Abstract
Modulation of intracellular pH is widely implicated in the control of cell growth and metabolism, yet little is known about intracellular pH and brain function. To determine how stimulation of brain may affect the intracellular pH of mammalian glial cells, rat cortical astrocytes were studied for the first time in vivo using pH-sensitive electrodes of submicron caliber. Stimulation of the cortical surface caused a cytoplasmic alkaline shift of tenths of a pH within seconds. Cessation of induced electrical activity was followed by pH recovery and a small acid rebound. Recordings obtained during cortical-spreading depression revealed similar but generally larger intracellular pH shifts. Production of metabolic acids is known to occur when the brain is stimulated and has led to the long-held presumption that brain cells accordingly become more acidic. The observation that glia initially become more alkaline during electrical activity is thus paradoxical. The correlation of glial alkalinization with evoked electrical activity suggests that modulation of intracellular pH of glia may have important functional implications.
Keywords: intracellular pH, pH microelectrodes, acid-base balance, glia, spreading depression
Glial cells play an important role in regulation of the brain cell microenvironment, contributing to, among other functions, the stabilization of interstitial-ion activities (20). Reflecting this role, glia are thought to increase their metabolic rate in response to neuronally derived increases in interstitial K+ activity (21, 25). The physiological variable that relates glial metabolism to elevations in external K+ is unknown, but hydrogen-ion activity is one potential candidate. In muscle, for example, a small rise in intracellular pH can significantly augment glycolytic rate (9), and similar pH changes in other tissues are thought to activate a variety of cellular processes (5). In brain, interstitial pH transients are known to accompany electrical activity (13), but whether and how intracellular pH shifts correlate with function is poorly understood, largely because direct measurements of intracellular pH in the mammalian central nervous system have only recently become possible (14, 15). Accordingly, we have monitored the intracellular pH of mammalian astrocytes in vivo during evoked activity and cortical-spreading depression. Our measurements demonstrate that electrical activity of nerve cells is accompanied by a profound and rapid increase in glial intracellular pH.
METHODS
Adult Wistar rats were anesthetized with halothane, mounted in a stereotaxic holder, and artificially ventilated. Arterial glucose, respiratory gases, and pH were stabilized prior to recordings. Electrodes were inserted through a craniotomy into frontal cortex bathed with warm (35–37°) Ringer solution. Ringer was gassed with 5% CO2 and 95% O2 (pH 7.35 at 25°C) (17) and had the following composition (mM): 108 NaCl, 3 KC1, 26 NaHCO3, 1.5 CaCl2, 1.4 MgCl2, 5 glucose, 8 sucrose, and 10 sodium gluconate (13).
Double-barrel, pH-sensitive electrodes (tip diam <1 µm) were pulled from borosilicate glass in either an eccentric (30) or “figure eight” configuration. When filled with 3 M KC1, pH barrels had a resistance of 20–40 MΩ. Silanization with N,N-dimethyltrimethylsilylamine was performed with a modification of the technique described by Borelli et al. (4), using a hot-air gun to heat the silane-treated electrode. Ion-sensitive barrels were backfilled with tridodecylamine-based proton exchanger (2) to form a column of exchanger several millimeters long, then finally backfilled with phosphate buffer (pH 7.3). Reference barrels contained 0.6 M K2SO4 or 0.5 M KCl. Electrode responses to step pH changes were 95% complete in 5–10 s. Brain recordings were referenced to superfusate pH. Calibration in phosphate buffers (pH 6.0–7.4) was performed before and after recordings from cells and proved similar, with a slope response of 48–54 mV/pH. Electrodes were insensitive to CO2 (0–5%). Reference potentials were continuously subtracted from pH-barrel potentials to yield the pH signal. Electrical stimuli were delivered to the cortical surface with an optically isolated bipolar electrode.
Cells were identified as glia by a high membrane potential and lack of injury or synaptic discharge. Further characterization was obtained by staining such elements with horseradish peroxidase (HRP). Single-barrel electrodes were filled with 9% HRP (Boehringer-Mannheim, FRG) in 0.5 M KC1 and phosphate buffer (pH 7.6). HRP was electrophoretically injected into selected cells (pulses of 840 ms/s, 10–30 nA-min), then brains were fixed by intracardiac perfusion with phosphate-buffered Formalin. Vibratome sections (75 µm) were processed with 3,3′-diaminobenzidine according to the method of Chan and Nicholson (6).
