Abstract
Background
Imatinib, given concurrently or alternating with chemotherapy, has improved the response and survival of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) but relapses are still frequent. The aim of this study was to evaluate the feasibility and results of giving imatinib concurrently with intensive chemotherapy, stem cell transplantation and post-transplant imatinib maintenance therapy in patients with newly diagnosed Ph+ ALL.
Design and Methods
This was a phase II study of patients with newly diagnosed Ph+ ALL given standard chemotherapy, together with imatinib (400 mg/day) until stem cell transplantation, followed by imatinib maintenance therapy for all patients regardless of the molecular status of the disease.
Results
Of the 30 patients included, 27 (90%) achieved complete remission, one was resistant to treatment and two died during induction therapy. The percentages of major and complete molecular responses were 86% and 21% after induction, and 81% and 65% after consolidation, respectively. Similar results were observed assessing minimal residual disease by flow cytometry. Of the 27 patients who achieved complete remission, 21 underwent stem cell transplantation (16 allogeneic, 5 autologous). Imatinib (400 mg/day) could be administered after transplantation for a median of 3.9 months in 12 patients, although it was interrupted in 10 patients (in 2 cases because of side effects of the drug). Nine patients relapsed, four before and five after stem cell transplantation and eight patients died of transplant-related causes. With a median follow-up of 4.1 years, the probabilities (95% CI) of disease-free and overall survival were 30% (15% to 45%) and 30% (16% to 45%), respectively.
Conclusions
These results confirm that imatinib is an effective first-line treatment for adult Ph+ ALL when given concurrently with chemotherapy, making stem cell transplantation feasible in a high proportion of patients. However, post-transplantation imatinib administration was limited, mainly because of transplantation-derived complications rather than drug-specific toxicity.
Keywords: acute lymphoblastic leukemia, Philadelphia chromosome, BCR-ABL, imatinib, intensive chemotherapy, stem cell transplantation, imatinib maintenance
Introduction
The Philadelphia chromosome is the most frequent single recurrent cytogenetic abnormality in acute lymphoblastic leukemia (ALL) in adults and together with the t(4;11) rearrangement is the most adverse prognostic factor in these patients.1,2 The Philadelphia chromosome carries the BCR-ABL fusion gene, which encodes the p190BCR-ABL and p210BCR-ABL oncoproteins that are targeted by the tyrosine kinase inhibitor, imatinib.
Concurrent or alternating use of imatinib together with intensive chemotherapy for remission induction and consolidation is associated with a high frequency of morphologic (95%–100%) and molecular (50%) complete remissions3–8 in young adults with Philadelphia chromosome-positive (Ph+) ALL. These features have translated into a significantly higher probability of survival than that obtained in historical controls with similar chemotherapy schedules without imatinib. Consequently, the combination of imatinib and chemotherapy is currently considered the standard treatment for Ph+ ALL.9
In young patients with a histocompatible donor, allogeneic stem cell transplantation (SCT) is usually recommended in first complete remission when feasible, especially in patients with no residual disease or minimal residual disease (MRD); alternatively, the transplant can be carried out immediately after detection of disease at the molecular level. However, with prolonged follow-up a substantial proportion of young patients and practically all elderly patients relapse.10–12 Some of the relapses are late, making careful molecular follow-up of these patients mandatory.
There is little information on the feasibility and efficacy of giving imatinib as maintenance therapy to prevent relapse after SCT in patients already treated with imatinib and chemotherapy prior to the transplant. Uncontrolled studies13 and ongoing clinical trials14 indicate that the use of imatinib is feasible, with a low relapse rate and an acceptable transplant-related mortality, but problems with tolerability may lead to early discontinuation of imatinib or limit the intensity of the dose that can be given to some patients. On the other hand, whether this drug needs to be given to patients without residual disease after SCT, the optimal duration of imatinib treatment and the feasibility and the efficacy of combinations of imatinib with other agents as maintenance therapy are all unknown.9
We undertook a prospective phase II study to evaluate the efficacy of concurrent administration of imatinib and intensive chemotherapy during remission induction and consolidation, and the feasibility and efficacy of imatinib after SCT in a series of 30 patients with newly diagnosed Ph+ ALL.
Designs and Methods
Eligibility
Patients up to 65 years of age with newly diagnosed Ph+ ALL were eligible for the CSTIBES02 study if they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, adequate renal and hepatic function (serum creatinine < 2 mg/dL and bilirubin < 2 mg/dL), an adequate cardiac status and a negative pregnancy test. Patients with serious underlying medical problems were excluded as were those who had received investigational anti-leukemic therapy within the preceding 7 days. Females of childbearing potential were required to use an effective method of contraception. The trial was activated in April 2003 and was closed for follow-up in September 2008.
Study design and therapy
This was a prospective, non-randomized, phase II trial conducted by the Spanish PETHEMA (Programa Español de Tratamiento en Hematología) and GETH (Grupo Español de Trasplante Hemopoyético) groups. The protocol was reviewed and approved by the institutional review board of each of the participating centers and was conducted in accordance with the Declaration of Helsinki. Patients provided written consent before entering the study. The trial was registered at http://www.clinicaltrials.gov with the number NCT00388895.
