Abstract
Plants under stress from both biotic and abiotic sources produce increased levels of ethylene, which is perceived by ethylene receptors and triggers cellular responses further downstream. Protein phosphorylation and dephosphorylation were implicated in the regulation of ethylene induction by stresses based on studies using protein kinase and phosphatase inhibitors. However, the kinase(s) involved remains to be determined. Using a conditional gain-of-function transgenic system, we demonstrate that the activation of SIPK, a tobacco mitogen-activated protein kinase (MAPK), by NtMEK2DD, an active mutant of the upstream kinase of SIPK, resulted in a dramatic increase in ethylene production. The increase in ethylene after the activation of SIPK coincided with a dramatic increase in 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) activity, which was followed by the activation of a subgroup of ACS and ACC oxidase (ACO) genes, suggesting that either the activation of unidentified ACS(s) or post-transcriptional regulation is involved. Infection with Tobacco mosaic virus (TMV), which is known to activate the SIPK cascade and induce ethylene biosynthesis, also induced the same ACSs and ACOs. After ethylene production in NtMEK2DD plants, strong activation of ETHYLENE-RESPONSE FACTOR (ERF) genes was observed, similar to the effect in NN tobacco plants infected with TMV. In contrast to previous reports, no major increase in jasmonic acid (JA) and methyl jasmonate (MJ) was detected after the activation of SIPK/WIPK in NtMEK2DD transgenic plants. These results suggest that the induction of ethylene but not JA/MJ is involved in plant defense responses mediated by the NtMEK2-SIPK/WIPK pathway.
INTRODUCTION
Ethylene plays important roles in plant responses to abiotic and biotic stresses besides its functions in plant growth and development. Recent genetic analyses revealed a number of important components in the ethylene signaling pathway, from the receptors that sense ethylene to the transcription factors that activate downstream gene expression (Kende, 1993; Kieber, 1997; Johnson and Ecker, 1998; Chang and Shockey, 1999; Bleecker and Kende, 2000; Wang et al., 2002). Ethylene-regulated processes are initiated mostly by an increase in ethylene synthesis (Kende, 2001). Therefore, there are earlier signaling events that regulate ethylene production after the sensing of environmental and/or endogenous cues in plants. Despite major progress in our understanding of the ethylene biosynthetic pathway and signaling components downstream of ethylene, the signal transduction pathways that modulate ethylene biosynthesis in plants under stress are largely unknown.
The two key steps in ethylene biosynthesis are the conversion of S-adenosyl-l-Met to 1-aminocyclopropane-1-carboxylic acid (ACC) and the oxidative cleavage of ACC to form ethylene (Yang and Hoffman, 1984; Kende, 1993; Zarembinski and Theologis, 1994). The enzymes that catalyze these two reactions are ACC synthase (ACS) and ACC oxidase (ACO), respectively. Both enzymes are encoded by small gene families. In general, the basal activity of ACS is very low in tissues that do not produce significant amounts of ethylene. By contrast, ACO activity is thought to be constitutively present in most vegetative tissues. Upon stimulation, ACS activity is induced rapidly. Therefore, ACS is considered to be the rate-limiting step in ethylene biosynthesis and is the major regulatory step in the induction of ethylene (Yang and Hoffman, 1984; Kende, 1993). Recent studies have demonstrated that the activity of ACS can be regulated at the post-translational level by protein phosphorylation and dephosphorylation, which potentially could alter the turnover rate of ACS protein (Spanu et al., 1994; Tatsuki and Mori, 2001; Wang et al., 2002; Chae et al., 2003). In addition, accelerated ethylene biosynthesis in plants frequently is associated with the induction of ACS and ACO gene activation, which is responsible at least in part for the increased levels of enzyme activity under some conditions (McKeon et al., 1995; Bleecker and Kende, 2000; Wang et al., 2002). Furthermore, individual members of the ACS and ACO gene families are regulated differentially in response to specific stimuli, suggesting that they play different roles in enhanced ethylene production in response to different environmental or endogenous cues (McKeon et al., 1995; Bleecker and Kende, 2000; Wang et al., 2002).
Mitogen-activated protein kinase (MAPK) cascades are important mediators of signal transduction in cells. They convert signals generated at the receptors/sensors to cellular responses (Widmann et al., 1999; Davis, 2000; Chang and Karin, 2001). An increasing number of studies have demonstrated that SIPK and WIPK, two tobacco MAPKs, as well as their orthologs in other plant species, are activated in plants under various stress conditions, such as wounding, osmotic shock, high salinity, drought, UV irradiation, ozone, extreme temperature, and pathogen infection (Mizoguchi et al., 1997; Tena et al., 2001; Zhang and Klessig, 2001; Jonak et al., 2002; MAPK Group, 2002). The activation of SIPK by these stresses occurs within minutes, representing one of the earliest responses in plants under stress, which potentially allows this MAPK pathway to regulate a variety of intermediate and late defense responses. Based on the fact that SIPK is activated by multiple stresses, it was speculated that this MAPK might control a subset of common defense responses (Zhang and Klessig, 2001). One such common response in plants under all of these stress stimuli is the production of ethylene.
Despite a good correlation between the activation of SIPK and the accelerated production of ethylene during plant stress responses, these two events have never been linked together. Here, we provide conditional gain-of-function evidence for a role of SIPK in regulating the biosynthesis of ethylene using steroid-inducible promoter:NtMEK2DD transgenic plants. NtMEK2DD is an active mutant of NtMEK2, the upstream kinase of SIPK and WIPK (Yang et al., 2001). Induction of NtMEK2DD by the application of dexamethasone (DEX) led to the rapid activation of endogenous SIPK, which was followed by a dramatic increase in ethylene production. By contrast, the appearance of WIPK activity was delayed, excluding its involvement in the initial phase of ethylene production. After the increase in ethylene in NtMEK2DD plants, ethylene-responsive genes such as ETHYLENE-RESPONSIVE-FACTORS (ERFs) were induced, similar to the situation in NN tobacco plants infected with Tobacco mosaic virus (TMV). In contrast to previous reports, no significant increase of jasmonic acid (JA) and methyl jasmonate (MJ) was detected after the activation of SIPK/WIPK in NtMEK2DD plants. These results suggest that the induction of ethylene but not JA/MJ is involved in signaling plant defense responses downstream of the NtMEK2-SIPK/WIPK pathway.
RESULTS
Activation of the NtMEK2-SIPK/WIPK Cascade Dramatically Induces Ethylene Biosynthesis
Transgenic tobacco plants carrying NtMEK2DD, an active mutant of the upstream MAPKK of SIPK and WIPK, were generated recently (Jin et al., 2003). As a control, plants carrying NtMEK2KR, an inactive mutant of NtMEK2, also were generated. A steroid-inducible promoter was used to control the transgene expression to mimic the activation of the MAPK pathway by stresses, which also allowed us to determine the sequence of events after MAPK activation (Yang et al., 2001; Jin et al., 2003). Among the 19 NtMEK2DD lines that showed transgene induction after DEX application, two showed visible morphological alterations in the absence of DEX when plants became older, which is a result of higher basal expression of the NtMEK2DD transgene (data not shown). After passing the 10-leaf stage, plants from these two lines showed epinasty, a stunted apex, shortened internodes, an enlarged stem diameter, and adventitious roots (Figure 1), suggesting the involvement of ethylene (Reid, 1995). As a result, we examined whether the expression of NtMEK2DD affects the rate of ethylene biosynthesis.
Figure 1.
NtMEK2DD Transgenic Plants with Higher Basal Expression Show Phenotypes Characteristic of Ethylene Overproduction after Passing the 10-Leaf Stage.
(A) Morphology of young NtMEK2DD and NtMEK2KR transgenic tobacco plants used in the experiments. These plants were ∼6 weeks old after vegetative propagation.
