Abstract
Background
Bone marrow mesenchymal stem cells support proliferation and differentiation of hematopoietic progenitor cells in vitro. Since these cells constitute a rare subset of bone marrow cells, mesenchymal stem cell preparations for clinical purposes require a preparative step of ex vivo multiplication. The aim of our study was to analyze the influence of culture duration on mesenchymal stem cell supportive activity.
Design and Methods
Mesenchymal stem cells were expanded for up to ten passages. These cells and CD34+ cells were seeded in cytokine-free co-cultures after which the phenotype, clonogenic capacity and in vivo repopulating activity of harvested hematopoietic cells were assessed.
Results
Early passage mesenchymal stem cells supported hematopoietic progenitor cell expansion and differentiation toward both B lymphoid and myeloid lineages. Late passage mesenchymal stem cells did not support hematopoietic progenitor cell and myeloid cell outgrowth but maintained B-cell supportive ability. In vitro maintenance of NOD/SCID mouse repopulating cells cultured for 1 week in contact with mesenchymal stem cells was effective until the fourth passage of the mesenchymal cells and declined thereafter. The levels of engraftment of CD34+ cells in NOD/SCID mice was higher when these cells were co-injected with early passage mesenchymal stem cells; however mesenchymal cells expanded beyond nine passages were ineffective in promoting CD34+ cell engraftment. Non-contact cultures indicated that mesenchymal stem cell supportive activity involved diffusible factors. Among these, interleukins 6 and 8 contributed to the supportive activity of early passage mesenchymal stem cells but not to those of late passage cells. The phenotype, as well as fat, bone and cartilage differentiation capacity, of mesenchymal stem cells did not change during their culture.
Conclusions
Extended culture of mesenchymal stem cells alters the ability of these cells to support hematopoietic progenitor cells without causing concomitant changes in their phenotype or differentiation capacity.
Keywords: human bone marrow mesenchymal stem cells, NOD/SCID-repopulating cells, progenitor cells of B lymphoid
Introduction
Umbilical cord blood is a valid alternative to bone marrow or mobilized peripheral blood as a source of hematopoietic stem cells for clinical transplantation. However the use of cord blood hematopoietic stem cells in adult recipients is limited by the small numbers of cells present in a single unit. Numerous studies have attempted to expand hematopoietic stem cells ex vivo in cell cultures supplemented with various combinations of hematopoietic cytokines. Although large numbers of committed progenitors can be obtained in a short period of 7 to 10 days, expansion of true long-term repopulating cells has been difficult to achieve.
Mesenchymal stem cells (MSC) are non-hematopoietic cells which can typically differentiate into adipocytic, osteocytic and chondrocytic tissues. They may also participate in the formation of hematopoietic stem cell niches.1,2 Indeed, human MSC have been reported to secrete various hematopoietic growth factors and support hematopoiesis in vitro. In cytokine-supplemented cultures of CD34+ cells, the expansion of nucleated cells as well as colony-forming cells is greater when MSC are included in the culture than when they are not.3 Differentiation of primitive progenitor cells occurs mainly along the myeloid lineage, but B lymphoid development has also been reported.4,5 In such co-culture systems, repopulating cells, assayed in xenogeneic recipients, are maintained or modestly expanded.6,7 The relative contributions of the cytokines and the feeder cells in the overall supportive activity in these studies have not, however, been clearly determined. We have previously reported that stromal cell derived factor 1 (SDF-1) may participate in the cell cycle-promoting activity of MSC toward co-cultured CD34+ cells.8
Another aspect of the hematopoiesis-supporting activity of MSC lies in their ability to enhance bone marrow homing of co-injected hematopoietic stem and progenitor cells.9,10 In this procedure, improved hematopoietic reconstitution can be achieved without ex vivo expansion of hematopoietic progenitors. In the NOD/SCID mouse model, bone marrow homing is particularly enhanced when limiting numbers of CD34+ cells are infused.11 The capacity of MSC to improve engraftment of CD34+ cells can be enhanced by stimulation with cytokines that increase CXCR4 expression by MSC.12
Since they account for a very small fraction of bone marrow cells, preparations of MSC for clinical purposes require a preliminary step of ex vivo multiplication. While considerable expansion of MSC is often needed to obtain large cell doses starting from a bone marrow aspirate, the optimal duration of MSC culture has not been defined. It has recently been reported that bone marrow homing of MSC decreases with prolonged ex vivo culture.13 Whether other properties of MSC relevant to their hematopoiesis-supporting activity are modified ex vivo remains to be established.14
In the present study, we analyzed the influence of culture duration on MSC. The phenotype and differentiating capacity of MSC preparations were assessed after up to ten passages. In parallel, the ability of MSC to support differentiation and self-renewal of cord blood hematopoietic stem/progenitor cells was assayed in cytokine-free co-cultures. Enhancement of bone marrow homing was studied by co-transplantation of MSC and unmanipulated cord blood CD34+ cells into NOD/SCID mice.