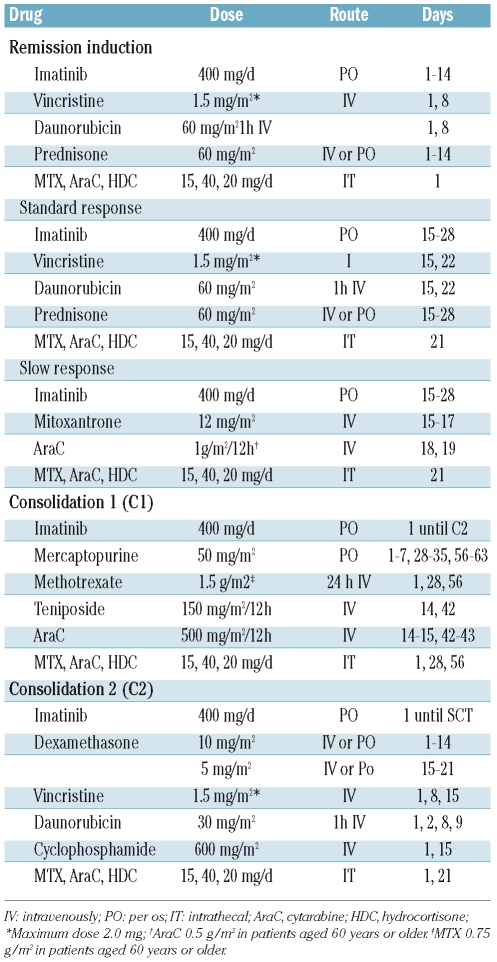
The treatment schedule is shown in Table 1. Remission induction therapy consisted of weekly vincristine and daunorubicin, and daily prednisone and imatinib (400 mg/day from day 1). Bone marrow aspiration was performed on day 15: patients with a standard response (≤5% blast cells or hypocellular bone marrow) continued with the same schedule up to day 28, whereas those with a slow response (>5% blast cells) received mitoxantrone and cytarabine together with imatinib (Table 1). Treatment was considered to have failed in patients who did not attain complete remission, and these patients were excluded from the study. The first cycle of consolidation therapy (C1) consisted of high-dose methotrexate, mercaptopurine, high-dose cytarabine, teniposide and imatinib (400 mg/day), and the second consolidation cycle (C2) included vincristine, daunorubicin, dexamethasone, cyclophosphamide and imatinib (400 mg/day) (Table 1). Full hematologic recovery, defined as a neutrophil count of at least 1×109/L and a platelet count of at least 50×109/L, was required for the initiation of each course of consolidation. Central nervous system prophylaxis, consisting of intrathecal injections of methotrexate (15 mg), cytarabine (30 mg) and hydrocortisone (20 mg), was given during the remission induction cycle (days 1 and 22), C1 (days 1, 28 and 56) and C2 (days 1 and 15). Patients with evidence of central nervous system leukemia at diagnosis (≥5 leukemic blasts/μL of cerebrospinal fluid having ensured no contamination of the sample by peripheral blood) received intrathecal injections twice a week until two cerebrospinal fluid examinations showed that the blasts had disappeared. The C2 cycle was not mandatory if a donor was already available.
Table 1.
Chemotherapy schedule for patients with BCR-ABL-positive ALL in the CSTIBES02 study.
After C2, patients with a HLA-identical donor were submitted to allogeneic SCT. The remaining patients, as well as those with medical contraindications to allogeneic SCT, underwent autologous SCT. In this latter case, hematopoietic stem cell mobilization was achieved with granulocyte colony-stimulating factor after recovery from the C2 cycle. Following SCT, all patients were planned to receive imatinib (400 mg/day) from the time of full hematologic recovery for at least 1 year if molecular remission persisted.
The preparative regimen for autologous or allogeneic SCT (either from an HLA-identical sibling or from an unrelated donor) consisted of fractionated total body irradiation (total dose 13 Gy, from day −7 to −4), and cyclophosphamide (60 mg/kg days −3 and −2). For patients aged over 50 years a non-myeloablative conditioning regimen with fludarabine (30 mg/m2, from day −8 to −4) and melphalan (70 mg/m2, days −3 and −2) was recommended. The recommended conditioning regimen for the patients in whom unrelated cord blood was the source of hematopoietic stem cells included tiothepa (5 mg/kg days −7 and −6), fludarabine (50 mg/m2, days −5, −4 and −3), busulphan (3.2 mg/kg in intravenous infusion days −5, −4 and −3) and thymoglobulin (2 mg/kg days −5, −4, −3 and −2).
Dose modifications
For patients aged 60 years or older, the dose of cytarabine in the induction therapy was reduced to 500 mg/m2 every 12 h, while the dose of methotrexate in the C1 cycle was reduced to 0.75 g/m2.
The dose of imatinib could be modified, based on the following conditions. During the remission induction and consolidation courses, hematologic toxicity was essentially not considered a reason for reducing the dose of imatinib or suspending treatment with this tyrosine kinase inhibitor. In cases of grade 3 or 4 non-hematologic toxicity, administration of imatinib was interrupted until recovery to grade 1 or better and then resumed at a dose of 400 mg/day. If grade 3 or 4 toxicity recurred after resuming imatinib, the dose was reduced to 300 mg or 200 mg. Imatinib was not to be interrupted during the interval between induction and consolidation cycles or between the two consolidation courses or during the mobilization and collection of hematopoietic stem cells. The administration of imatinib was, however, suspended 15 days before SCT and resumed after full hematologic recovery had been achieved. If imatinib-related toxicity or intolerance occurred after the SCT, the dose could be reduced to 300 mg or 200 mg before considering permanent discontinuation of the therapy.
Supportive care
Decisions on hospital admissions, prophylaxis and management of infections, and transfusions were made in accordance with specific protocols of each participating hospital. Granulocyte colony-stimulating factor (5 μg/kg/day, subcutaneously) was administered to all patients who developed neutropenia following the induction and consolidation cycles. In cases of autologous or allogeneic SCT, granulocyte colony-stimulating factor was administered from day +7 after the transplant until sustained neutrophil recovery (white cell count >1×109/L) had been achieved.
Evaluation of patients
The primary objectives of this study were to determine the complete remission rate in this series of patients with Ph+ ALL, the proportion of patients reaching SCT in first molecular complete remission and the feasibility and efficacy of imatinib after SCT. The secondary aims were to assess the toxicity and safety of the therapy, response duration and survival. Pretreatment evaluations included clinical history and physical examination, complete blood count with differential, sequential multiple analysis-12, and bone marrow aspiration for cytology, flow cytometry and cytogenetic studies. Bone marrow aspirates were repeated on day 14 after the start of therapy, at the time of evaluating complete remission (usually on days 28 to 35 after starting chemotherapy), before the C2 cycle, before SCT, and every 3 months after SCT.