(B) Older NtMEK2DD transgenic plants with higher basal expression show ethylene-induced phenotypes. Photographs were taken after plants passed the 10-leaf stage. Images at right show the lower portion of the stems of NtMEK2DD plants exhibiting adventitious roots.
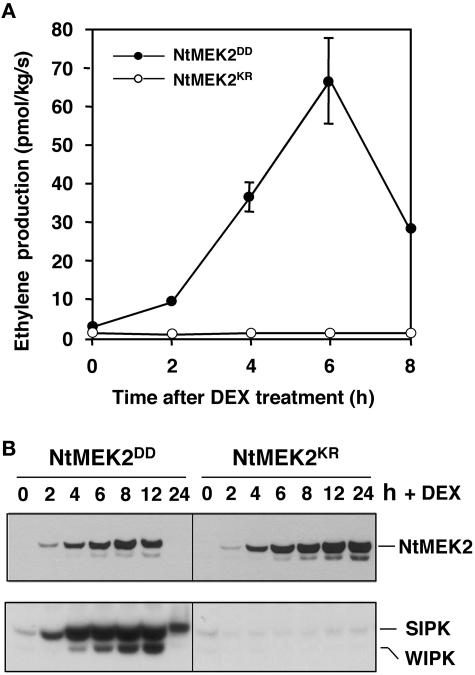
Young NtMEK2DD plants at ∼6 weeks old were sprayed with 30 μM DEX to induce transgene expression. At this stage, NtMEK2DD plants had no obvious phenotype (Figure 1A). As controls, NtMEK2KR plants were treated side by side. At various times after DEX treatment, ethylene production rates in newly fully expanded leaves were measured. As shown in Figure 2A, there was a dramatic increase in ethylene biosynthesis in NtMEK2DD plants after the application of DEX, reaching a maximum rate of ∼65 pmol·kg−1·s−1 in 6 h, an increase of >40-fold. By contrast, no increase was observed in control NtMEK2KR plants (Figure 2A). The basal level of ethylene production in NtMEK2DD plants before DEX application was approximately twofold that in NtMEK2KR plants (Figure 2A). It is likely that the morphological alterations that we observed in older plants (Figure 1B) are a result of long-term exposure to this low increased level of ethylene, which may explain why young plants do not have an obvious phenotype (Figure 1A).
Figure 2.
Activation of Endogenous SIPK by NtMEK2DD Induces Ethylene Production in Transgenic Tobacco Plants.
(A) The activation of the NtMEK2-SIPK cascade leads to increased ethylene production in NtMEK2DD transgenic tobacco. Vegetatively propagated T1 NtMEK2KR (open circles) and NtMEK2DD (closed circles) transgenic plants were treated with DEX (30 μM). Ethylene production rates were measured at the times indicated. Average values from triplicate experiments are shown. Vertical bars indicate standard deviations where they are larger than the symbols.
(B) Induction of NtMEK2DD expression by DEX activates endogenous SIPK and WIPK. The expression of Flag-tagged NtMEK2DD and NtMEK2KR after DEX treatment was monitored by immunoblot analysis using anti-Flag antibody (top gel). The kinase activities of endogenous SIPK and WIPK were determined by an in-gel kinase assay with myelin basic protein as the substrate (bottom gel). The up-shift of SIPK band at 24 h in NtMEK2DD plants is attributable to the loss of ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco) during cell death. The abundant large subunits of Rubisco form a major band in the gel, which pushes the SIPK band down slightly.
Immunoblot analysis with an anti-Flag antibody revealed that NtMEK2DD and NtMEK2KR were induced to similar levels (Figure 2B), demonstrating that ethylene biosynthesis is related to NtMEK2DD rather than to the DEX-inducible system. An in-gel kinase assay revealed a rapid activation of endogenous SIPK ∼2 h after DEX treatment, concurrent with the increase in ethylene biosynthesis (Figure 2). By contrast, the appearance of WIPK activity was delayed to 4 h after DEX treatment, suggesting that WIPK was not involved in the initial stage of ethylene production. The induction of NtMEK2KR failed to activate endogenous MAPKs, which correlates with its inability to induce ethylene biosynthesis (Figure 2). For the results shown in Figure 2B, 30 μg of protein was loaded in each lane and a longer exposure time was used to increase the sensitivity of immunoblot and in-gel kinase assays. Under our standard assay conditions, the transgene expression was barely visible at 2 h after the application of DEX (Jin et al., 2003).
Similar ethylene induction was observed in NtMEK2DD transgenic lines that do not show morphological alterations throughout the life cycle. These plants also have lower basal levels of ethylene production (data not shown). This result suggests that the accelerated ethylene biosynthesis is not limited to the transgenic lines that showed morphological alterations, although the initial link was identified in these plants. In addition, similar results were observed in transgenic Arabidopsis plants (Ren et al., 2002) after the activation of AtMPK6/AtMPK3, the orthologs of SIPK/WIPK, demonstrating that the induction of ethylene biosynthesis after the activation of stress-responsive MAPK is not a phenomenon limited to tobacco plants (data not shown). Based on the fact that the accelerated ethylene production after NtMEK2DD expression was rapid and concurrent with the activation of SIPK, we conclude that it is an active process directly related to SIPK activation, rather than a secondary effect associated with a hypersensitive response–like cell death in NtMEK2DD plants, which occurs ∼14 h after DEX treatment (Jin et al., 2003). The use of a conditional expression system further reduced the possibility of nonspecific effects that are sometimes associated with the constitutive overexpression system.
Increased Ethylene Production in NtMEK2DD Plants after DEX Treatment Coincides with a Dramatic Increase in ACS Activity
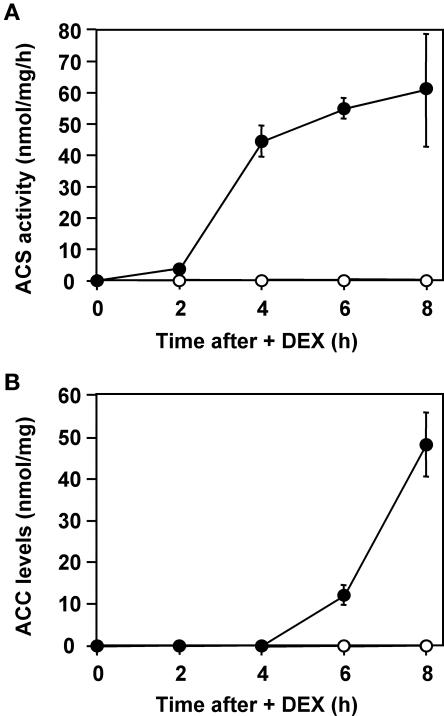
Among all of the defense responses that we observed in NtMEK2DD plants after DEX application, the increase in ethylene production was one of the earliest events after the activation of endogenous SIPK. In general, the rate-limiting step of ethylene synthesis in plants is the conversion of S-adenosyl-l-Met to ACC by ACS (Kende, 1993). As a result, we measured ACS activity in protein extracts prepared from both NtMEK2DD and control NtMEK2KR transgenic plants after DEX treatment. As shown in Figure 3A, there was a significant increase in ACS activity at 2 h after the application of DEX in NtMEK2DD plants, reaching 3.7 nmol·mg−1·h−1 in the total protein extract, which is approximately one-third of the level of ACS activity in wounded tomato fruit (Tatsuki and Mori, 2001). In another 2 h, ACS activity in NtMEK2DD plants increased to more than five times the activity in wounded tomato fruit. This dramatic increase in ACS activity correlated closely with the activation of SIPK and was concurrent with the increase in ethylene production (Figure 2). In NtMEK2KR plants or NtMEK2DD plants before DEX application, ACS activity was below the detection limit under our assay conditions. This result suggests that the increase in ACS activity is responsible for the increased rates of ethylene biosynthesis in NtMEK2DD plants.