Design and Methods
Cell isolation and preparation
Human bone marrow samples were obtained from normal adult volunteers. Mononuclear cells were isolated by centrifugation over Ficoll-Paque™ Plus (GE Healthcare, Diegem, Belgium) and washed in Dulbecco’s phosphate-buffered saline (DPBS; Lonza, Verviers, Belgium). Cells were seeded at a density of 5×105 cells/cm2 in mesenchymal stem cell growth medium (MSCGM; Lonza). MSCGM is a ready-to-use medium supplemented with 10% serum. Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2. MSC were selected by plastic adherence and elimination of non-adherent cells 24 h after cell seeding. When cultures reached 90% confluence, cells were detached with trypsin-EDTA solution (Lonza), and sub-cultured at 5×103 cells/cm2 for ten passages. All experiments assessing the influence of passage number on the properties of MSC were done using three independent MSC preparations, with the exception of the secretion profile assays which were done with MSC from only two donors.
Human umbilical cord blood samples were obtained following full-term vaginal delivery. All samples were processed within 24 h after delivery. Mononuclear cells were isolated by centrifugation over Ficoll-Paque™ Plus and washed in DPBS. CD34+ cells were purified using magnetic activated cell separation (MACS) CD34 isolation kits (Miltenyi Biotech, Gladbach, Germany). The purity of the selected cells was always higher than 95%.
All material was acquired with informed consent and used according to the guidelines established by the Ethical Committee of the University of Liège.
Flow cytometric analysis of mesenchymal stem cell preparations
The following antibodies were used for phenotypic characterization of MSC: anti-CD13, anti-CD14 and anti-CD36 conjugated to fluorescein isothiocyanate (FITC), anti-CD11b, anti-CD49a, anti-CD73, anti-CD90, anti-CD106, anti-CD146, anti-CD271, and anti-SSEA4 conjugated to phycoerythrin (PE) (all from BD Biosciences, Erembodegem, Belgium), PE-anti-CD105 (Serotec, Düsseldorf, Germany), PE-anti-STRO1 (Santa Cruz Biotechnology, Heidelberg, Germany), anti-CD45 (BD Biosciences), and anti-mouse IgG (Jackson ImmunoResearch, Suffolk, England) conjugated to allophycocyanin (APC), and purified anti-GD2 (BD Biosciences). Cells were incubated with antibodies or isotype-matched control IgG for 30 min at 4°C in the dark. Cells were washed in DPBS and fixed in DPBS 1% formaldehyde (Vel, Leuven, Belgium). Data were acquired on a BD FACSCanto II flow cytometer and analyzed using BD FACSDiVa™ software.
Mesenchymal stem cell differentiation assays
Fat, bone and cartilage differentiation assays were carried out as described by Pittenger et al.15 and revealed by staining with oil red O, alizarin red and toluidine blue, respectively.