Quantitative real-time polymerase chain reaction (RT-PCR) assays for BCR-ABL were performed on bone marrow and peripheral blood samples using TaqMan technology in accordance with the guidelines approved in the Europe Against Cancer Program.15 The assays were carried out in a central laboratory participating in this program. Standard curves were produced for major and minor BCR-ABL breakpoint variants from 10-fold dilution series of five different plasmid concentrations (200,000, 20,000, 200, 20 and 2 copies/μL). To minimize variability in the results due to differences in the efficiency of cDNA synthesis and RNA integrity among the patients’ samples, the absolute BCR-ABL copy number was normalized to the expression of the GUS housekeeping gene, chosen according to the Europe Against Cancer Program.16 The normalized values of the BCR-ABL copies in each sample are reported as BCR-ABL copy numbers. All experiments were carried out in duplicate. At least 105 GUS plasmid equivalents were required in a sample to consider a negative PCR result valid; otherwise, the sample was not useful for minimal residual disease studies. All reactions were performed on an ABI PRISM 7700 DNA Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Fluorescence spectra were continuously monitored and analyzed by the SDS system (Applied Biosystems, software version 1.9). Samples were collected at diagnosis, at day 14 of remission induction therapy, at the time of evaluation of complete remission, following the C1 cycle, before SCT and every 3 months after SCT. Negative results were not confirmed by nested PCR.
Concurrent detection of minimal residual disease (MRD) was performed by multiparametric flow cytometry (FACScan, Becton/Dickinson Biosciences, San José, CA, USA) in the same bone marrow sample and laboratory using quadruple staining, as previously described.17 Briefly, erythrocyte-lysed whole bone marrow samples were stained with an identical panel of four-color (fluoroscein isothiocyanate, phycoerythrin, peridin cholophyllcyanin 5, allophycocyanin) combinations of monoclonal antibodies aimed at the specific identification of blast cells,18 as follows: CD10/CD20/CD19/CD34, CD10/CD19/CD38/CD34, CD10/CD34/CD19/CD45, CD10/CD13/CD19/CD34, and CD10/CD33/CD19/CD34. All monoclonal antibodies were purchased from Becton Dickinson. In order to increase the sensitivity of the analysis, flow cytometric data from follow-up samples was acquired in two consecutive steps. Briefly, in the first step, all the cells present in the sample were analyzed and at this point at least 20,000 events/tube were measured. Subsequently, in a second step, a multiparametric live-gate was used to acquire more specific data on leukemic cells, which may be present at low frequencies in the sample. For this purpose, a low to intermediate SSC/CD19+ and SSC/CD34+ antigen live gate was applied and information was collected for at least 105 bone marrow nucleated cells. Data analysis was based on the identification of cells with aberrant phenotypic features.18 Either the LYSIS II or Cell-Quest software program (Becton Dickinson Biosciences) was used for data acquisition. The PAINT-A-GATE PRO software program (Becton Dickinson Biosciences), with a polynomial SSC transformation function, was used for further data analysis.
Toxicity was evaluated on the basis of the National Cancer Institute Expanded Common Toxicity Criteria (NCI-CTC) version 2.0. Studies on ABL kinase domain mutations were not routinely performed.
Response criteria
Complete remission was considered to have been achieved when all the following criteria were fulfilled: less than 5% blasts in bone marrow, no leukemic blasts in the peripheral blood, recovery of peripheral blood neutrophil counts to at least 1.5×109/L and platelet counts to at least 100×109/L and no evidence of extramedullary leukemia. Other defined outcomes were induction death (if death occurred after starting therapy and without the patient fulfilling the criteria for complete remission or resistant disease), resistant disease (if the patient survived the induction treatment period but the leukemia persisted or re-grew) and relapse (if disease recurred at any site after the patient had achieved a complete remission). A major molecular response was defined as the achievement of less than 50 copies of ABL-BCR in the bone marrow (as assessed by quantitative RT-PCR), in the presence of cytological complete remission, no MRD (assessed by flow cytometry with a threshold of 0.01%) and the hematologic criteria for complete remission. A complete molecular response was defined as the absence of BCR-ABL copies and no MRD assessed by flow cytometry with a threshold of 0.001%.
Statistical analysis
Disease-free survival was measured from the date of the complete remission until documented relapse or death in remission from any cause. Overall survival was measured from the date of starting therapy until death from any cause. Transplant-related mortality was defined as death occurring after SCT in relapse-free patients. Disease-free and overall survival curves were plotted according to the method of Kaplan and Meier19 and the differences analyzed by the log-rank test.20 Patients undergoing SCT were not censored at the time of transplantation. The Statistical Package for Social Sciences (SPSS) 12.0 software (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Patients’ characteristics
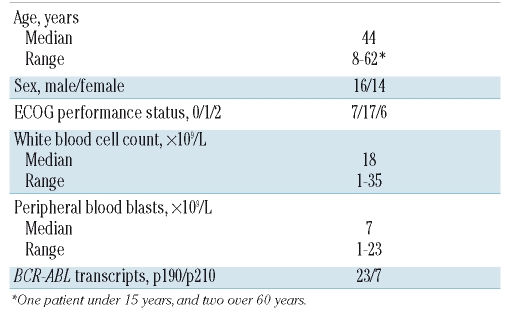
Between April 2003 and November 2004, 30 patients with newly diagnosed Ph+ ALL entered the study. The patients’ characteristics are listed in Table 2. The p190BCR-ABL transcript (e1a2) was expressed in 23 (80%) of the 30 patients and a p210BCR-ABL transcript (b2a2 or b3a2) in the remaining seven (20%). The median age of the group was 44 years and there was only one patient under 15 years old and two over 60 years old. Sixteen (53.3%) of the patients were male.