RESULTS
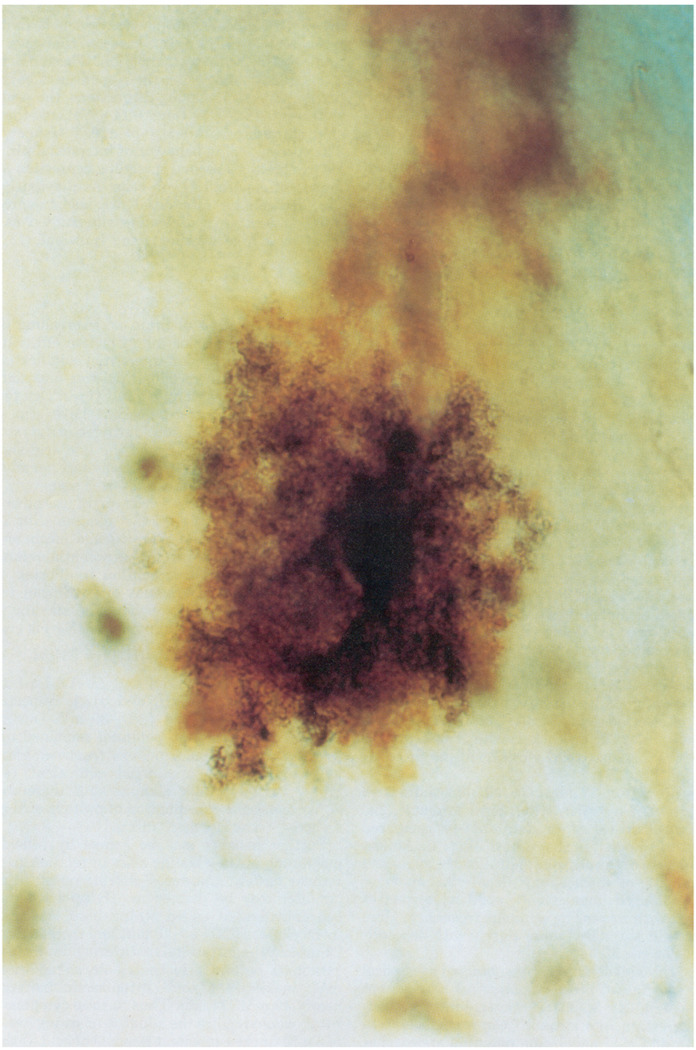
Recordings were obtained at cortical depths of 0–1,500 µm from the pial surface. To test whether both barrels of pH microelectrodes were intracellular, recordings were obtained with identical micropipettes containing electrolyte in each side. Both barrels always entered and exited cells simultaneously and recorded identical intracellular potentials. In agreement with previous reports (29), injection of HRP into such cells (Fig. 1) consistently stained protoplasmic astrocytes (7). Their resting intracellular pH was 7.10 ± 0.03, with a membrane potential of 76 ± 1 mV (mean ± SE, n = 25). All animals were normothermic (36.7 ± 0.1°C). Corresponding arterial blood physiological variables were Po2, 113 ± 5 Torr, Pco2, 37 ± 2 Torr (pH 7.41 ± 0.01), glucose 8.6 ± 0.3 mM, and hematocrit 44 ± 1% (±SE, n = 14 animals).
FIG. 1.
Protoplasmic astrocyte from rat neocortex stained with horseradish peroxidase. Injection of horseradish peroxidase into cells with electrical characteristics analogous to those studied with pH electrodes consistently stained protoplasmic astrocytes as shown here. Diameter of cell in horizontal direction is ∼50 µm.