Mesenchymal stem cell secretion profile assays
At each passage, at 90% confluence, the medium was changed and incubated for an additional 10 days. The MSC-conditioned medium was collected and frozen at −20°C for further analyses. In some experiments, confluent MSC were stimulated with 10 ng/mL interleukin (IL)-1α every other day for 10 days. RayBio® human cytokine antibody arrays (RayBiotech, Inc, Boechout, Belgium) were used according to the provided protocols to analyze the presence of bone morphogenetic protein (BMP)-4, BMP-7, Flt-3 ligand, granulocyte colony-stimulating factor (G-CSF), granulocyte-monocyte colony-stimulating factor (GM-CSF), IL-10, IL-15, IL-1α, IL-2, IL-3, IL-6, IL-7, IL-8, leukemia inhibitory factor (LIF), L-selectin, stem cell factor (SCF), SDF-1, thrombopoietin (TPO), vascular cell adhesion molecule (VCAM)-1, intracellular adhesion molecule (ICAM)-1, matrix metalloprotease (MMP)-2, MMP-3, MMP-10, MMP-13, and tissue inhibitor of metalloprotease (TIMP)-4 in conditioned medium. The concentrations of IL-6 and IL-8 were measured using cytometric bead array kits (BD Biosciences) according to the manufacturer’s recommendations. Samples were analyzed in a BD FACSCanto II flow cytometer and analyzed by using BD FCAP Array software (BD Biosciences). Results are expressed as picograms per milliliter.
Long-term cultures of CD34+ cells and mesenchymal stem cells
Five thousand CD34+ cells were plated either in contact with confluent MSC or in transwells seeded with MSC. Cultures were maintained for 3 weeks. Thereafter, the persistence of primitive progenitors was assessed by transferring cells into a semi-solid medium to allow development of secondary hematopoietic colonies. As a control, uncultured CD34+ cells were transferred directly into the semi-solid medium. The long-term culture medium consisted of α-MEM supplemented with 8% horse serum, 8% fetal bovine serum, 0.2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (all from Lonza), 0.2 mM inositol (Sigma-Aldrich), and 0.1 mM 2-mercaptoethanol (Invitrogen, Merelbeke, Belgium). Cultures were maintained at 37°C in 5% CO2 with weekly changes of half the medium. In some experiments, cultures were supplemented every other day with 1 μg/mL of anti-IL6 or anti-IL8 function-blocking antibodies (R&D, Abingdon, UK). After 3 weeks, cultures were trypsinized. First, a third of the cell suspension was transferred in duplicate progenitor assays into a semi-solid medium consisting of MethoCult® GF+ H4435 (Stem Cell Technologies). After an additional 2 weeks of incubation at 37°C secondary colony-forming cells were scored. The remaining two-thirds of the cells were harvested for FACS analysis. Absolute numbers of CD34+, CD10+, CD19+, CD11b+ and CD33+ cells grown in cultures were determined using TruCount tubes (BD Biosciences). APC-conjugated anti-CD34, PE-Cy7-conjugated anti-CD10, APC-Cy7-conjugated anti-CD19, PE-conjugated anti-CD11b and peridin chlorophyll-cyanin 5.5-conjugated anti-CD33 (all from BD Biosciences) were used. Cells were incubated with antibodies or isotype-matched control IgG for 30 min at 4°C in the dark. Cells were washed in DPBS and fixed in DPBS 1% formaldehyde. Data were acquired on a BD FACSCanto II flow cytometer and analyzed using BD FACSDiVa™ software.
Assessment of B-cell maturation
The stage of maturation of B cells generated after 3 weeks of co-culture in contact with MSC was assessed by flow cytometry analysis and real-time quantitative polymerase chain reaction (PCR) analysis of B-specific transcripts. For FACS analysis, the following antibodies were used: FITC-conjugated anti-kappa, FITC-conjugated anti-IgM, PE-conjugated anti-lambda, PE-conjugated anti-CD79a (all from BD Biosciences). After cell fixation and permeabilization using a BD Cytofix/Cytoperm™ Fixation/Permeabilization kit, cells were incubated with antibodies or isotype-matched control IgG for 30 min at 4°C in the dark. Data were acquired on a BD FACSCanto II flow cytometer and analyzed using BD FACSDiVa™ software.