Table 2.
Main characteristics of the 30 patients in the series.
Treatment efficacy
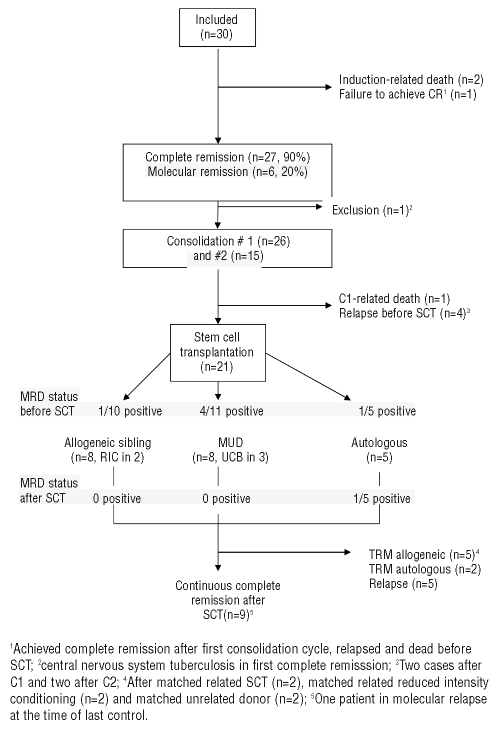
Figure 1 shows the progression of the patients along the trial. Twenty-seven (90%) of the 30 patients achieved a complete remission. This complete remission rate is higher than that observed in the ALL93 trial (70%, P=0.045), in which the same chemotherapy, but without imatinib, was used. One patient had resistant leukemia and two patients died in the remission induction period from infections. Of the 27 patients who achieved a complete remission, 20 had a standard response already detectable at day 14, while the other seven had a slow response. One patient was removed from the study before C1 because of disseminated central nervous system tuberculosis. Twenty-six patients received the first consolidation cycle (C1) and 15 the second one (C2). One patient died after the C1 cycle due to infection. Four patients relapsed before SCT, two of them after C1 and two after C2.
Figure 1.
Flow chart of the patients included in the trial. MUD: matched unrelated donor; RIC: reduced intensity conditioning; UCB: unrelated cord blood; TRM transplant-related mortality.
Transplantation outcome
SCT (allogeneic, 16; autologous, 5) was performed in 21 out of 27 (78%) patients who achieved a complete remission. The median time from complete remission to SCT was 187 days (range, 129 to 297 days). Conditioning regimens included cyclophosphamide and total body irradiation (n=16), and tiothepa, fludarabine, busulphan and thymoglobulin in the three patients receiving an unrelated cord blood transplant, and a reduced intensity regimen with fludarabine and melphalan in two additional patients. As regards the allogeneic transplants, the donor was an HLA identical sibling for eight patients and an alternative donor for the other eight patients, including three who received hematopoietic stem cells from umbilical cord blood. Engraftment was successful in all patients. Non-relapse transplant-related deaths occurred in eight patients after related donor SCT (n=2), after non-myeloablative related donor SCT (n=2), after matched unrelated donor SCT (n=2) and after autologous SCT (n=2). The main causes of death in these patients included early death before hematologic recovery (n=2), graft-versus-host disease (GVHD) (n=4), post-transplant lymphoproliferative disorder (n=1) and pneumococcal pneumonia after complete hematologic recovery following an autologous SCT (n=1). Five patients had a relapse in bone marrow (n=4) or in bone marrow and the central nervous system (n=1) between 5 and 27 months after SCT (4 allogeneic and 1 autologous).
Minimal residual disease measurements
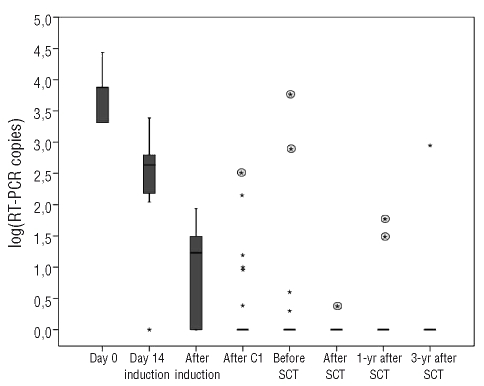
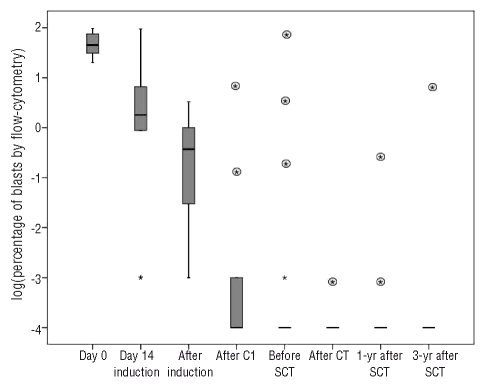
Quantitative RT-PCR showed that the bone marrow contained less than 50 copies of BCR-ABL in 6 out of 29 (21%) evaluable patients on day 14 and in 24 out of 28 (86%) patients at the time of complete remission (Figure 2). The median BCR-ABL copy number decreased 1 log on day 14 with an additional 2 log reduction at the time of complete remission. In turn, levels of MRD under 0.01%, as assessed by flow cytometry, were observed in 5 out of 29 (17%) patients on day 14 and in 12 of 28 (43%) evaluable patients at the end of induction (Figure 3). As in the PCR study, there was a median 3 log reduction in the level of MRD during the remission induction period. There was an additional mean reduction of 2 logs in residual disease before the onset of C2. Before C2, 11 out of 26 (42%) patients were PCR negative and 15 out of 26 (58%) cases were negative for MRD assessed by flow cytometry. Before SCT, 15 out of 23 (65%) patients were PCR-negative and 14 out of 23 (61%) were negative for MRD assessed by flow cytometry. No significant reduction was observed in MRD between the onset of C2 and the time of SCT. After SCT, 15 out of 19 patients (79%) achieved a complete molecular response according to the RT-PCR results and 17 out of 19 patients according to the flow cytometry assessment (Figures 2 and 3). All patients in molecular complete remission before and after SCT according to both assessment techniques (n=6) remained in continuous complete remission at last follow-up. Conversely, detection of less than a major response before or after SCT by either of the two techniques was invariably associated with relapse. Post-SCT major response was associated with relapse only if PCR and flow cytometry results were concordant. A one log increase in MRD both before and after SCT was predictive of relapse, whichever technique had detected the increment. Among patients undergoing an autologous SCT only one was MRD-positive before the procedure and relapsed early afterwards; none of the other four relapsed although two died of other causes.