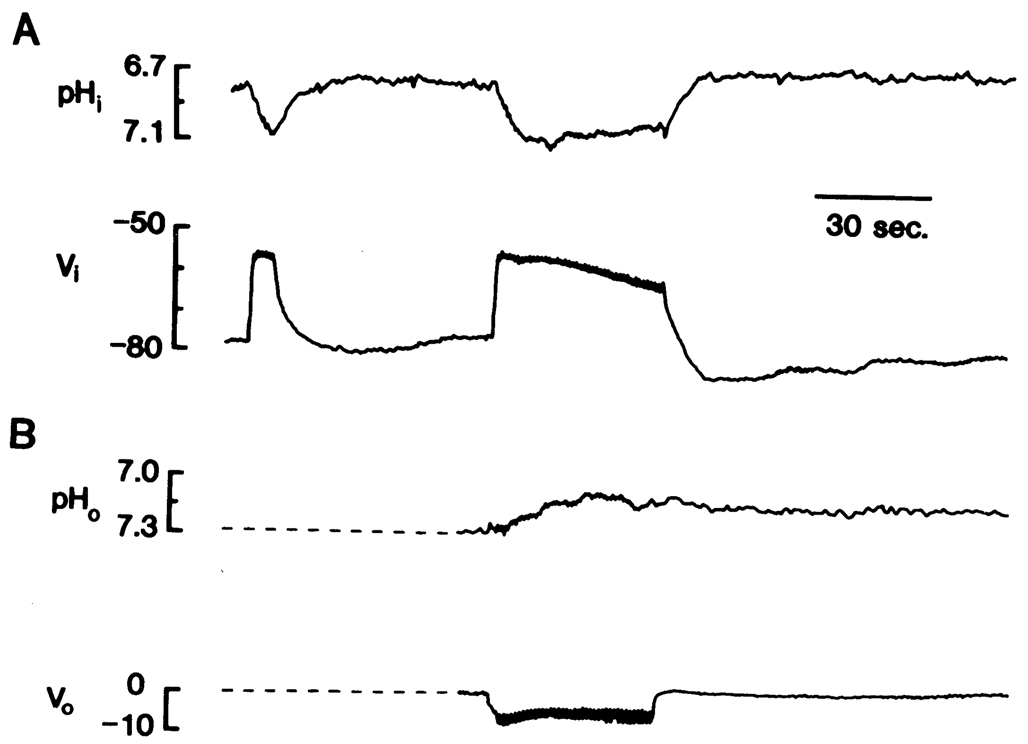
The characteristic response of astrocytes to cortical stimulation consisted of a rapid membrane depolarization and a somewhat slower increase in intracellular pH (Fig. 2A). The behavior of the glial membrane potential has been well described and mainly reflects the effect of a change in K+ activity on a membrane selectively permeable to K+ (22). Thus, during electrical stimulation, the glial membrane depolarized due to a rise in external K+ (22), whereas removal of stimulation resulted in a hyper-polarization, presumably reflecting the accumulation of internal K+ (3) and decline of external K+ below normal (10). In the example shown, pH increased by 0.24. After stimulation, intracellular pH recovered and occasionally acidified beyond its original level, a process that appeared to be related to stimulus duration. Hence, with maintained stimulation (43 s, Fig. 2A), intracellular pH reached a maximum, slowly became less alkaline, then rebounded to a more acid level than base line when stimulation ceased. The corresponding behavior of interstitial pH was studied by repeating the stimulus after withdrawal of electrodes from cells. The interstitial pH response was a slow acidification, with a less precise relation to stimulus onset and offset than the intracellular response (Fig. 2B).
FIG. 2.
Intracellular and interstitial responses to cortical stimulation. Stimulus trains (200-µs pulses at 20 Hz for 0.8 s) were delivered once per second. A: upper trace is intracellular pH (pHi), bottom trace is intracellular potential (Vi). Stimuli were delivered first for 6 s then for 43 s. B: upper trace is interstitial pH (pHo), bottom trace is interstitial potential (Vo). Stimuli were delivered for 41 s following withdrawal from cell recorded in A. Note stimulus train artifacts on Vi and Vo records. All potentials in millivolts with respect to remote electrode.
In 22 recordings from 17 glial cells, alkaline shifts were consistently evoked by stimulation. Since stimulus efficacy varies with cortical depth, data are best compared with respect to the electrical response (Fig. 3). Among all cells the degree of the alkaline shift directly correlated with the amount of intracellular depolarization (r = 0.63). In a prolonged impalement, repetitive responses were initiated with increasing stimulus frequency. Here the alkaline shift correlated more closely with depolarization (r = 0.94, see Fig. 3).
