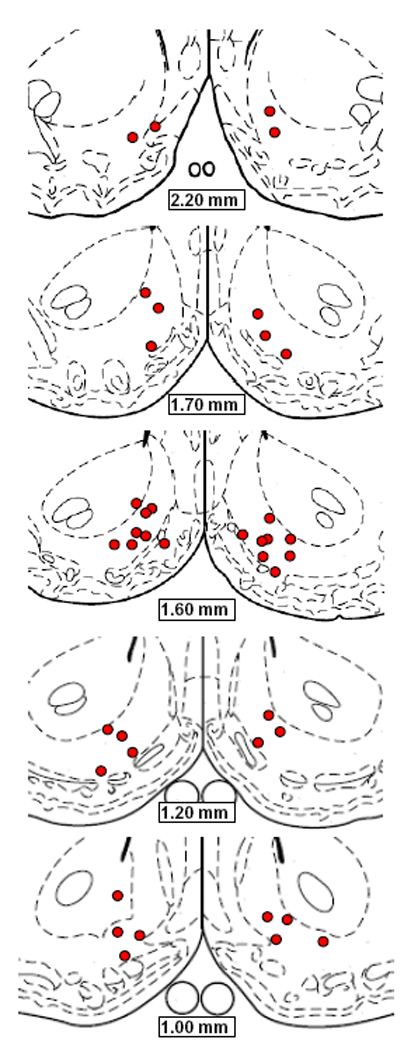
Schematic diagrams adapted from Paxinos and Watson (1998), indicating microinjection sites in NAc shell of subjects tested in Experiment 4.
DISCUSSION
While previous studies provided evidence of increased behavioral and striatal cellular responsiveness to d-amphetamine and SKF-82958 in FR rats, the present results begin to connect the behavioral and tissue studies by demonstrating that direct injection of either drug into the NAc medial shell exerts a reward-potentiating effect that is enhanced by FR. In addition, elimination of the enhanced response to d-amphetamine by pretreatment with a low dose of SCH-23390 suggests that upregulation of postsynaptic D-1 DA receptor function may be sufficient to explain the enhanced behavioral response to released DA as well as direct D-1 DA receptor stimulation.
Convergent results of in vivo microdialysis, microinjection mapping, and intracerebral drug self-administration studies attribute a critical role to NAc medial shell DA transmission in the primary reinforcing effects of abused drugs and the incentive motivational processes via which amphetamine invigorates instrumental responding reinforced by contingent, or potentiated by noncontingent, delivery of conditioned stimuli (Pontieri et al. 1995; Carlezon and Wise 1996; McBride et al. 1999; Parkinson et al. 1999; Taylor and Horger 1999; Wyvell and Berridge 2000; Rodd-Henricks et al. 2002; Ikemoto et al. 2005). Physiologically, D-1 DA receptors in NAc appear to be fundamentally involved in the selection of neuronal ensembles that encode specific goal-directed behaviors (Pennartz et al. 1994; Surmeier et al. 2007). In the absence of DA input, responses of NAc neurons to environmental cues –and cue-elicited behavioral responses- are abolished (Yun et al 2004b). A substantial proportion of NAc neurons display a significant change in firing rate in association with initiation of goal-directed behavior. Taha and Fields (2006) have described a subpopulation distributed among recording sites in medial shell and medial core that displayed long-lasting inhibition that preceded the initiation and continued through completion of sucrose-seeking and sucrose-licking, whether the behavior was spontaneous or learned. They also identified NAc neurons that fired prior to and during reward-directed movements with directional selectivity (Taha et al. 2007). In the particular case of lever pressing for rewarding electrical brain stimulation, DA surges and coincident firing patterns in NAc shell precede and continue through each ICSS response (Cheer et al. 2007). Local infusion of the D-1 DA receptor antagonist, SCH-23390, blocked the ICSS-related neuronal firing as well as the ICSS behavior. Thus, the present finding that D-1 DA agonist microinjection in NAc shell enhances ICSS and does so to a greater extent in FR than AL rats may reflect an upregulated mechanism for selection and expression of goal-directed behavior.
Past microinjection mapping and lesion studies have produced conflicting results as to which NAc subdivision mediates the motor-activating effects of psychostimulants and DA receptor agonists (e.g., Parkinson et al. 1999; Sellings and Clarke 2003). However, a previous striatal mapping study using SKF-82958 identified NAc shell as the subregion most responsive to locomotor-activating effects (Swanson et al. 1997). The present findings are consistent with NAc shell localization of D-1 receptor-mediated locomotor activity. Further, the finding that a 2.0 µg dose of SKF-82958 was adequate to potentiate reward and induce hyperactivity in FR subjects, but was without effect on either measure in AL subjects, strengthens the conclusion that FR is associated with behaviorally significant changes in D-1 DA receptor function in NAc shell. Moreover, the selective efficacy of 2.0 µg SKF-82958 in potentiating reward and increasing motor activity in FR rats is suggestive of a common neural basis of reward-potentiating and motor-activating effects, both of which may reflect facilitation of the “approach” behavior that is prepotent in the corresponding context.