Figure 2.
Progression of MRD of individuals assessed by RT-PCR. Values are expressed on a logarithmic scale and negative values set at 0. Boxes represent medians and quartiles; dots represent patients with outlier values. Rounded asterisks represent values for individual patients who relapsed after that control. Out of eight patients in sustained complete remission only one had a positive PCR in a pre-SCT evaluation and another a single positive PCR in a post-SCT control.
Figure 3.
Progression of MRD of individuals as assessed by flow cytometry. Values are expressed on a logaritmic scale and negative values set at minus 4. Boxes represent medians and quartiles; dots represent patients with outlier values. Rounded asterisks represent values for individual patients who relapsed after that control. Out of eight patients in sustained complete remission only one had positive flow cytometry in a pre-SCT evaluation and none was positive in any post-SCT controls.
Imatinib after stem cell transplantation
Imatinib was started following SCT in 13 out of 21 patients (62%). The median time from SCT to initiation of imatinib therapy was 3.9 months. Four out of five patients who had undergone autologous SCT were able to start imatinib maintenance therapy, which was continued up to the last control (at 40 and 60 months) in two patients or death in the other two. The remaining patient did not start imatinib therapy because of persistent severe thrombocytopenia after the transplant, followed by early post-SCT relapse. The main reasons for not initiating imatinib among patients who underwent allogeneic SCT were early transplant-related mortality (n=4, including the patient with post-transplant lymphoproliferative disease), severe GVHD (n=1) and the patients’ decision (n=2). The median time on imatinib therapy for the remaining nine patients who underwent allogeneic SCT was 9 months (range, 1 to 27). The post-SCT dose of imatinib was reduced to 200 mg in three patients (two were able to continue imatinib treatment to last control and another patient had to discontinue treatment). Imatinib therapy was interrupted due to relapse (n=3), severe chronic GVHD (n=2), grade 3–4 toxicity (n=2, hematologic in one patient and gastrointestinal in the other), non-relapse death (n=1) and patient’s choice (n=1) after 24 month of continuous imatinib treatment.
Survival
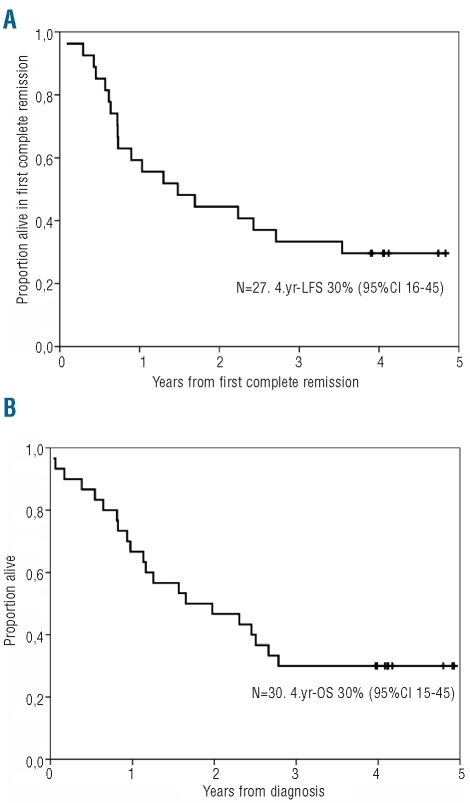
Nine patients remain alive after 3 years of follow-up, eight with a molecular complete response and one with molecular relapse. After a median follow-up of 4.1 years (range, 3.9–4.8), the median disease-free and overall survival were 1.5 years and 1.7 years, respectively. The probabilities of disease-free and overall survival were 30% (95% CI 16% to 45%) and 30% (95%CI 15% to 45%), respectively. (Figure 4). The survival of these patients was significantly better than that of an historical cohort of 37 patients included in the ALL93 trial in which patients were treated with chemotherapy and SCT but not imatinib (Online Supplementary Figure S1).
Figure 4.
Probabilities of overall survival (A) and disease-free survival (B) of the patients included in the CSTIBES02 trial. Black dots in figures A and B indicate patients alive and alive in complete remission, respectively.