Figure 3.
The Induction of Ethylene Biosynthesis in NtMEK2DD Plants Coincides with a Dramatic Increase in ACS Activity.
(A) Rapid induction of ACS activity in NtMEK2DD plants after DEX treatment. ACS activity in total protein extracts from NtMEK2KR (open circles) and NtMEK2DD (closed circles) transgenic plants was determined as described in Methods. Average values from triplicate experiments are shown. Vertical bars indicate standard deviations where they are larger than the symbols.
(B) Delayed accumulation of ACC in NtMEK2DD plants after DEX treatment. The amount of ACC in the total protein extracts from NtMEK2KR (open circles) and NtMEK2DD (closed circles) transgenic plants was determined as described in Methods. Average values from triplicate experiments are shown. Vertical bars indicate standard deviations where they are larger than the symbols.
In NtMEK2DD plants, ethylene production peaked at 6 h after DEX application and decreased thereafter (Figure 2A), although the ACS activity stayed high (Figure 3A). In addition, we detected a significant accumulation of ACC in total extracts from NtMEK2DD plants at 6 h after DEX treatment (Figure 3B). These results indicate that at this stage, ACO activity, rather than ACS activity, is the limiting factor in ethylene biosynthesis. An alternative explanation is that the deteriorating physical state of the cells limited ethylene production, although a hypersensitive response–like cell death was not clearly visible at 8 h after DEX application.
Delayed Activation of a Subset of ACS and ACO Genes in NtMEK2DD Plants
The induction of ethylene biosynthesis by various stresses frequently is associated with the activation of ACS and ACO gene expression, which is believed to be responsible, at least in part, for the increase of ACS and ACO activities in certain cases (Yang and Hoffman, 1984; McKeon et al., 1995). The paradigm for the action of stress-responsive MAPKs in yeast and animals is that the activated MAPK phosphorylates transcription factors, which in turn activate the expression of downstream genes and eventually change the physiology of cells (Widmann et al., 1999; Davis, 2000; Chang and Karin, 2001; Hazzalin and Mahadevan, 2002). As a result, we sought to determine if the increase in ACS activity and the accelerated production of ethylene after SIPK activation are caused by the activation of ACS and ACO expression.
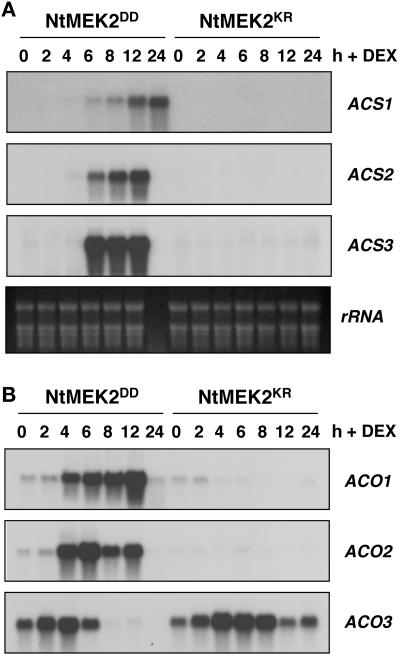
A search of the databases and literature revealed three known ACS genes (NtACS1, NtACS2, and NtACS3) and three known ACO genes (NtACO1, NtACO2, and NtACO3) in tobacco. Reverse transcription–PCR was used to amplify fragments from these genes, which were used as probes in RNA gel blot analysis. As shown in Figure 4A, there was significant induction of NtACS1, NtACS2, and NtACS3 expression in NtMEK2DD plants after DEX treatment. By contrast, none of these ACS genes was induced in NtMEK2KR control transgenic plants. Because the activation kinetics of all three ACSs that we examined occurred after the increase in ACS activity (Figures 3A and 4A), we concluded that the activation of these ACS genes is not involved in the initial increase in ACS activity and ethylene biosynthesis.
Figure 4.
Induction of ACS and ACO Expression in NtMEK2DD Transgenic Plants after DEX Application.
(A) Delayed activation of ACS genes after MAPK activation in NtMEK2DD plants. Leaves were collected from NtMEK2DD or NtMEK2KR transgenic plants at the times indicated after DEX (30 μM) treatment, and total RNA was extracted. Ten micrograms of total RNA per lane was separated on 1.2% formaldehyde-agarose gels and transferred to nylon membranes. Three identical blots were prepared and hybridized with α-32P-dCTP random primer–labeled NtACS1, NtACS2, and NtACS3 inserts. Equal loading of RNA was confirmed by ethidium bromide staining of the rRNA. The RNA degradation at 24 h in NtMEK2DD plants was attributable to cell death.
(B) Rapid activation of ACO genes in NtMEK2DD transgenic plants. The same blots shown in (A) were stripped and reprobed with α-32P-dCTP random primer–labeled NtACO1, NtACO2, and NtACO3 inserts.
We observed a more rapid induction of ACO gene expression in the NtMEK2DD plants after the application of DEX. As shown in Figure 4B, NtACO1 and NtACO2 were induced specifically in NtMEK2DD transgenic plants between 2 and 4 h after the application of DEX, which was ∼2 h before the activation of ACS expression (Figure 4). The expression of NtACO3, however, was independent of MAPK activation. Its mRNA level increased and then decreased in both NtMEK2DD and NtMEK2KR plants (Figure 4B, bottom gel). The more profound decrease of NtACO3 mRNA at the late time points in NtMEK2DD plants likely was a result of the prolonged activation of SIPK and/or the delayed activation of WIPK. For different ACOs, there clearly are additional levels of regulation, possibly at the translational or post-translational level, because relatively high levels of NtACO3 mRNA (Figure 4B) did not correlate with a high rate of ethylene production (Figure 2). Alternatively, the availability of ACC, the substrate of ACO, is the determinant.
The Same Subset of ACSs and ACOs Is Activated by TMV Infection, Which Is Known to Induce SIPK/WIPK and Ethylene Biosynthesis
Ethylene biosynthesis is induced by a number of biotic and abiotic stimuli, including wounding, osmotic/salt stress, and pathogen attack (McKeon et al., 1995; Johnson and Ecker, 1998; Bleecker and Kende, 2000; Wang et al., 2002), all of which activate SIPK alone or both SIPK and WIPK (Zhang and Klessig, 2001). To provide correlative evidence for the significance of NtMEK2-SIPK/WIPK–mediated ACS and ACO activation, we examined their expression in resistant NN tobacco plants infected with TMV. Here, we took advantage of the reversible, high-temperature inhibition of TMV-induced defense responses in tobacco plants. At 32°C, TMV-infected tobacco plants fail to show resistance responses, and the virus can multiply and move systemically. Upon shifting these plants to lower temperatures (22°C), all defense responses are induced rapidly in a more synchronous manner (de Laat and van Loon, 1983; Zhang and Klessig, 1998a).
