We read with interest the recent perspective article by Klein.1 Genetic analysis in individuals with severe congenital neutropenia (SCN) indicates that 60% of cases were attributable to heterozygous mutation in ELA2 gene encoding neutrophil elastase.2 Homozygous mutation in HAX1 gene has been identified in patients with autosomal recessive form of SCN (Kostmann syndrome).3 Patients with ELA2 or HAX1 mutations reveal a similar morphological and similar clinical phenotype suggesting that there may be common downstream molecular events caused by both mutations.
Interestingly, 28 out of 39 (72%) patients with HAX1 mutations reported were in SCN kindred of Middle eastern descent indicative of consanguinity or intermarrying within specific population groups.3–5 Five Japanese SCN patients are described6 and only a few cases of true European descent have been documented so far.3,5,7
In the present study we describe the first 2 Italian SCN patients carrying two novel HAX1 mutations associated to neurodevelopment abnormalities.
Genomic DNA was extracted from peripheral blood of the patients and the components of their families. Sequencing analysis of HAX1 was performed as previously reported,3 after excluding mutations in ELA2 gene.8
In the first kindred the patient, a 4-year old boy, started presenting infections at the age of nine days. After persistent neutropenia and recurrent infections at seven months of life a Kostmann syndrome was diagnosed on the basis of neutrophil count (0.1×109/L) and on bone marrow analysis showing severe ipoplasia of myeloid serie, hyper-eosinophilia and arrest at the promyelocyte stage. No cytogenetic abnormalities were found. He was started on G-CSF therapy with an average dose of 7.5 μg/kg once a day in order to maintain neutrophil count between 1.5 to 3.0×109/L. At 18 months of age, signs of a developmental delay were noted involving motor, language and social areas. Neurological diagnostics revealed pathological EEG but normal MRI; he has not presented seizures.
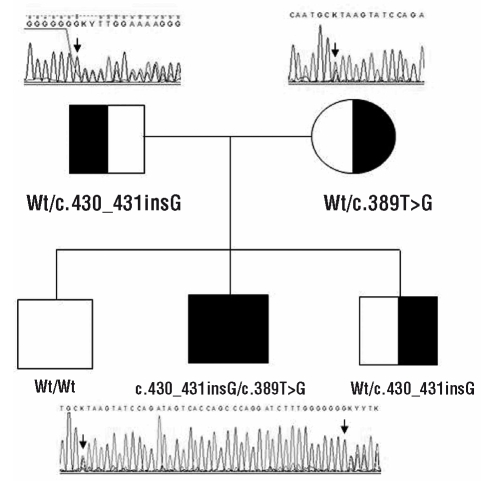
A compound heterozygous mutation within exon 3 of HAX1 gene has been found. It consisted of a frame-shift mutation c.430_431insG leading to a premature stop codon Val144GlyfsX5 inherited from his father and a mis-sense mutation c.389T>G generating a non-conservative amino acid substitution Leu130Arg inherited from his mother. This Leu130Arg substitution is a novel variation that was not detected in 90 healthy controls, so excluding the possibility of a polymorphic change. Both parents and one brother, heterozygous carriers of the c.430_431insG, had no detectable clinical phenotype (Figure 1).
Figure 1.
Sequence chromatograms of kindred 1. The filled symbol denotes the affected patient who is compound heterozygous for two mutations, each of them inherited from one of the parents. Both parents and one brother are heterozygous carriers (half-filled symbol). Mutations are indicated with black arrows. Square symbols denote male family members, the circle denotes a female.
In the second kindred, the patient, a 7-year old boy, was diagnosed at the age of 21 months after a persistent neutropenia and a long history of severe recurrent infections. Bone marrow aspiration showed myeloid dysplasia and arrest at myelocyte stage. Cytogenetic analysis was normal. He was started on G-CSF therapy with an average dose of 5 μg/kg once a day in order to maintain neutrophil count between 1.5 to 3.0×109/L. Patient development has been delayed since infancy, with mental and psychomotor retardation, serious walking impairment, and severe bilateral myopia. No episode of seizure was reported in this patient. The sequencing analysis of HAX1 gene identified the homozygous mutation c.409C>T within exon 3, resulting in a premature stop codon p.Gln137X. This mutation is novel and heterozygous carrier status was confirmed in healthy parents and his sister. No consanguinity was reported among parents, but their origins are from the same geographical area.