For real-time quantitative PCR analysis, RNA was isolated using an RNeasy Blood & Tissue Kit (Qiagen, KJ Venlo, the Netherlands). The concentration and purity of the RNA were estimated by optical density measurements. To avoid genomic DNA contamination, RNA was treated with RNase-free DNaseI (Fementas GMBH, St.Leon-Rot, Germany) at 37°C for 30 min. cDNA synthesis was performed using a RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas). cDNA was analyzed by real-time PCR, with amplification performed using the ABI Prism 7700 sequence detection system (Applied Biosystems, Lennik, Belgium). A 20 μL reaction mixture containing 10 μL of Power SYBR® Green PCR master mix (Applied Biosystems) and cDNA template and forward and reverse primers was used. Target genes were EBF (forward primer: GATACGGCTCTGCCGCAAT, reverse primer: CAGCTGAGCCGTTGAGGAA), PAX5 (forward primer: TGGAGGATCCAAACCAAAGG, reverse primer: GGCAAACATGGTGGGATTTT), RAG1 (forward primer: CATCAAGCCAACCTTCGACAT, reverse primer: CAGGACCATGGACTGGATATCTC), TdT (forward primer: TTGCCCTGTTGGGATGGA, reverse primer: TCCGCTCATGTGTGGCATAG), and VPREB (forward primer: GACATCGGTGTGTACAGCGTCTA, reverse primer: TGGCTCTTGTCTGATTGTGAGAA). The reference gene was β2-microglobulin (forward primer: GAGTATGCCTGCCGTGTG, reverse primer: AATCCAAATGCGGCATCT). Relative quantities of target genes were calculated using the relative standard curve method recommended by Applied Biosystems.
Transplantation of human cells into NOD/SCID mice
Two-month old NOD/SCID mice were sublethally irradiated with 300 cGy from a 137Cs source (GammaCell 40, Nordion, Ontario, Canada). Four hours before injection, 10 days and 21 days after engraftment, the mice received 20 μL of anti-asialo GM1 antiserum (Wako Chemicals GmbH, Neuss, Germany) by intraperitoneal injection. The mice were injected with 1.5×105 CD34+ uncultured cells or the expansion product of 1.5×105 CD34+ cells co-cultured for 1 week in contact with confluent MSC harvested at passage (P)1 to P10 in long-term culture medium. Other mice were transplanted with the expansion product of 1.5×105 CD34+ cells co-cultured for 1 week in transwells seeded with confluent MSC. For co-transplantation experiments, mice were infused with 1.5×105 uncultured CD34+ cells and 1.5×105 MSC harvested at P1 to P10. Cells were infused by intravenous tail injection. After 6 weeks, the mice were killed by cervical dislocation and bone marrow cells were harvested from the femora and tibiae by flushing the bones with Iscove’s modified Dulbecco’s medium (Lonza) with 10% fetal bovine serum. Mononuclear low-density cells were isolated by centrifugation over Ficoll-Paque™ Plus, washed and resuspended in the same medium with 10% fetal bovine serum. In order to assess engraftment, bone marrow cells from recipient mice were pelleted and incubated with various mouse anti-human monoclonals for 30 min at 4°C in the dark. APC-conjugated anti-human CD45, FITC-conjugated anti-human CD33 and PE-conjugated anti-human CD19 (all from BD Biosciences) were used. Cells were washed in DPBS and fixed in DPBS 1% formaldehyde. Data were acquired on a BD FACSCanto II flow cytometer and analyzed using BD FACSDiVa™ software. Positive cells were identified by comparison with isotypic controls. All experiments were conducted in accordance with the recommendations of the Animal Ethics Committee of the University of Liège.