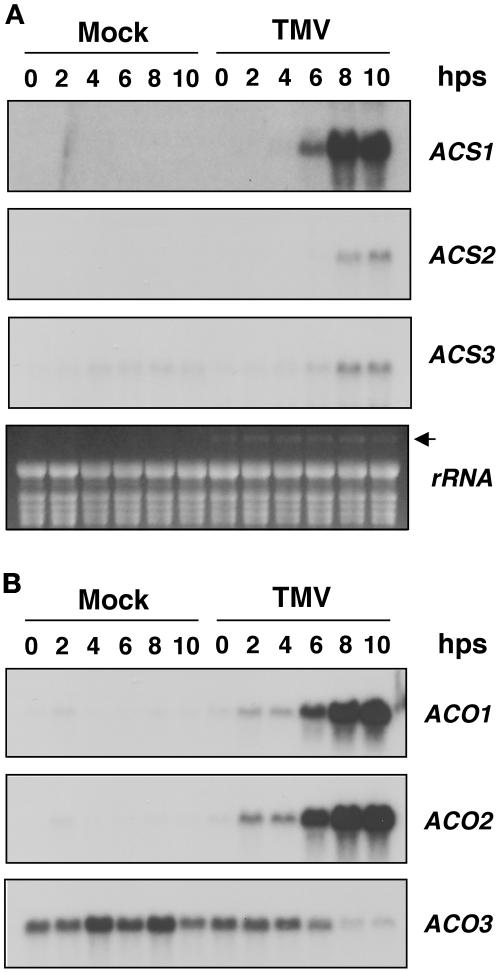
The induction of all three ACS genes lagged behind that of ACOs in tobacco infected with TMV, which is similar to their expression patterns in NtMEK2DD plants after the application of DEX. As shown in Figure 5A, the expression of NtACS1, NtACS2, and NtACS3 was induced 6 to 8 h after the temperature shift. By contrast, NtACO1 and NtACO2 expression was induced 2 h after the temperature shift and peaked at ∼6 h in TMV-infected plants. NtACO3 was not induced by TMV, and its level fluctuated in mock-inoculated plants. At late time points, the expression of NtACO3 was downregulated in TMV-infected plants, which again is similar to that in NtMEK2DD plants (Figure 4B). The expression of NtACSs, NtACO1, and NtACO2 was undetectable in mock-inoculated plants and in TMV-infected plants kept at 32°C (Figure 5, 0 h), suggesting that their activation is part of the resistance response. Previously, we demonstrated that the activation of SIPK/WIPK also is specific for the resistance response. At high temperature (32°C), no activation of SIPK/WIPK was observed (Zhang and Klessig, 1998a). After the temperature shift, SIPK activity started to increase in 1 to 2 h, which preceded the activation of NtACO1 and NtACO2. The appearance of WIPK at ∼3 h occurred after the induction of these two ACOs (Zhang and Klessig, 1998a). This correlative evidence suggests that TMV-induced NtACSs, NtACO1, and NtACO2 expression and ethylene biosynthesis might be mediated by the NtMEK2-SIPK/WIPK pathway. The resistance-specific induction of NtACO1 and NtACO2 by TMV described here is consistent with previous reports (Knoester et al., 1995; Ohtsubo et al., 1999; Guo et al., 2000).
Figure 5.
The Same ACS and ACO Genes Are Activated during the Resistance Response of Tobacco against TMV Infection.
(A) Delayed activation of ACS genes in TMV-infected tobacco (NN) plants after a temperature shift. Leaves of resistant tobacco plants (NN) were inoculated with TMV or mock-inoculated. The plants were kept at 32°C for 48 h. The temperature in the growth chamber then was decreased to 22°C, and leaf samples were collected at the hours indicated after shift (hps). Total RNA was prepared, and the steady state levels of ACSs were determined by RNA gel blot analysis. An ethidium bromide–stained gel was used to show equal loading of the samples. The arrow indicates the position of viral RNA.
(B) Rapid activation of ACO genes in TMV-infected tobacco (NN) plants after a temperature shift. The same blots shown in (A) were stripped and reprobed with α-32P-dCTP random primer–labeled NtACO1, NtACO2, and NtACO3 inserts.
Ethylene-Responsive Genes Are Turned on by the NtMEK2-SIPK/WIPK Cascade after Ethylene Biosynthesis
Many ethylene-induced responses involve changes in gene expression. The cloning of ETHYLENE-INSENSITIVE3 (EIN3) provided direct evidence for nuclear regulation in the ethylene signal transduction pathway (Chao et al., 1997; Wang et al., 2002). Recently, Arabidopsis ETHYLENE-RESPONSE-FACTOR1 (ERF1) was identified as a transcription factor downstream of EIN3 that binds to the ethylene-responsive element and regulates the expression of other ethylene-responsive genes (Solano et al., 1998). The expression of ERF1 itself is induced by ethylene.
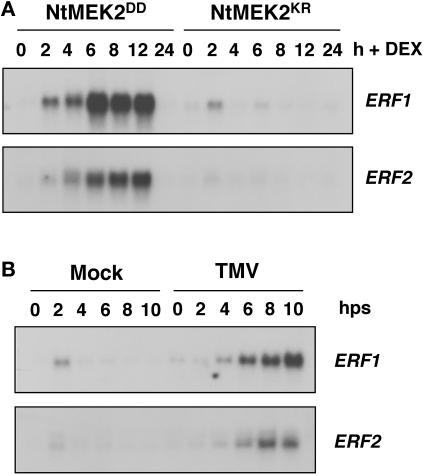
To determine if MAPK activation-induced ethylene production also results in similar responses, we examined the expression of NtERF1 and NtERF2, two tobacco homologs of Arabidopsis ERF1 (Ohme-Takagi et al., 2000). As shown in Figure 6A, both NtERF1 and NtERF2 were induced in NtMEK2DD but not NtMEK2KR transgenic plants 2 to 4 h after DEX treatment, which is immediately after the increase in ethylene production (Figure 2). The expression of NtERF1 and NtERF2 also was induced by TMV infection after the temperature shift, suggesting that it is part of the resistance response (Figure 6B). These results indicate that MAPK-mediated ethylene production is sufficient to induce responses further downstream of ethylene.
Figure 6.
Activation of ERF Genes by NtMEK2DD and TMV Infection.
(A) Induction of ERF expression in NtMEK2DD transgenic plants after the activation of SIPK. Identical blots were prepared from the same total RNA preparations used in Figure 4. The steady state mRNA levels of NtERF1 and NtERF2 were determined by RNA gel blot analysis.
(B) Activation of the same ERF genes by TMV in tobacco plants after a temperature shift. Identical blots were prepared from the same total RNA preparations used in Figure 5. The steady state mRNA levels of NtERF1 and NtERF2 were determined by RNA gel blot analysis. hps, hours postshift.
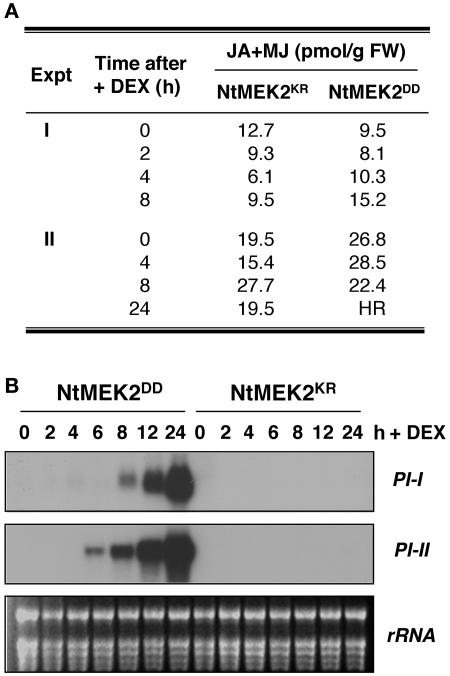
JA/MJ Levels Were Not Increased after SIPK/WIPK Activation, although Proteinase Inhibitor Transcripts Were Induced
It was reported previously that WIPK regulates wounding-induced JA and MJ biosynthesis, which in turn controls the expression of PROTEINASE INHIBITOR (PI) (Seo et al., 1995, 1999). As a result, we sought to determine if the activation of SIPK/WIPK by NtMEK2DD leads to an increase of JA/MJ levels in plants. Samples were collected from both NtMEK2DD and NtMEK2KR plants at various times after the application of DEX. As shown in Figure 7A, there was no apparent increase in JA and MJ over the basal level in either NtMEK2DD or NtMEK2KR plants. The highest level detected was within the range of basal levels normally present in tobacco plants. Based on this finding, we concluded that JA/MJ is not a downstream target of the NtMEK2-SIPK/WIPK pathway. Nonetheless, we did observe a strong induction of PI-I and PI-II expression in NtMEK2DD plants after the activation of SIPK/WIPK (Figure 7B). This result indicates that the activation of PI genes after MAPK activation is not mediated by the induction of JA/MJ. Previous studies demonstrated that ethylene also could induce the expression of PIs (Balandin et al., 1995; Hiraga et al., 2000). As a result, we speculate that the PI activation that we observed in NtMEK2DD transgenic plants after SIPK/WIPK activation is mediated by ethylene.