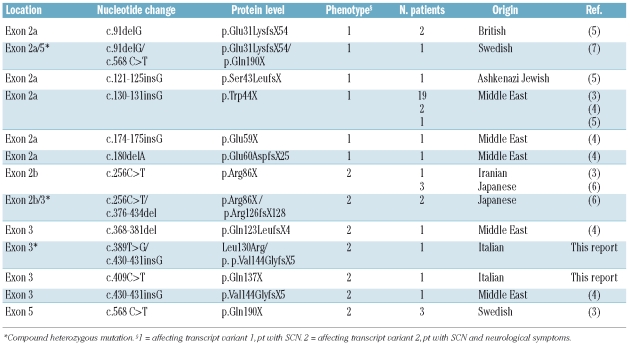
So far only 10 HAX1 mutations are described. The known HAX1 mutations reported up to date, included the new mutations that we have described, are listed in Table 1.
Table 1.
Known mutations in HAX1.
As shown in the Table, analysis of the patients’ genotypes and phenotypes revealed a striking correlation: mutations affecting transcript variant 1 only were associated with SCN, whereas mutations affecting both transcript variants 1 and 2 caused SCN and neurological symptoms, including epilepsy and neurodevelopmental delay.7 This correlation is confirmed also in our patients. In fact, all the mutations are founded within exon 3, affecting transcript variants 1 and 2 of HAX1 gene; both patients presented neurodevelopment abnormalities with mental and psychomotor retardation.
Our study describing the first 2 Italian patients with SCN due to HAX1 mutations indicates that mutations of this gene are not limited to patients with specific ethnic origin. HAX1 is a ubiquitously expressed gene but its mutations are relatively uncommon. Given this, the description of all new patients and the determination of whether the type of mutation impacts on phenotype and/or susceptibility to leukemic transformation, adds new and precious information needed to better characterize the clinical features of SCN-HAX1 mutated patients and the role of HAX1 protein.
Acknowledgments
the authors would like to thank M. Di Duca for technical support, and B. Caruzzo for secretarial assistance.
Footnotes
Funding: this work was supported by Compagnia di San Paolo, ERG s.p.a, SAAR Depositi Oleari Portuali.
References
- 1.Klein C. Molecular basis of congenital neutropenia. Haematologica. 2009;94(10):1333–6. doi: 10.3324/haematol.2009.012260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz M, Duan Z, Korkmaz B, Lee HH, Mealiffe ME, Salipante SJ. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 2007;109(5):1817–24. doi: 10.1182/blood-2006-08-019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein C, Grudzien M, Appaswamy G, Germeshausen M, Sandrock I, Schaffer AA, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease) Nat Genet. 2007;39(1):86–92. doi: 10.1038/ng1940. [DOI] [PubMed] [Google Scholar]
- 4.Germeshausen M, Grudzien M, Zeidler C, Abdollahpour H, Yetgin S, Rezaei N, et al. Novel HAX1 Mutations in patients with severe congenital neutropenia reveal isoform-dependent genotype-phenotype associations. Blood. 2008;111(10):4954–7. doi: 10.1182/blood-2007-11-120667. [DOI] [PubMed] [Google Scholar]
- 5.Smith BN, Ancljff PJ, Pizzey A, Khwaja A, Linch DC, Gale R. Homozygous HAX1 mutations in severe congenital neutropenia patients with sporadic disease: a novel mutation in two unrelated British kindreds. Br J Haematol. 2008;144(5):762–70. doi: 10.1111/j.1365-2141.2008.07493.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa N, Okada S, Miki M, Shirao K, Kihara H, Tsumura M, et al. Neurodevelopmental abnormalities associated with severe congenital neutropenia due to the R86X mutation in the HAX1 gene. J Med Genet. 2008;45(12):802–7. doi: 10.1136/jmg.2008.058297. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson G, Elinder G, Malmgren H, Trebinska A, Grzybowska E, Dahl N, et al. Compound heterozygous hax1 Mutations in a Swedish patient with severe congenital neutropenia and no neurodevelopmental abnormalities. Pediatr Blood Cancer. 2009;53 (6):1143–6. doi: 10.1002/pbc.22131. [DOI] [PubMed] [Google Scholar]
- 8.Lanciotti M, Caridi G, Rosano C, Pigullo S, Lanza T, Dufour C. Severe congenital neutropenia: a negative synergistic effect of multiple mutations of ELA2 gene. Br J Haematol. 2009;146(5):573–582. doi: 10.1111/j.1365-2141.2009.07787.x. [DOI] [PubMed] [Google Scholar]