FIG. 3.
Stimulus-evoked intracellular alkaline shifts vs. depolarization. Data were obtained from 17 glial cells in frontal cortex. Open symbols and broken trace are data and regression line, respectively, from single cell (r = 0.94). Solid symbols are data from all other cells. Solid trace is regression line for all data (n = 22, r = 0.63).
The size of the glial depolarization obtained by cortical stimulation was limited because interstitial K+ does not normally rise >10–12 mM (11). However, during cortical-spreading depression, interstitial K+ can reach levels >40 mM (32, 23), and glial membrane potential approaches 0 mV (28). Under these conditions the above findings would predict a large intracellular alkaline shift. A recording during spreading depression is shown in Fig. 4A. As the wave of spreading depression reached the recording site, the glial membrane rapidly depolarized. Concurrently a large alkaline shift occurred that gave way to an acid rebound with repolarization. These pH transients were usually larger than the responses to cortical stimulation. For 16 spreading depressions, the peak alkaline shift was 0.28 ± 0.05 (range 0.11–0.78), and the final acid shift above base line was 0.21 ± 0.05 (range 0.10–0.39). The corresponding behavior of interstitial pH was studied by eliciting a second spreading depression after withdrawal of electrodes from cells. As previously reported the predominant interstitial response to spreading depression was an acidification interrupted early in its onset by a brief alkaline-going transient (13, 19) (Fig. 4B).
FIG. 4.
Intracellular and interstitial responses to cortical-spreading depression. Two consecutive high-frequency stimulus trains (2-ms pulses at 100 Hz for 0.8 s) were delivered to cortical surface to elicit cortical-spreading depression. A: upper trace is intracellular pH, bottom trace is intracellular potential. Note that initiating stimuli gave rise to early intracellular alkaline transient (arrow) and depolarization. B: upper trace is interstitial pH, bottom trace is interstitial potential. Cortical spreading was initiated 20 min after withdrawal of electrode from cell recorded in A. Dotted trace in A represents estimate of transmembrane potential during cortical-spreading depression, obtained by graphic subtraction of Vo from Vi. All potentials are in millivolts with respect to remote electrode. See Fig. 2 for definitions.
DISCUSSION
Both stimulation of brain and spreading depression cause increased production of lactate (18, 19) and CO2 (12, 18) and result in an interstitial acidification (13, 19, 31). In neurons, intracellular pH was reported to fall in response to depolarizing stimuli (1, 8). Our preliminary experiments on cortical neurons have also shown an intracellular acidification during cortical stimulation. This neuronal response, and the late acid shift of glia, may relate to the generation of these metabolic acids. The glial alkaline shift, however, is seemingly paradoxical, making hypotheses for its origin less obvious.
Although the cause of the alkaline shift is not yet known, available data permit some mechanisms to be excluded. If the glial membrane potential remained more negative than the hydrogen-ion equilibrium potential, the electrochemical gradient for hydrogen ions would favor net acid entry throughout the alkaline shift. The hydrogen ion equilibrium potential (EH+) is given by
With a range of 6.8–7.4 and 7.0–7.3 for intracellular and interstitial pH (pHi and pHo), respectively, EH+ would fall between +24 and −30 mV. During cortical stimulation, membrane potential remained negative to this range, excluding a passive transmembrane flux of hydrogen ions or a charged equivalent (e.g., . The alkaline shift therefore requires either metabolic consumption of protons or the action of an active transport mechanism.
Further insights may be gained by considering the magnitude of the alkaline shift in relation to the intracellular buffering power. Buffering power in millimoles per liter may be defined as ΔB/ΔpH where B is the concentration of added strong base. At a pH of 7.0, intracellular is ∼10 mM and therefore contributes 23 mM to the intracellular buffering power (24). Nonbi-carbonate buffers may contribute an additional 15–20 mM, making the total cytoplasmic buffering power nearly 40 mM (24, 26). Thus to raise cytoplasmic pH by >0.20 would require the addition of strong base in excess of 8 mM or the equivalent removal of acid. If the alkaline shift originated from a proton-consuming reaction, a similar concentration of substrate would have to be consumed within a matter of seconds. Available data neither support nor exclude such a process. However, stimulus-evoked shifts of this order have been reported for intracellular K+, Na+, and Cl− in mammalian cortical glia (3). How a similar acid-base flux might occur remains to be elucidated.