Toxicity
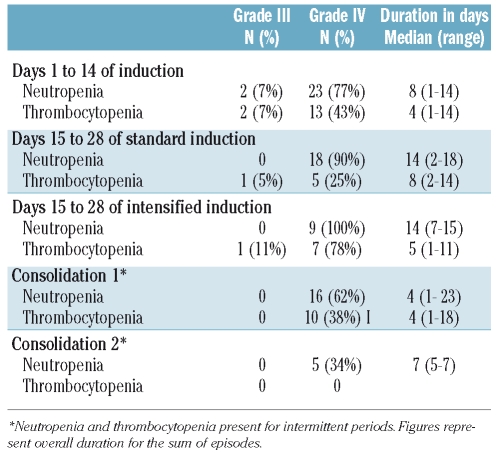
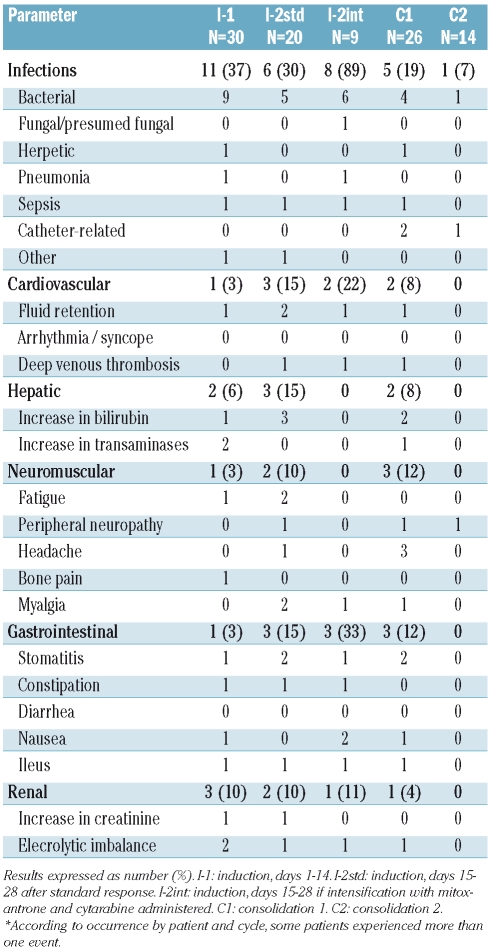
Table 3 shows the hematologic toxicity that occurred during the induction and consolidation periods. Grade III–IV neutropenia was observed in almost all induction cycles, especially in patients receiving intensified induction. The frequency and duration of severe neutropenia was lower after the C1 and C2 cycles. The frequency of severe thrombocytopenia throughout the study was low and no cases of severe thrombocytopenia were observed in the C2 cycle. Table 4 shows the cases of grade III–IV non-hematologic toxicity. The most frequent adverse events were infections, especially bacterial one, followed by hepatic and gastrointestinal toxicity. Other frequent grade I–II toxicities were hepatic adverse events, fluid retention, mucositis and wasting syndrome.
Table 3.
Hematologic toxicities of concurrent chemotherapy and imatinib mesylate in induction and post-induction courses.
Table 4.
Non-hematologic, grade III and IV toxicities of concurrent chemotherapy and imatinib mesylate in induction and post-induction courses*.
No major toxic events were observed during the mobilization of peripheral blood hematopioetic stem cells, either for autologous SCT (n=5) or as a back-up prior to an unrelated donor or umbilical cord blood SCT (n=8). During this period the patients continued on imatinib therapy and the collection of hematopoietic stem cells was adequate in all cases.
Interruption of post-SCT imatinib maintenance therapy was directly associated with imatinib in two out of ten patients (one each with grade III gastrointestinal and hematologic toxicity) and a likely association with imatinib and other drugs was established in another case (microangiopathic thrombocytopenia). No other grade III or higher toxicity attributed to imatinib was recorded.
Discussion
The results of this study confirm that imatinib is an effective first-line treatment for de novo adult Ph+ ALL when given concurrently with chemotherapy and allows SCT to be performed in a substantial proportion of patients in major or complete molecular remission. However, in the present trial prophylactic imatinib could not be given as scheduled after SCT in a considerable proportion of patients, mainly because of transplant-related events. Imatinib-specific toxicity was not an issue for most patients and, particularly in the setting of autologous SCT, the tyrosine kinase inhibitor could be administered safely for a prolonged period.
As occurred in other contemporary trials, combining chemotherapy and imatinib (in concurrent or intermittent schedules) provided better results in terms of cytologic and molecular complete responses,3–8,21 time to hematologic response and survival than those in historical controls treated with chemotherapy without imatinib.22–24 It is of note that the clearance of MRD was mainly observed during the induction and the first consolidation cycle (C1), with no further decrease thereafter. The fact that two out of the four patients in the present study who relapsed before SCT did so after the C2 cycle raises the question of whether an earlier transplant would have avoided such relapses. Both flow cytometric and molecular biology detection of MRD appeared to be highly predictive of both pre-SCT relapse (4 out of 8 patients relapsing before SCT when MRD > 0.01% after C1) and post-SCT relapse (5 out of 5 relapsed at any level of detectable MRD)
As a result of an improved complete remission rate, 50% to 86% of patients with Ph+ ALL were able to undergo allogeneic SCT in first complete remission in several trials combining imatinib and intensive chemotherapy.3–5,21 We used a relatively low dose of imatinib (400 mg/day) to ensure continuous treatment during initial therapy, obtaining similar short-term results (complete remission rate, molecular remission rate at the end of induction, and proportion of cases submitted to allogeneic SCT in a MRD negative status) to those of contemporary trials in which imatinib was given at a dose of 600 mg/day.
No definitive consensus has been reached on the need to systematically perform allogeneic SCT in patients with a good molecular response.9 In our study, SCT (allogeneic when possible) was scheduled for all patients who achieved complete remission and was actually performed in 74% of them. It is of note that 65% or 61% (PCR or flow cytometry) of the patients in our series were in complete molecular remission at the time of SCT. There is evidence that a good quality remission before SCT is associated with a lower probability of post-transplant relapse.25
Despite encouraging short-term results, relapses are observed in a very significant proportion of patients treated with the combination of imatinib and chemotherapy, even after allogeneic SCT, resulting in a decrease in the probability of survival down to 30%–50%10,11 in cohorts with an extended follow-up, as occurred in the present trial, which is one with the longest follow-up reported to date. The occurrence of relapses after SCT provides the rationale for evaluating the usefulness of ‘maintenance’ therapy. There is evidence suggesting that pre-SCT treatment with imatinib does not have detrimental effects on engraftment, the occurrence and severity of GVHD, or on transplant-related organ toxicity. Uncontrolled studies have shown that imatinib can also be safely administered early after myeloablative allogeneic SCT at a dose intensity comparable to that used in primary therapy,13,29 and there is preliminary evidence that imatinib can also be administered safely after autologous SCT.30 In a study by Wassmann et al.,26 half of the patients with Ph+ ALL who received imatinib because they had MRD after SCT experienced prolonged disease-free survival, which could be anticipated by the rapid achievement of molecular complete remission. This makes imatinib a good candidate for maintenance therapy after SCT, with the objective of preventing relapses, although the need for systematic administration of imatinib after allogeneic SCT has not been adequately addressed yet.