Figure 7.
Activation of Endogenous SIPK/WIPK by NtMEK2DD Induces the Expression of PI Genes but Not JA/MJ Biosynthesis.
(A) Activation of endogenous SIPK/WIPK in NtMEK2DD transgenic plants did not lead to an increase in JA/MJ levels. Transgenic NtMEK2DD and NtMEK2KR plants were sprayed with 30 μM DEX. Leaves were collected at the times indicated after DEX treatment, and JA/MJ levels were determined as described in Methods. Data from two independent experiments (Expt I and II) are shown. HR denotes the hypersensitive response–like cell death, which occurred 24 h after DEX application in NtMEK2DD plants. As a result, JA/MJ levels could not be determined. FW, fresh weight.
(B) Induction of PI-I and PI-II expression after SIPK/WIPK activation in NtMEK2DD plants. Transgenic plants were sprayed with 30 μM DEX. Leaves were collected at the times indicated after DEX treatment, and total RNA was extracted. Ten micrograms of total RNA per lane was separated on a 1.2% formaldehyde-agarose gel and transferred to a nylon membrane. The blot was hybridized sequentially with α-32P-dCTP random primer–labeled cDNA inserts. Equal loading of RNA was confirmed by ethidium bromide staining of the rRNA.
DISCUSSION
Ethylene plays important roles in various aspects of the plant life cycle, including growth, development, and response/adaptation to adverse environmental conditions. An increasing variety of stress stimuli that induce ethylene production, such as pathogen infection, wounding, ozone, UV irradiation, drought, high osmolarity, ozone, and oxidative stresses, have been shown to rapidly activate SIPK in tobacco and SIPK orthologs in other plant species (Mizoguchi et al., 1997; Tena et al., 2001; Zhang and Klessig, 2001). However, the causal relation between SIPK activation and ethylene production has not been reported previously. Here, we provide gain-of-function evidence for the involvement of this specific MAPK cascade in the regulation of ethylene biosynthesis in plants under stress.
Our conclusion that SIPK is the downstream target of NtMEK2DD in mediating ethylene induction in NtMEK2DD transgenic plants is based on the following evidence. First, the chance for NtMEK2DD to act on substrate(s) other than MAPKs is very slight. To date, there is no evidence that a MAPKK can function through a non-MAPK. This is related to the unique dual specificity of MAPKKs (i.e., they phosphorylate both Thr and Tyr in the TXY motif), which limits their substrates to MAPKs. Second, among all of the MAPKs that we tested, only SIPK and WIPK can be phosphorylated and activated by NtMEK2DD both in vitro and in vivo (Yang et al., 2001). Similar specificity was observed with their Arabidopsis orthologs. AtMEK4 and AtMEK5, the orthologs of NtMEK2, can act only on AtMPK6 and AtMPK3, the orthologs of SIPK and WIPK, respectively, among all of the MAPKs tested (Asai et al., 2002; Ren et al., 2002). Third, the activation of WIPK by NtMEK2DD occurred after ethylene induction (Figure 2), excluding its involvement in the initial phase of ethylene induction in NtMEK2DD plants. Very recently, we found that in an atmpk6 knockout background, NtMEK2DD-induced ethylene production was greatly compromised (our unpublished data).
Correlative evidence from NN tobacco plants infected with TMV also supports a role for SIPK in stress-induced ethylene production. In temperature-shift experiments, ethylene production starts at ∼2 h in TMV-infected plants (de Laat and van Loon, 1983), which is preceded by the activation of SIPK (Zhang and Klessig, 1998a). Both events are gene-for-gene dependent and specific for the resistance response (de Laat and van Loon, 1983; Zhang and Klessig, 1998a). In addition, we observed the activation of NtERF1 and NtERF2 in both NtMEK2DD plants after DEX treatment and NN tobacco plants infected with TMV after the temperature shift (Figure 6). These two transcription factors are responsive to ethylene and are involved in the regulation of ethylene-responsive defense genes (Ohme-Takagi et al., 2000).
Mechanism of SIPK-Mediated Ethylene Induction
The exact mechanism underlying SIPK-mediated ethylene induction is unclear at present. We observed rapid induction of NtACO1 and NtACO2 expression and delayed activation of NtACS1, NtACS2, and NtACS3 expression after SIPK activation in both steroid-inducible promoter:NtMEK2DD plants after DEX application and NN plants that resist TMV infection. However, the increase in ethylene biosynthesis appeared to precede the induction of these genes. This result suggests that these genes may not be the initial targets of the SIPK cascade that leads to ethylene biosynthesis. In general, the rate-limiting step of ethylene synthesis in plants is the conversion of S-adenosyl-l-Met to ACC by ACS (Kende, 1993). Because we did not examine all of the ACS genes in tobacco, it is possible that the activation of other unidentified ACSs, whose expression precedes the increase in ethylene biosynthesis after SIPK activation, is responsible for the accelerated ethylene biosynthesis. Alternatively, post-translational regulation of ACS activity is involved. The very rapid increase in ACS activity after SIPK activation (Figures 2B and 3A) favors the mode of post-translational regulation, although direct evidence is lacking. We cannot perform inhibitor studies in our conditional gain-of-function system because transgene induction requires de novo transcription and translation.
Protein phosphorylation and dephosphorylation were implicated in the regulation of ethylene induction based on pharmacological studies using protein kinase and phosphatase inhibitors (Spanu et al., 1994; Felix et al., 2000; Wang et al., 2002). Treatment of tomato suspension cells with fungal elicitor induces a rapid increase in ACS activity, which can be blocked by the addition of the protein kinase inhibitors K-252a and staurosporine (Spanu et al., 1994). In addition, treatment of cells with calyculin A, a protein phosphatase inhibitor, stimulates ACS activity in the absence of fungal elicitor and accelerates the rate of ACS increase in elicitor-treated cells. We speculate that the SIPK ortholog in tomato might be the kinase involved. The effects of protein kinase and phosphatase inhibitors on ACS activity in tomato parallel their effects on SIPK activation by fungal elicitors in tobacco. SIPK is activated rapidly by various elicitors, which could be inhibited rapidly by the addition of the kinase inhibitors K-252a or staurosporine (Zhang et al., 1998, 2000; Liu et al., 2003). Furthermore, we found that calyculin A can mimic fungal elicitor to activate SIPK (Liu et al., 2003). SIPK-like MAPK activity was reported in tomato, although the corresponding gene has not been identified (Stratmann and Ryan, 1997). However, based on the high similarity between tobacco SIPK and Arabidopsis MPK6 in response to stress stimuli, it is reasonable to assume that the tomato SIPK ortholog has similar properties, because tobacco and tomato are more closely related.