A rise in intracellular pH during cortical stimulation has a number of implications for glial cell function. In the face of metabolic demands associated with a rise in interstitial K+, an intracellular alkaline shift could enhance glycolytic rate (9), increasing the availability of energy resources during neuronal activity. Under pathological conditions, such as the onset of ischemia, bicarbonate generated with an alkaline shift would increase the intracellular buffering power of glia and thereby mitigate the ultimate ischemic acidosis (13, 16, 17). Since gap junctional conductance can be markedly increased by cytoplasmic alkalinization (27), clearance of external K+ through the glial syncytium (22) could be enhanced. Thus shifts in acid-base status might rapidly modify the functional state of glial cells in response to demands imposed by surrounding neurons.
The importance of intracellular pH modulation has been highlighted in studies of metabolism, growth, and fertilization, where transformation of the functional state of cells has been associated with cytoplasmic alkalinization (5). Our results raise the possibility that acid-base status may be used to rapidly modify the functional state of glial cells. Thus the observed lability of glial intracellular pH may play an important role for the normal and pathophysiological behavior of glia.
Acknowledgments
We thank Dr. F. Plum for reading and commenting on the manuscript.
This study was supported by National Institutes of Neurological and Communicative Disorders and Stroke Grants NS-19108 and NS-00767 (Teacher Investigator Development Award) as well as a Redel and Du Pont Foundation Grant to R. P. Kraig. M. Chesler was supported by National Institute of Neurological and Communicative Disorders and Stroke Training Grant NS-07141.
REFERENCES
- 1.Ahmed Z, Connor JA. Intracellular pH changes induced by calcium influx during electrical activity in Molluscan neurons. J. Gen. Physiol. 1980;75:403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammann D, Lanter F, Steiner RA, Schulthess P, Shijo Y, Simon W. Neutral carrier based hydrogen ion selective microelectrodes for extra- and intracellular studies. Anal. Chem. 1981;53:2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- 3.Ballanyi K, Grafe P, Ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J. Physiol. Lond. 1987;382:159–174. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borelli MJ, Carlini WG, Dewey WC, Ransom RB. A simple method for making ion-selective microelectrodes suitable for intracellular recording in vertebrate cells. J. Neurosci. Methods. 1985;15:141–154. doi: 10.1016/0165-0270(85)90051-2. [DOI] [PubMed] [Google Scholar]
- 5.Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am. J. Physiol. 1984;246:R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. (Regulatory Integrative Comp. Physiol. 15) [DOI] [PubMed] [Google Scholar]
- 6.Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J. Physiol. Lond. 1986;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesler M, Kraig RP. Direct recording of intracellular pH from glial cells of rat brain in vivo (Abstract) J. Cereb. Blood Flow Metab. 1987;7(Suppl1):S114. [Google Scholar]
- 8.Enders W, Ballanyi K, Serve G, Grafe P. Excitatory amino acids and intracellular pH in motorneurons of the isolated spinal cord. Neurosci. Lett. 1986;72:54–58. doi: 10.1016/0304-3940(86)90617-8. [DOI] [PubMed] [Google Scholar]
- 9.Fidelman ML, Seeholzer SH, Walsh KB, Moore RD. Intracellular pH mediates action of insulin on glycolysis in frog skeletal muscle. Am. J. Physiol. 1982;242:C87–C93. doi: 10.1152/ajpcell.1982.242.1.C87. (Cell Physiol. 11) [DOI] [PubMed] [Google Scholar]