Microinjection of SKF-82958 into NAc core was also effective in lowering the ICSS threshold and this tissue site appeared more sensitive than shell based on the magnitude of response to the 2.0 µg dose. However, sensitivity did not differ between feeding groups. On the other hand, d-amphetamine which reverses the DA transporter and increases extracellular DA concentrations produced a reward-potentiating effect that was greater in FR than AL rats, though it was insensitive to the low dose of SCH23390 that reversed the effect in NAc shell. This finding suggests that behaviorally important neuroadaptations are present in NAc core but differ mechanistically from those in shell, as previously suggested by the finding of increased extracellular DA in NAc core, but not shell, of FR rats injected systemically with d-amphetamine (Cadoni et al. 2003). A limit to interpretation of the present results is the absence of data relating to D-2 DA receptor function. Transmission at D-1 DA receptors in NAc interacts cooperatively with transmission at D-2 receptors. For example, rats will self-administer a mixture of D-1 and D-2 receptor agonists into NAc shell, but will not self-administer the agonists individually (Ikemoto et al. 1997). Similarly, a mixture of agonists elicits locomotor activity more effectively than either agonist alone (Plaznik et al 1989). Changes in D-2 receptor function could plausibly be a factor in the ICSS experiments; ICSS (Cheer et al. 2007) and noncontingent rewarding electrical stimulation in lateral hypothalamus (Hernandez et al. 2008) increase DA release in NAc and inevitably entail stimulation of D-2 receptors. Moreover, systemic and intraventricular injection of the D-2 DA receptor agonist, quinpirole, produces enhanced reward-potentiating and motor-activating effects in FR relative to AL rats (Carr et al. 2001; Carr et al. 2003). Thus, tentative conclusions regarding increased postsynaptic D-1 DA receptor function in NAc shell and presynaptic neuroadaptations in NAc core must be tempered by the lack of information regarding D-2 (and D-3) receptor function.
The DA innervation of medial shell arises from cell bodies in the posteromedial VTA and appears to be fundamentally involved in incentive motivational states that invigorate reward-directed behavior (for recent review, see Ikemoto 2007). The inferred upregulation of postsynaptic D-1 DA receptor function in FR subjects may represent compensation for a decrease in basal DA transmission which adaptively conserves behavior and energy, except when signals relating to contexts or cues associated with a possibility of food acquisition are positively gated to the pathway. Whether past findings consistent with decreased DA neural activity in FR subjects (Pothos et al. 1995; Haberny and Carr 2005b; Pan et al., 2006) are localized to medial shell is not known.
The DA innervation of NAc core arises from lateral VTA and appears to be particularly involved in reward-directed associative learning (e.g., Kelley 2004) and instrumental responding maintained by conditioned stimuli (Fuchs et al. 2004; Floresco et al. 2008). If medial shell is viewed as an amplifier of reward-directed responses (Ito et al. 2004), neuroanatomically upstream from NAc core in the hierarchy of information flow (Haber et al. 2000), a decrease in basal DA tone in medial shell may be sufficient to increase the amount of cytoplasmic DA in NAc core (Pothos et al. 1995) based on long term decreased DA release, and thereby increase the amount of DA released per storage vesicle in exocytosis (Pothos 2002). This may contribute to the increased behavioral response to locally applied d-amphetamine in the absence of increased D-1 receptor sensitivity.
Cellular inhibition in NAc medial shell is a predominant D-1 DA receptor-dependent underpinning of ICSS (Cheer et al. 2007), and neuronal inhibition in NAc has been proposed to permissively gate appetitive and consummatory responses (Taha and Fields 2006). Several compatible pharmacological findings suggest the release of reward-directed behavior by widespread inhibition of neurons in NAc. Microinjection of the AMPA/kainate receptor antagonist, DNQX, in NAc shell elicited a strong feeding response (Maldonado-Irizarry et al. 1995), and microinjection of CNQX plus AP-5 (concurrent blockade of AMPA/kainate and NMDA receptors) in shell or medial core disinhibited uncued and nonreinforced lever pressing in a cue-evoked food-seeking task (Yun et al 2004a). In addition, inactivation of NAc medial shell with combined microinjection of GABA-A and GABA-B agonists enhanced cue-induced reinstatement of instrumental responding for sucrose (Floresco et al. 2008) and cocaine (Di Ciano et al. 2007) during extinction. These results may illuminate the present finding that DNQX and 1-na spermine selectively lowered the theta-0 reward threshold in FR subjects. The decrease in slope and x-axis intercept (theta-0) of rate-frequency curves is indicative of a preferential increase in responding for low frequency, previously subthreshold, brain stimulation. The similar effects of DNQX and 1-na spermine indicate that blockade of GluR1 may be sufficient to reproduce the effect of the broad spectrum AMPA/kainate receptor antagonist. One interpretation of this result would be that AMPAR blockade selectively increased the reward efficacy of weak stimulation in FR subjects. An alternative, in line with the NAc shell inactivation studies, is that the stimulation remained subthreshold but GluR1 blockade disinhibited cue-induced responding for nonrewarding stimulation. In this case, the increased involvement of GluR1 in regulation of ICSS in FR subjects might lead to the prediction that slopes of FR subjects’ pre-injection rate-frequency curves would be steeper than those of AL subjects. This was not observed, but an unbiased test of this prediction was precluded by the training protocol in which brain stimulation intensities were adjusted with the goal of matching baseline rate-frequency curves of all subjects.