An ongoing randomized trial by Wassmann et al.14 is comparing systematic versus MRD-triggered administration of imatinib after SCT: the preliminary results show a low rate of relapses in both arms, suggesting that the administration of imatinib early after molecular relapse is equally effective as the systematic administration of the drug as long as close molecular follow-up (i.e. every 3 weeks) of the patients after SCT is ensured. However, imatinib was discontinued in 48% of the cases, mostly because of gastrointestinal toxicity and GVHD. The impact of allogeneic SCT toxicity was even more striking in the present trial, accounting for a significant proportion of non-relapse mortality and significantly limiting post-SCT exposure to imatinib. The relatively high transplant-related mortality may explain the slightly lower survival observed in our study in comparison with that of other similar trials.3–8,21
Given the frequency of ABL domain mutations in young27 and elderly28 patients with Ph+ ALL at diagnosis, second generation tyrosine kinase inhibitors (dasatinib, nilotinib) may be alternative candidates for prophylactic post-SCT treatment. Although ABL mutation studies were not performed in the present trial, our results suggest that the main issue in the post-SCT setting is tolerance to treatment rather than the emergence of resistant clones.
Inferences on the influence of imatinib on transplant-related mortality cannot be made from the results of our trial, although such an influence is unlikely. Imatinib was well tolerated during induction and consolidation and after SCT. Early transplant-related mortality and GVHD were the major causes of inability to start or continue post-transplant imatinib maintenance therapy. In fact, exposure to imatinib was minimal in a substantial proportion of patients submitted to allogeneic SCT mainly because of transplant-related events. The heterogeneous circumstances of the patients undergoing SCT, particularly differences in MRD status, the different sources of stem cells used, and the variety in post-SCT complications, are considerable limitations to the interpretation of results. Nevertheless, interruption of imatinib maintenance therapy because of toxicity of the drug was rare, with complications of the allogeneic SCT being the main cause of imatinib suspension. In our trial the two patients with the longest exposure to imatinib had undergone autologous SCT. In this regard, lymphocyte-depleted allogeneic SCT or even autologous SCT appears to be a sensible alternative if imatinib maintenance therapy is to be attempted. Whether such maintenance treatment is capable of compensating for a weak graft-versus-leukemia effect of an undepleted graft remains an unresolved question that can only be answered by specifically designed trials. The significant proportion of patients obtaining molecular remission before SCT and the greater possibility of maintenance imatinib therapy in the patients in our trial would argue in favor of such strategies. Unfortunately, too few patients underwent autologous SCT in our trial to enable strong conclusions to be drawn.
In summary, our trial confirms the efficacy of therapy programs combining intensive chemotherapy, imatinib and SCT. However, we have shown that the systematic administration of imatinib early after allogeneic SCT is limited by the occurrence of transplant-related events. Even when imatinib is given, discontinuation is frequent, again due to transplant-related events rather than to the inherent toxicity of the drug. These factors should be taken into account when planning to give maintenance therapy with imatinib or other tyrosine kinase inhibitors, either alone or in combination, after allogeneic SCT. An autologous SCT might be an adequate alternative approach, which would be worth exploring in specifically designed trials.
Supplementary Material
Acknowledgments
imatinib was kindly provided by Novartis Pharmaceuticals (Basel, Switzerland). We would like to thank Felipe Prósper (Clínica Universitaria, Navarra, Spain) for his help in the design of the study.
Appendix
The following institutions and physicians participated in this trial: Institut Català d’Oncologia-Hospital Germans Trias i Pujol, Badalona (Josep-Maria Ribera, Albert Oriol, Evarist Feliu), Clínico, Salamanca (Marcos González, Belén Vidriales, Jesús-Maria Hernández-Rivas), Sant Pau, Barcelona (Salut Brunet, Jorge Sierra), Clínic, Barcelona (Jordi Esteve), Clínico San Carlos, Madrid (Eloy del Potro, Joaquín Díaz-Mediavilla), Universitario, Alicante (Concepción Rivas, Pascual Fernández-Abellan), Clínico Virgen de la Victoria, Málaga (María-José Moreno, Maria-Paz Queipo), Clínico, Valencia (Mar Tormo), Puerta del Mar, Cádiz (Maria-Victoria Martín-Reina), Institut Català d’Oncologia-Duran y Reynals, Barcelona (Josep Sarrá), Virgen del Rocío, Sevilla (Ricardo Parody), Ramón y Cajal, Madrid (Jaime Pérez de Oteyza), Marqués de Valdecilla, Santander (Encarnación Bureo), and Central de Asturias (Eva Martínez-Revualta, Consuelo Rayón), Spain.
Footnotes
Funding: supported in part by grants P-EF/08 from the Jose Carreras Leukemia Foundation and RD06/0020/1056 from Red Temática de Investigación Cooperativa en Cáncer.
The online version of this article has a supplementary appendix.
Authorship and Disclosures
J-MR designed the trial; J-MR and AO analyzed the data and wrote the paper; MG and BV performed the studies of minimal residual disease; J-MR, AO, SB, JE, EdP, CoR, M-JM, MT, VM-R, JS, RP, JPdO, EB and M-TB all qualified as authors according to the WAME criteria, provided the patients included in the trial and followed them clinically. JE received honoraria from Novartis as speaker of a symposium on ALL. The other authors reported no potential conflicts of interest. The preliminary results of this study were presented at the 43rd Annual Meeting of the American Society of Hematology, December 2004, San Diego, USA.