- 10.Heinemann U, Lux HD. Undershoots following stimulus induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res. 1975;93:63–76. doi: 10.1016/0006-8993(75)90286-3. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann U, Lux HD. Ceiling of stimulus-induced rises in extracellular potassium concentration in the cerebral cortex of the cat. Brain Res. 1977;120:231–249. doi: 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- 12.Kraig RP, Cooper AJL. Bicarbonate and ammonia changes in brain during spreading depression. Can. J. Physiol. Pharmacol. 1987;65:1099–1104. doi: 10.1139/y87-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in the cerebellar microenvironment. J. Neurophysiol. 1983;49:831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- 14.Kraig RP, Nicholson C. Acidosis of presumed glia during ischemia (Abstract) Soc. Neurosci. Symp. 1986;12:65. [Google Scholar]
- 15.Kraig RP, Nicholson C. Profound acidosis in presumed glia during ischemia. In: Raichle ME, Powers WJ, editors. Cerebrovascular Diseases (15th Princeton-Williamsburg Conference) New York: Raven; 1987. pp. 97–102. [Google Scholar]
- 16.Kraig RP, Pulsinelli WA, Plum F. Hydrogen ion buffering in brain during complete ischemia. Brain Res. 1985;342:281–290. doi: 10.1016/0006-8993(85)91127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraig RP, Pulsinelli WA, Plum F. Carbonic acid buffer changes during complete brain ischemia. Am. J. Physiol. 1986;250:R348–R357. doi: 10.1152/ajpregu.1986.250.3.R348. (Regulatory Integrative Comp. Physiol. 19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mcilwain F, Bachelard HS. Biochemistry and the Central Nervous System. London: Churchill Livingstone; 1971. chapt. 4; p. 69. [Google Scholar]
- 19.Mutch WAC, Hansen AJ. Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. J. Cereb. Blood Flow Metab. 1984;4:17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson C. Dynamics of the brain cell microenvironment. Neurosci. Res. Program Bull. 1980;18:177–322. [PubMed] [Google Scholar]
- 21.Orkand PM, Bracho H, Orkand RK. Glial metabolism: alterations by potassium levels comparable to those during neural activity. Brain Res. 1973;55:467–471. doi: 10.1016/0006-8993(73)90315-6. [DOI] [PubMed] [Google Scholar]
- 22.Orkand RK, Nicholls JG, Kuffler SW. Effects of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- 23.Prince DA, Lux HD, Neher E. Measurement of extracellular potassium activity in cat cortex. Brain Res. 1973;50:489–495. doi: 10.1016/0006-8993(73)90758-0. [DOI] [PubMed] [Google Scholar]
- 24.Roos A, Boron WF. Intracellular pH. Physiol. Rev. 1981;61:296–433. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 25.Salem RD, Hammerschlag R, Bracho H, Orkand RK. Influence of potassium ions on accumulation and metabolism of [14C]glucose by glial cells. Brain Res. 1975;86:499–503. doi: 10.1016/0006-8993(75)90903-8. [DOI] [PubMed] [Google Scholar]
- 26.Siesjo BK, Messeter K. Factors determining intracellular pH. In: Siesjo BK, Sorenson SC, editors. Ion Homeostasis of the Brain. Copenhagen: Munksgaard; 1971. pp. 244–262. [Google Scholar]
- 27.Spray DC, Harris AL, Bennett MVL. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science Wash. DC. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- 28.Sugaya E, Takato M, Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of the cat. J. Neurophysiol. 1975;38:822–841. doi: 10.1152/jn.1975.38.4.822. [DOI] [PubMed] [Google Scholar]
- 29.Takato M, Goldring S. Intracellular marking with Lucifer Yellow CH and horseradish peroxidase of cells electrophysiologically characterized as glia in the cerebral cortex of the cat. J. Comp. Neurol. 1979;186:173–188. doi: 10.1002/cne.901860205. [DOI] [PubMed] [Google Scholar]
- 30.Thomas RC. Eccentric double micropipette suitable both for pHi microelectrodes and for intracellular iontophoresis. J. Physiol. Lond. 1985;371:24P. [Google Scholar]
- 31.Urbanics R, Leninger-Follert E, Lubbers DW. Time course of changes of extracellular H+ and K+ activities during and after direct electrical stimulation of the brain cortex. Pfluegers Arch. 1978;378:47–53. doi: 10.1007/BF00581957. [DOI] [PubMed] [Google Scholar]
- 32.Vyskocil F, Kriz N, Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization. Brain Res. 1972;39:255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]