Possible mechanisms underlying the phosphorylation/dephosphorylation regulation of ACS include the change in ACS activity, the change in ACS stability, or both (Spanu et al., 1994; Tatsuki and Mori, 2001). Recent genetic and in vivo functional analyses demonstrated that the C terminus of ACS5 plays an important role in regulating the stability of ACS and, therefore, total cellular ACS activity (Vogel et al., 1998; Woeste et al., 1999; Chae et al., 2003). Whether ACS5 is a target of the SIPK pathway remains to be determined. If the regulation of ACS activity by phosphorylation is involved in the accelerated production of ethylene in NtMEK2DD plants, the kinase could be either SIPK itself or other unidentified downstream kinases. The C terminus of tomato ACS2 was shown to be phosphorylated by a calcium-dependent protein kinase in vitro (Tatsuki and Mori, 2001). In animals, several kinases have been shown to act downstream of MAPK (Widmann et al., 1999). Besides the potential role of the NtMEK2-SIPK/WIPK pathway in signaling ethylene biosynthesis during the tobacco resistance response, we speculate that this MAPK cascade is involved in the upregulation of ethylene biosynthesis in response to other stresses, because the same MAPK cascade is activated rapidly by these stresses (Tena et al., 2001; Zhang and Klessig, 2001).
A Subset of SIPK-Induced Defense Responses Likely Is Mediated by Ethylene
In conditional gain-of-function transgenic plants, we observed both defense gene activation and hypersensitive response–like cell death after the application of DEX inducer (Yang et al., 2001; Zhang and Liu, 2001; Jin et al., 2003). The activation of defense genes was an early event after MAPK activation. By contrast, hypersensitive response–like cell death occurred only after prolonged activation of SIPK and the delayed appearance of WIPK activity. At this time, we do not have evidence to implicate the accelerated production of ethylene in the NtMEK2DD-induced hypersensitive response–like cell death. Attempts to block cell death in NtMEK2DD plants after the application of DEX using inhibitors of ethylene biosynthesis and perception gave inconclusive results because these inhibitors by themselves caused cell death in control plants (data not shown).
Figure 8 depicts our current model of the role of ethylene in MAPK-induced defense gene activation. We observed strong induction of several groups of defense-related genes, including 3-hydroxy-3-methylglutaryl coenzyme A reductase, PIs, and several basic pathogenesis-related genes in NtMEK2DD transgenic plants after DEX treatment (Figure 7) (Yang et al., 2001; C.Y. Kim and S. Zhang, unpublished data). The activation of some of these defense-related genes was reported to be mediated by ethylene (Balandin et al., 1995; Yang et al., 1997; Dong, 1998; Hiraga et al., 2000; Wang et al., 2002). NtERFs are likely to be involved in the induction of at least a subset of defense genes in NtMEK2DD plants after DEX treatment or tobacco plants infected with TMV (Figure 6). This speculation is consistent with recent studies of Arabidopsis ERF1, the overexpression of which is sufficient to induce the antimicrobial defensin gene PDF1.2 (Solano et al., 1998; Berrocal-Lobo et al., 2002).
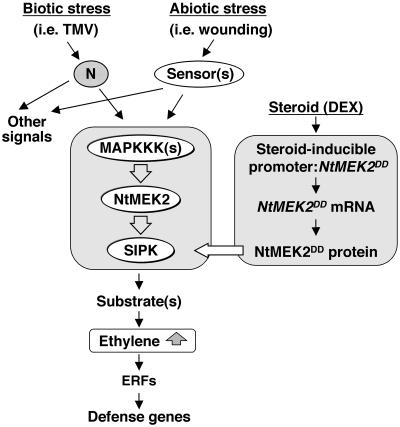
Figure 8.
Model of the Role of the SIPK Cascade in Stress-Induced Ethylene Biosynthesis and Defense Gene Activation.
External stresses from either biotic or abiotic sources are sensed by plant cells by means of R proteins/receptors/sensors. Signals generated from these interactions lead to the rapid activation of NtMEK2 by unidentified MAPKKK(s). Active NtMEK2 phosphorylates and activates the preexisting SIPK, which rapidly induces ethylene production through either a transcriptional or a post-transcriptional mechanism, or both. The perception of increased ethylene production by ethylene receptors triggers downstream responses, including the activation of ERFs, which leads to the expression of a subset of defense genes. In NtMEK2DD transgenic plants, the use of steroid-inducible promoters allowed us to mimic the activation of endogenous SIPK by stresses and, therefore, to examine the responses mediated by this MAPK pathway. The signal transduction pathway downstream of ethylene was simplified. N, N resistance gene product.
Could NtMEK2-SIPK Also Function Downstream of CONSTITUTIVE-TRIPLE-RESPONSE 1 (CTR1) and Mediate Ethylene-Induced Responses?
Genetic studies in Arabidopsis revealed that CTR1, a Raf MAPKKK homolog, functions downstream of ethylene (Kieber et al., 1993; Huang et al., 2003). It is interesting for us to discover that the NtMEK2-SIPK pathway is involved in the induction of ethylene biosynthesis. Very surprisingly, it was reported recently that alfalfa SIMKK-SIMK, the ortholog of the tobacco NtMEK2-SIPK and Arabidopsis AtMEK4/AtMEK5-AtMPK6 pathways, functions downstream of CTR1 (Ouaked et al., 2003). One major piece of supporting evidence for this conclusion is that hyperactive SIMKK transgenic Arabidopsis plants phenotypically copy ctr1. This result is inconsistent with SIMKK being the downstream MAPKK of CTR1. If SIMKK is downstream of CTR1, the gain-of-function hyperactive SIMKK transgenic plants should give phenotypes opposite that of the loss-of-function ctr1 mutant. In light of our findings, it is possible that ethylene is constitutively overproduced in hyperactive SIMKK transgenic plants, which could lead to the same phenotype as that of ctr1.
Our inducible promoter:NtMEK2DD transgenic plants with higher basal expression showed ethylene-induced phenotypes when plants became older, which is, in a sense, analogous to the phenotypes of Cauliflower mosaic virus 35S:SIMKK transgenic Arabidopsis plants. Although the initial clue that led to our discovery was from transgenic lines with higher basal transgene expression, the conclusion reported here is based on comparing ethylene production before and after DEX application. More importantly, NtMEK2DD transgenic lines with no visible phenotype throughout the life cycle also showed increased levels of ethylene biosynthesis after the application of DEX. The use of a conditional transgenic system in this report (Figure 8) has two advantages. First, it allowed us to mimic the stress-induced activation of SIPK and WIPK by the application of DEX. Second, it allowed us to determine the sequence of events after the application of inducer. By contrast, it is not possible to determine the sequence of events using a constitutive overexpression system.
Besides the hyperactive SIMKK transgenic plants, the report by Ouaked et al. (2003) also provided other biochemical evidence to support their conclusion. Is it possible that the same MAPKK-MAPK (i.e., NtMEK2-SIPK in tobacco and SIMKK-SIMK in alfalfa) could function both upstream of ethylene in regulating ethylene biosynthesis in response to stresses (Figure 8) and downstream of CTR1 in transmitting ethylene-induced signal (see Figure 6 in Ouaked et al., 2003)? There is evidence that a single MAPKK or MAPK can function in different MAPK pathways by forming complexes with different components (Widmann et al., 1999; Davis, 2000; Chang and Karin, 2001). It remains to be determined how the same MAPKK-MAPK can function both upstream and downstream of ethylene if both models are correct.
Is WIPK Involved in Wounding-Induced JA Induction and PI Activation?
Based on the fact that WIPK activity appeared later than the increase in ethylene, we conclude that the accelerated production of ethylene in NtMEK2DD plants after the application of DEX likely is a result of SIPK activation (Figure 2). Similarly, the appearance of WIPK activity in TMV-infected tobacco also occurred after ethylene induction (de Laat and van Loon, 1983; Zhang and Klessig, 1998a). However, the possible involvement of WIPK at a later stage cannot be excluded. Our recent studies revealed that SIPK and WIPK are linked at two different levels. First, they share the same upstream kinase, NtMEK2 (Yang et al., 2001). Second, the transcriptional activation of WIPK gene and the increase in WIPK protein in response to stress are regulated by SIPK (Liu et al., 2003). As a result, it is difficult to draw a definitive conclusion regarding the function of WIPK in ethylene induction.