The inferred increase in GluR1 involvement in ICSS of FR subjects may reflect an upregulation of basal GluR1 channel conductance and/or surface expression, or a transient DA-dependent increase during ICSS responding. DA, amphetamine, and sucrose ingestion all lead to phosphorylation of NAc GluR1 (Snyder et al. 2000; Rauggi et al. 2005) which enhances AMPA currents and facilitates rapid insertion into the postsynapse (Barry and Ziff 2002; Man et al. 2007; Snyder et al. 2000). Recently, this laboratory observed that systemically administered SKF-82958 led to greater phosphorylation of GluR1 in NAc of FR relative to AL rats (Carr, Chau, Gustafson, in progress). Consequently, a primary upregulation of D-1 receptor function may secondarily increase involvement of GluR1 in regulating ICSS in FR subjects.
Together, results of the present experiments raise the possibility that neuroadaptations induced by FR in NAc medial shell increase the focus and vigor of reward-directed behavior in two ways: by upregulating D-1 DA receptor function involved in selection and expression of goal-directed behavior, and increasing GluR1-mediated activation of cells that suppress nonreinforced behavior. The net effect of this combination would have clear adaptive value in the energy-deficient subject. However, it may also contribute to the enhanced ability of abused drugs to usurp brain reward circuitry (Volkow and Wise, 2005) and reinforce self-administration.
Acknowledgments
This research was supported by DA03956 from NIDA/NIH.
REFERENCES
- Barry MR, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Krahne L, Carr KD. A progressive ratio schedule of self-stimulation testing reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a "sensitizing" regimen of d-amphetamine. Psychopharmacol. 2004;175:106–113. doi: 10.1007/s00213-003-1768-4. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacol. 1996;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91:459–472. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim G-Y, Cabeza de Vaca S. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacol. 2001;154:420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol. 1984;4:47–88. [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacol. 2008;33:1414–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neurosci. 2008;154:877–884. doi: 10.1016/j.neuroscience.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005a;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Comparison of basal and D-1 dopamine receptor agonist-stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Molec Brain Res. 2005b;141:121–127. doi: 10.1016/j.molbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Rajabi H, Stewart J, Arvanitogiannis A, Shizgal P. Dopamine tone increases similarly during predictable and unpredictable administration of rewarding brain stimulation at short inter-train intervals. Behav Brain Res. 2008;188:227–232. doi: 10.1016/j.bbr.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav. Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical ‘feeding’ and ‘rewarding’ systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- Pan Y, Haberny LY, Berman Y, Meller E, Carr KD. Synthesis, protein levels, activity and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Plaznik A, Stefanski R, Kostowski W. Interaction between accumbens D1 and D2 receptors regulating rat locomotor activity. Psychopharmacol. 1989;99:558–562. doi: 10.1007/BF00589908. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Behav Brain Res. 2002;130:203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauggi R, Scheggi S, Cassanelli A, Graziella de Montis M, Tagliamonte A, Gambarana C. The mesolimbic dopaminergic response to novel palatable food consumption increases dopamine-D1 receptor-mediated signaling with complex modifications of the DARPP-32 phosphorylation pattern. J Neurochem. 2005;92:867–877. doi: 10.1111/j.1471-4159.2004.02920.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WK. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Sellings LH, Clarke PB. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg A, Valee CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Nicola SM, Fields HL. Cue-evoked encoding of movement planning and execution in the rat nucleus accumbens. J Physiol. 2007;584:801–818. doi: 10.1113/jphysiol.2007.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa A, Yamazaki O, Kanai H, Ishida N, Kato N, Yamauchi T. Potent and long-lasting anticonvulsant effects of 1-naphthylacetyl spermine, an analogue of Joro spider toxin, against amygdaloid kindled seizures in rats. Brain Res. 1996;706:173–176. doi: 10.1016/0006-8993(95)01334-2. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacol. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Carlezon WA., Jr Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Liu X, Zhang G, Parelkar NK, Arora A, Haines M, Fibuch EE, Mao L. Phosphorylation of glutamate receptors: a potential mechanism for the regulation of receptor function and psychostimulant action. J Neurosci Res. 2006;84:1621–1629. doi: 10.1002/jnr.21050. [DOI] [PubMed] [Google Scholar]
- Wise RA. Lateral hypothalamic electrical stimulation: does it make animals ‘hungry’? Brain Res. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004a;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004b;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]


