Very recently Montesinos et al. reported on the incidence of central nervous system (CNS) involvement at first relapse in patients with acute promyelocytic leukemia (APL) who had been treated with all-trans retinoic acid (ATRA) and anthracycline monochemotherapy without intrathecal prophylaxis.1 Although this study showed a relatively low incidence of CNS involvement at first relapse, controversy over treatment options remains. The introduction of ATRA and more recently arsenic trioxide (ATO) has changed treatment options and outcome for APL.2–3 In the setting of relapsed APL, ATO is currently regarded as the preferential remission induction therapy. However, for patients achieving complete remission (CR) thereafter, appropriate consolidation strategies have not yet been defined.4 Autologous hematopoietic stem cell transplantation (HSCT) is one treatment option in relapsed APL. Here, we report a patient who had been diagnosed with relapsed APL involving the CNS and who achieved a second CR after ATO salvage therapy. Mobilization of peripheral blood stem cells (PBSC) was accomplished using a combination of granulocyte-colony stimulating factor (G-CSF) and CXCR4 blockade.
A 40-year old woman experienced extramedullary relapse of APL while on maintenance therapy after having achieved CR with ATRA containing induction chemotherapy. Due to multilocular CNS manifestation as well as molecular bone marrow involvement, ATO was started (5 cycles, 0.15 mg/kg, day 1–13) in parallel with local irradiation. Liposomal cytarabine (7 applications, 50 mg absolute/week) was applied intrathecally in order to treat meningeosis. After a molecular analysis of the bone marrow had shown negativity for PML-RARα transcripts after ATO and intrathecal therapy, G-CSF mobilization was started out of steady state in order to collect PBSC for autologous HSCT. While the WBC peaked at 31 Gpt/L, only 6/μL CD34+ cells could be measured in the peripheral blood. The corresponding apheresis yield was only 0.9×106/kg CD34+ PBSC. In order to achieve a target of >2×106/kg CD34+ PBSC, the patient received the CXCR4 antagonist AMD3100 subcutaneously at a dose of 240 μg/kg ten hours prior to the next apheresis in addition to G-CSF within a compassionate use program. CXCR4 blockade led to an increase in WBC (44 Gpt/L) and CD34+ count (9/μL) with a subsequent harvest of 1.2×106/kg CD34+ PBSC. Interestingly, both apheresis products were found to be PML-RARα-PCR negative (Figure 1). Sensitivity of nested PCR for PML-RARα was achieved according to the minimal target sensitivity of 10−4.5 Three weeks later, myeloablative conditioning containing 12 Gy total body irradiation (day −6 to −4) and 120 mg/kg of intravenous cyclophosphamide (day −3 to −2) was performed and followed by reinfusion of PBSC on day 0. Fast and stable trilineage engraftment was documented with neutrophils >0.5 Gpt/L and platelets > 50 Gpt/L on day +14 and +16, respectively. Three years later (day +1,144 after autologous HSCT) the patient remains in complete hematologic remission without clinical signs of extramedullary disease.
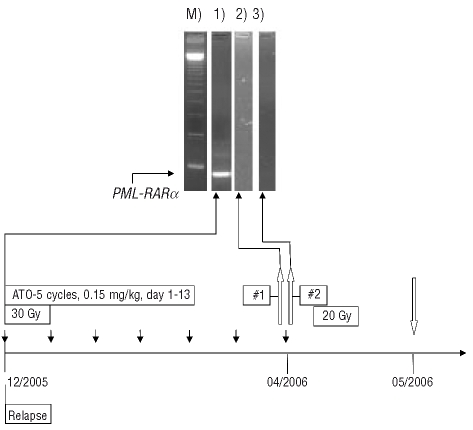
Figure 1.
PML-RARα specific PCR–M) marker, lane 1) before ATO treatment, lane 2) G-CSF mobilized stem cells, lane 3) G-CSF + AMD3100 mobilized stem cells. Lanes were processed to secure clearness since other patient samples were performed routinely in parallel. Graph: treatment course after relapse. Local irradiation with 30 Gy starting December 2005 and 20 Gy starting in April 2006. Black arrows indicate intrathecal application of liposomal cytarabine. White arrows pointing upward indicate apheresis #1 with G-CSF and apheresis #2 with G-CSF and AMD3100. White arrow pointing downward indicates autologous SCT after myeloablative conditioning with 12 Gy TBI and intravenous cyclophosphamide.
Arsenic trioxide has recently been shown to play an emerging role in relapsed and refractory APL with the majority of patients achieving a complete molecular remission.3,6 Following molecular CR after ATO treatment, subsequent collection of PBSC and autologous HSCT after myeloablative chemotherapy is recommended but discussed controversially with regard to the best consolidation strategy.4,7 Harvesting a satisfactory amount of CD34+ PBSC after repetitive chemotherapy regimens might be challenging. Sequential therapy with ATO might even decrease the hematopoietic capacity. Application of AMD3100 in addition to G-CSF displays a possible option to compensate for poor HSC mobilization. Albeit, leukemic blasts are known to express CXCR4 and could, therefore, become potential targets of AMD3100.8 Data in a murine model suggest that AMD3100 administration leads to an increased time-dependent mobilization of APL blasts by interrupting the CXCR4-SDF-1 axis.9 But for AML in general no clinical trials exist in order to confirm or disprove whether mobilizing leukemic stem cells reflect a relevant problem in this setting. DiPersio et al. advised caution and stated that AMD3100 might not be intended for mobilization and harvest in patients with leukemia.10
Our limited experience in this patient suggests at least that in case of molecular remission, no apparent mobilization of PML-RARα positive cells occurred. Whether different subsets of leukemic stem and progenitor cells might be differentially targeted by CXCR4 is unknown. Next leukemic blast mobilization in vitro was strictly time-dependent in the murine model with a peak of circulating APL blasts after three hours and return to baseline after 12 h. Furthermore, APL blast mobilization in an in vivo model seems to be influenced by the respective microenvironment since extramedullary blasts with exclusive intraperitoneal expansion were not shown to be circulating after AMD3100 administration.9 Our case shows that even after intensified ATO treatment due to relapsed APL, PML-RARα negative PBSC can be obtained by using the competitive CXCR4 antagonist AMD3100 to increase the number of harvested cells. These cells proved not to be contaminated by clonogenic APL cells allowing successful autologous HSCT which induced prolonged remission without further maintenance therapy.
References
- 1.Montesinos P, Diaz-Mediavilla J, Deben G, Prates V, Tormo M, Rubio V, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal orophylaxis. Haematologica. 2009;94(9):1242–9. doi: 10.3324/haematol.2009.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–8. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 3.Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94(10):3315–24. [PubMed] [Google Scholar]
- 4.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 5.van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 6.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 7.Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:391–9. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 8.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23(1):43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 9.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–14. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]