The expression of PI-I and PI-II was increased greatly after the activation of SIPK/WIPK in NtMEK2DD plants. However, no significant increase in the levels of JA/MJ were observed after SIPK/WIPK activation (Figure 7). This result is contradictory to previous reports (Seo et al., 1995, 1999). In these two reports, WIPK was reported to mediate the biosynthesis of JA in wounded tobacco plants, which in turn activated PI expression. This conclusion was based on the study of transgenic plants overexpressing WIPK. However, not all transgenic lines overexpressing WIPK showed PI expression, and the phenotype was not heritable (Seo et al., 1999). Similar WIPK transgenic plants that we constructed did not show increased WIPK activity, although WIPK protein was overexpressed (Zhang and Liu, 2001; S. Zhang and D.F. Klessig, unpublished data). This is because WIPK, like other MAPKs, requires phosphorylation activation by its upstream kinase. In unstimulated cells, the activity of upstream MAPKK is not sufficiently high to activate WIPK (Liu et al., 2003). Our biochemical analysis demonstrated that the major wounding-activated MAPK is SIPK rather than WIPK, although the expression of WIPK is induced by wounding (Zhang and Klessig, 1998b). Based on previous reports that PI expression also can be induced by ethylene (Balandin et al., 1995; Hiraga et al., 2000), we conclude that the PI activation that we observed in NtMEK2DD transgenic plants is a result of ethylene production. These results suggest that the increase in ethylene but not JA is involved in plant defense responses mediated by the NtMEK2-SIPK/WIPK cascade.
METHODS
Plant Growth and Treatments
Wild-type tobacco (Nicotiana tabacum cv Xanthi nc [NN]) and transgenic plants in the same background (Jin et al., 2003) were grown at 22°C in a growth room programmed for a 14-h-light cycle. Fully expanded leaves of ∼6-week-old tobacco plants were used for experiments. Transgene expression was induced by spraying with dexamethasone (DEX; 30 μM). Samples for protein and RNA preparation were collected at various times after DEX treatment, quick-frozen in liquid nitrogen, and stored at −80°C until use. Infection with Tobacco mosaic virus and temperature-shift experiments were performed as described previously (Zhang and Klessig, 1998a).
Quantitation of Ethylene Biosynthesis Rates and Jasmonic Acid/Methyl Jasmonate Levels
Ethylene production rates were determined by gas chromatography as described previously (Spollen et al., 2000). Briefly, at various times after DEX treatment, one newly fully expanded leaf was detached from the plant and placed in a 60-mL syringe, and the syringe was sealed with the plunger. After a 5-min incubation in a growth chamber, a 40-mL gas sample was injected into a cold trap and the ethylene was quantified using a gas chromatograph equipped with a photoionization detector. Samples from three plants were analyzed for each time point. Averages with standard deviations are presented. This experiment was repeated three times with two independent NtMEK2DD transgenic lines and NtMEK2KR controls.
Jasmonic acid and methyl jasmonate were extracted and measured as described previously using gas chromatography–mass spectrometry (Wang et al., 1999). Dihydrojasmonic acid was added to the sample as an internal standard. Two independent time courses were determined with similar results.
Protein Extraction, Immunoblot Analysis, and In-Gel Kinase Activity Assay
Protein was extracted from leaf tissue and stored at −80°C (Zhang and Klessig, 1998a). The concentration of protein extracts was determined using the Bio-Rad protein assay kit (Richmond, CA) with BSA as the standard. Immunoblot detection of Flag-tagged proteins and in-gel kinase activity assays were performed as described previously except that 30 μg of protein was used for the analyses (Zhang and Klessig, 1997; Yang et al., 2001).
1-Aminocyclopropane-1-Carboxylic Acid Synthase Activity Assay and the Determination of 1-Aminocyclopropane-1-Carboxylic Acid Levels
Total proteins were extracted in 2 volumes (w/v) of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) extraction buffer (100 mM Hepes, pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM DTT, 1 mM Na3VO4, 10 mM NaF, 50 mM β-glycerolphosphate, 10 μM pyridoxal 5′-phosphate, 10% glycerol, and complete protease inhibitors [EDTA free; Roche Molecular Biochemicals, Mannheim, Germany]). The ACS assay was performed using 150 μg of total protein in 1 mL of reaction buffer (100 mM KH2PO4, pH 8.0, 1 mM EDTA, 2 mM DTT, 10 μM pyridoxal 5′-phosphate, and 150 μM S-adenosyl-l-Met) at 22°C for 1.5 h (Chae et al., 2003). Total ACC contents in the ACS assay reaction and that carried over from the protein extracts were quantified (Lizada and Yang, 1979). After subtracting the ACC in the protein extract, ACS activity was calculated as nanomoles of ACC production per milligram of total protein per hour. The ACC content in leaf tissue was expressed as nanomoles of ACC per milligram of extractable protein. Two independent time courses were determined with similar results.
RNA Gel Blot Analysis
Total RNA was extracted using Trizol reagent (Life Technologies) according to the manufacturer's instructions. Poly(A)+ mRNA was purified from total RNA using the Oligotex mRNA Mini Kit (Qiagen, Valencia, CA). Reverse transcription–PCR was performed to amplify cDNAs from the poly(A)+ mRNA using primers specific to tobacco ACSs, ACOs, ERFs, and PIs. The reverse transcription–PCR products were cloned into pGEM-T vector (Promega, Madison, WI), sequenced, and used as probes. Ten micrograms of total RNA per lane was separated on 1.2% formaldehyde-agarose gels, transferred to an Immobilon-Ny+ membrane (Millipore, Bedford, MA), and hybridized with random primer–labeled cDNA fragments as described previously (Yang et al., 2001).
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Shuqun Zhang, zhangsh@missouri.edu.
Accession Numbers
The GenBank accession numbers for the genes mentioned in this article are as follows: NtACS1, X65982; NtACS2, X98492; NtACS3, AJ131836; NtACO1, X98493/Z29529; NtACO2, Z46349/AB012857; NtACO3, X83229; NtERF1, D38123; NtERF2, D38126; PI-I, Z12619; and PI-II, Z29537.
Acknowledgments
We thank N.-H. Chua for the pTA7002 vector. This research was supported by National Science Foundation Grants MCB-9974796 and IBN-0133220 (S.Z.), U.S. Department of Agriculture National Research Initiative Grant 2002-01661 (H.F. and D.H.), and the University of Missouri Food for the 21st Century Program (R.E.S.). C.Y.K. was supported by a Molecular Biology Postdoctoral Fellowship from the University of Missouri. E.T.T. was supported by a Predoctoral Fellowship from the U.S. Department of Agriculture National Needs Training Grant in Plant Biotechnology (98-38420-5834).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.011411.