References
- 1.Gleissner B, Göckbuget N, Bartram CR, Janssen B, Reider H, Janssen JW, et al. Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage acute lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5):1536–43. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 2.Radich JP. Philadelphia chromosome-positive acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15(1):21–36. doi: 10.1016/s0889-8588(05)70198-2. [DOI] [PubMed] [Google Scholar]
- 3.Wassmann B, Pfeifer H, Goekbuget N, Beelen DW, Beck J, Stelljes M, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108(5):1469–77. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 4.Yanada M, Takeuchi J, Sugiura I, Akiyama H, Usui N, Yagasaki F, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–6. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DA, Faderl S, Cortes J, O’Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, Lee JH, Choi SJ, Lee JH, Seol M, Lee YS, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2005;19(9):1509–16. doi: 10.1038/sj.leu.2403886. [DOI] [PubMed] [Google Scholar]
- 7.de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–13. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 8.Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2004;104(12):3507–12. doi: 10.1182/blood-2004-04-1389. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA. Philadelphia chromosome-positive acute lymphoblastic leukemia: a new era of challenges. Hematology Am Soc Hematol Educ Program. 2007:435–43. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 10.Thomas DA, Kantarjian HM, Cortes J, Ravandi F, Faderl S, Jones D, et al. Outcome after frontline therapy with the hyper-CVAD and imatinib mesylate regimen for adults with de novo or minimally treated Philadelphia (Ph) positive acute lymphoblastic leukemia (ALL) Blood. 2008;112(11):2931. [Google Scholar]
- 11.Yanada M, Sugiura I, Takeuchi J, Akiyama H, Maruta A, Ueda Y, et al. Prospective monitoring of BCR-ABL-1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143(4):503–10. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 12.Ottmann OG, Wassmann B, Pfeifer H, Giagounidis A, Stelljes M, Dührsen U, et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) Cancer. 2007;109(10):2068–76. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter PA, Synder DS, Flowers ME, Sanders JE, Gooley TA, Martin PJ, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109(7):2791–3. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wassmann B, Pfeifer H, Bethge W, Bornhauser J, Dengler J, Stadler D, et al. Up-front versus minimal residual disease triggered imatinib after stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukaemia: interim results of a randomized phase III GMALL study. Bone Marrow Transplant. 2009;43 (suppl 1):S48. [Google Scholar]
- 15.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 16.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe Against Cancer program. Leukemia. 2003;17(12):2474–86. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 17.Vidriales MB, Pérez JJ, Lòpez-Berges MC, Gutiérrez N, Ciudad J, Lucio P, et al. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunopheno-typic evaluation has high clinical value. Blood. 2003;101(12):4695–700. doi: 10.1182/blood-2002-08-2613. [DOI] [PubMed] [Google Scholar]
- 18.Tabernero MD, Bortoluci AM, Alaejos I, Lòpez-Berges MC, Rasillo A, Garcìa-Sanz R, et al. Adult precursor B-ALL with BCR/ABL gene rearrangements displays a unique immunophenotype based on the pattern of CD10, CD34, CD13 and CD38 expression. Leukemia. 2001;15(3):406–14. doi: 10.1038/sj.leu.2402060. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81. [Google Scholar]
- 20.Peto R, Pike MC. Conservatism of the approximation sigma (O-E)2/E in the logrank test for survival data or tumour incidence data. Biometrics. 1973;29(3):579–84. [PubMed] [Google Scholar]
- 21.Lee S, Kim YJ, Min CK, Kim HJ, Eom KS, Kim DW, et al. The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukaemia. Blood. 2005;105(9):3449–57. doi: 10.1182/blood-2004-09-3785. [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S, et al. Induction therapy by frequent administration of dox-orubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia. 2002;16(7):1259–66. doi: 10.1038/sj.leu.2402526. [DOI] [PubMed] [Google Scholar]
- 24.Ribera JM, Oriol A, Bethencourt C, Parody R, Hernàndez-Rivas JM, Moreno MJ, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHE-MA ALL-93 trial. Haematologica. 2005;90(10):1346–56. [PubMed] [Google Scholar]
- 25.Wassmann B, Pfeifer H, Scheuring UJ, Binckeback A, Gökbuget N, Atta J, et al. Early prediction of response in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) treated with imatinib. Blood. 2004;103(4):1495–8. doi: 10.1182/blood-2003-01-0154. [DOI] [PubMed] [Google Scholar]
- 26.Wassmann B, Pfeifer H, Stadler M, Bornhaüser M, Bug G, Scheuring UJ, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106(2):458–63. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 27.Pfeifer H, Wystub S, Wassmann B, Brueck P, Goekbuget N, Serve H, et al. Minimal residual disease and mutational status prior to and after SCT for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2008;112(11):702. [Google Scholar]
- 28.Pfeifer H, Wassmann B, Pavlova A, Wunderle L, Oldenburg J, Binckebanck A, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2007;110(2):727–34. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 29.Tiribelli M, Marin L, Calistri E, Geromin A, Damiani D, Fanin R. Imatinib mesylate (Glivec) pre-treatment does not have a negative effect on outcome of allogeneic hematopoietic stem cell transplantation in Philadelphia-positive leukemias. Bone Marrow Transplant. 2004;34(9):827–8. doi: 10.1038/sj.bmt.1704687. [DOI] [PubMed] [Google Scholar]
- 30.Wetzler M, Stock W, Donohue KA, Sher DA, Hoke EE, McCarty JM, et al. Autologous stem cell transplantation following sequential chemotherapy and imatinib for adults with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia. CALGB Study 10001. Blood. 2007;110(11):1282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.