References
- Asai, T., Tena, G., Plotnikova, J., Willmann, M.R., Chiu, W.-L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Balandin, T., van der Does, C., Albert, J.-M.B., Bol, J.F., and Linthorst, H.J.M. (1995). Structure and induction pattern of a novel proteinase inhibitor class II gene of tobacco. Plant Mol. Biol. 27, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo, M., Molina, A., and Solano, R. (2002). Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 29, 23–32. [DOI] [PubMed] [Google Scholar]
- Bleecker, A.B., and Kende, H. (2000). Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16, 1–18. [DOI] [PubMed] [Google Scholar]
- Chae, H.S., Faure, F., and Kieber, J.J. (2003). The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C., and Shockey, J.A. (1999). The ethylene-response pathway: Signal perception to gene regulation. Curr. Opin. Plant Biol. 2, 352–358. [DOI] [PubMed] [Google Scholar]
- Chang, L., and Karin, M. (2001). Mammalian MAP kinase signaling cascades. Nature 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Chao, Q., Rothenberg, M., Solano, R., Roman, G., Terzaghi, W., and Ecker, J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Davis, R. (2000). Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- de Laat, A.M.M., and van Loon, L.C. (1983). The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol. Plant Pathol. 22, 261–273. [Google Scholar]
- Dong, X. (1998). SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1, 316–323. [DOI] [PubMed] [Google Scholar]
- Felix, G., Regenass, M., and Boller, T. (2000). Sensing of osmotic pressure changes in tomato cells. Plant Physiol. 124, 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, A., Salih, G., and Klessig, D.F. (2000). Activation of a diverse set of genes during the tobacco resistance response to TMV is independent of salicylic acid: Induction of a subset is also ethylene independent. Plant J. 21, 409–418. [DOI] [PubMed] [Google Scholar]
- Hazzalin, C.A., and Mahadevan, L.C. (2002). MAPK-regulated transcription: A continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 3, 30–40. [DOI] [PubMed] [Google Scholar]
- Hiraga, S., Ito, H., Sasaki, K., Yamakawa, H., Mitsuhara, I., Toshima, H., Matsui, H., Honma, M., and Ohashi, Y. (2000). Wound-induced expression of a tobacco peroxidase is not enhanced by ethephon and suppressed by methyl jasmonate and coronatine. Plant Cell Physiol. 41, 165–170. [DOI] [PubMed] [Google Scholar]
- Huang, Y., Li, H., Hutchison, C.E., Laskey, J., and Kieber, J.J. (2003). Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 33, 221–233. [DOI] [PubMed] [Google Scholar]
- Jin, H., Liu, Y., Yang, K.-Y., Kim, C.Y., Baker, B., and Zhang, S. (2003). Function of a mitogen-activated protein kinase pathway in N-gene mediated resistance in tobacco. Plant J. 33, 719–731. [DOI] [PubMed] [Google Scholar]
- Johnson, P.R., and Ecker, J.R. (1998). The ethylene gas signal transduction pathway: A molecular perspective. Annu. Rev. Genet. 32, 227–254. [DOI] [PubMed] [Google Scholar]
- Jonak, C., Ökrész, L., Bögre, L., and Hirt, H. (2002). Complexity, crosstalk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 5, 415–424. [DOI] [PubMed] [Google Scholar]
- Kende, H. (1993). Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 283–307. [Google Scholar]
- Kende, H. (2001). Hormone response mutants: A plethora of surprises. Plant Physiol. 125, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber, J.J. (1997). The ethylene response pathway in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 277–296. [DOI] [PubMed] [Google Scholar]
- Kieber, J.J., Rothenberg, M., Roman, G., Feldmann, K.E., and Ecker, J. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441. [DOI] [PubMed] [Google Scholar]
- Knoester, M., Bol, J.F., van Loon, L.C., and Linthorst, H.J. (1995). Virus-induced gene expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Mol. Plant-Microbe Interact. 8, 177–180. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Jin, H., Yang, K.-Y., Kim, C.Y., Baker, B., and Zhang, S. (2003). Interaction between two mitogen-activated protein kinases during tobacco defense signaling. Plant J. 34, 149–160. [DOI] [PubMed] [Google Scholar]
- Lizada, M.C.C., and Yang, S.F. (1979). A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal. Biochem. 100, 140–145. [DOI] [PubMed] [Google Scholar]
- MAPK Group (2002). Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 7, 301–308. [DOI] [PubMed] [Google Scholar]
- McKeon, T.A., Fernández-Maculet, J.C., and Yang, S.-F. (1995). Biosynthesis and metabolism of ethylene. In Plant Hormones, P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 118–139.
- Mizoguchi, T., Ichimura, K., and Shinozaki, K. (1997). Environmental stress response in plants: The role of mitogen-activated protein kinases. Trends Biotechnol. 15, 15–19. [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi, M., Suzuki, K., and Shinshi, H. (2000). Regulation of ethylene-induced transcription of defense genes. Plant Cell Physiol. 41, 1187–1192. [DOI] [PubMed] [Google Scholar]
- Ohtsubo, N., Mitsuhara, I., Koga, M., Seo, S., and Ohashi, Y. (1999). Ethylene promotes the necrotic lesion formation and basic PR gene expression in TMV-infected tobacco. Plant Cell Physiol. 40, 808–817. [Google Scholar]
- Ouaked, F., Rozhon, W., Lecourieux, D., and Hirt, H. (2003). A MAPK pathway mediates ethylene signaling in plants. EMBO J. 22, 1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, M.S. (1995). Ethylene in plant growth, development, and senescence. In Plant Hormones, P.J. Davies, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 486–508.
- Ren, D., Yang, H., and Zhang, S. (2002). Cell death mediated by mitogen-activated protein kinase pathway is associated with the generation of hydrogen peroxide in Arabidopsis. J. Biol. Chem. 277, 559–565. [DOI] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano, R., Stepanova, A., Chao, Q., and Ecker, J.R. (1998). Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE 3 and ETHYLENE-RESPONSE-FACTOR 1. Genes Dev. 12, 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu, P., Grosskopf, D.G., Felix, G., and Boller, T. (1994). The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol. 106, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen, W.G., LeNoble, M.E., Samuels, T.D., Bernstein, N., and Sharp, R.E. (2000). Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 122, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann, J.W., and Ryan, C.A. (1997). Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 94, 11085–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuki, M., and Mori, H. (2001). Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J. Biol. Chem. 276, 28051–28057. [DOI] [PubMed] [Google Scholar]
- Tena, G., Asai, T., Chiu, W.-L., and Sheen, J. (2001). Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 4, 392–400. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P., Woeste, K.W., Theologis, A., and Kieber, J.J. (1998). Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 95, 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Avdiushko, S., and Hildebrand, D. (1999). Overexpression of a cytoplasm-localized allene oxide synthase promotes the wound-induced accumulation of jasmonic acid in transgenic tobacco. Plant Mol. Biol. 40, 783–793. [DOI] [PubMed] [Google Scholar]
- Wang, K.L.-C., Li, H., and Ecker, J.R. (2002). Ethylene biosynthesis and signaling networks. Plant Cell 14 (suppl.), S131.–S151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann, C., Gibson, S., Jarpe, M.B., and Johnson, G.L. (1999). Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Woeste, K.E., Ye, C., and Kieber, J.J. (1999). Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 119, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K.-Y., Liu, Y., and Zhang, S. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.F., and Hoffman, N.E. (1984). Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 35, 155–189. [Google Scholar]
- Yang, Y., Shah, J., and Klessig, D.F. (1997). Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Zarembinski, T.I., and Theologis, A. (1994). Ethylene biosynthesis and action: A case of conservation. Plant Mol. Biol. 26, 1579–1597. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Du, H., and Klessig, D.F. (1998). Activation of tobacco SIP kinase by both a cell wall–derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48 kD MAP kinase in tobacco. Plant Cell 9, 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. a). N resistance gene-mediated de novo synthesis and activation of a tobacco MAP kinase by TMV infection. Proc. Natl. Acad. Sci. USA 95, 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. b). The tobacco wounding-activated MAP kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95, 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (2001). MAPK cascades in plant defense signaling. Trends Plant Sci. 6, 520–527. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Liu, Y. (2001). Activation of salicylic acid–induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell 13, 1877–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Liu, Y., and Klessig, D.F. (2000). Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 23, 339–347. [DOI] [PubMed] [Google Scholar]