Homing of mesenchymal stem cells
Two-month old NOD/SCID mice were sublethally irradiated with 300 cGy from a 137Cs source. Four hours before injection, 10 days and 21 days after engraftment, the mice received 20 μL of anti asialo GM1 antiserum by intraperitoneal injection. Mice received 2×105 MSC from P3 or P7 by intravenous tail injection. After 24 h and 5 weeks, mice were killed by cervical dislocation and blood, bone marrow, spleen, lung, liver, kidney and small intestines were harvested and frozen. Genomic DNA was isolated from the mouse tissues using a DNeasy Blood & Tissue Kit (Qiagen). The concentration and purity of the DNA were estimated by optical density measurements. DNA was analyzed by real-time PCR, with amplification performed using the ABI Prism 7700 sequence detection system. A 20 μL reaction mixture containing 10 μL of Power SYBR® Green PCR master mix and 100 ng of DNA template and forward and reverse primers was used. The target gene was human albumin, the forward primer was TGAAACATACGTTCCCAAAGAGTTT, and the reverse primer was CTCTCCTTCTCAGAAAGTGTGCATAT. The reference gene was murine β-actin, the forward primer was CCTGTGGCATCCATGAAACTAC, and the reverse primer was CACTGTGTTGGCATAGAGGTCTTT. The relative quantity of human albumin gene was calculated using the comparative Ct method. A validation experiment was carried out to confirm that efficiency of amplification of the target and reference genes were similar. The absolute value of the slope of log input amount versus Ct was less than 0.1, as recommended by the manufacturer.
Statistical analysis
Results are reported as mean ± standard error of the mean (SEM). Comparisons were made using two-tailed Student’s t tests, Mann-Whitney tests or Z-tests with SigmaPlot and SigmaStat software (SPSS, Richmond, CA, USA).
Results
Mesenchymal stem cell expansion and characterization of the mesenchymal stem cell preparations
MSC were purified by plastic adhesion and expanded in MSCGM. Cells were passaged every 10 days. There were two to three doublings at each passage (Online Supplementary Figure S1). MSC proliferated for at least ten passages. Cells were harvested at each passage and their phenotype and differentiation potential assessed.
As early as the second passage, cells displayed a characteristic mesenchymal phenotype, being negative for CD11b, CD14, CD36 and CD45, weakly positive (mean channel fluorescence ratio, MCFR 1.5 to 3.5) for CD49a, CD105, CD106 and CD146 antigens, positive (MCFR 3.5 to 9) for CD13 and CD73, and strongly positive (MCFR > 25) for CD90 and GD2 antigens. Cells did not express antigens previously reported on MSC such as CD271, SSEA4 and STRO-1 antigens16–18 (Online Supplementary Figure S1). There were no consistent changes in phenotype for at least ten passages.
Differentiation into fat, bone and cartilage was assessed by transferring MSC into specific induction media.15 Lipid vacuoles, calcium deposits and chondrogenic differentiation were revealed with oil red O, alizarin red, and toluidine blue, respectively. The ability to differentiate into these three tissues was present in every MSC preparation tested, up to P10 (data not shown). All MSC preparations used to assess the ability of these cells to support hematopoiesis, as described below, were confirmed to have the same phenotype and differentiation capacity.
Cytokine secretion profile
The presence of various hematopoietic cytokines, adhesion molecules and metalloproteases in MSC-conditioned medium was assayed at several passages by cytokine arrays (detection limit: 4–25 pg/mL). In baseline conditions IL-6, IL-8, SDF-1, VCAM-1, ICAM-1, MMP-2, MMP-3, MMP-13 and TIMP-4 were detected in MSC-conditioned medium. When MSC were stimulated with IL1-α, an additional secretion of GM-CSF, LIF and MMP-10 was observed. Both the baseline and IL1α-stimulated secretion profiles were maintained up to P10 without variations in the nature of molecules detected. Next, the expression of IL-6 and IL-8, the two most abundantly secreted molecules, was quantified by flow cytometric bead arrays. We observed that the level of IL-6 secretion was 4.95±1.27 ng/mL after P1-3, increased to 24.0±8.5 after P4-6 (P<0.001) and was maintained until P10. Conversely, IL-8 secretion decreased progressively from 8.1±3.6 ng/mL after up to P3, to 4.7±1.4 ng/mL after P4-6, 4.3±0.7 at P7-8 and 2.3±0.6 ng/mL after P9 or 10 (P<0.01 versus P1-3; Online Supplementary Figure S1).
Hematopoiesis supporting ability of mesenchymal stem cells in long-term cultures
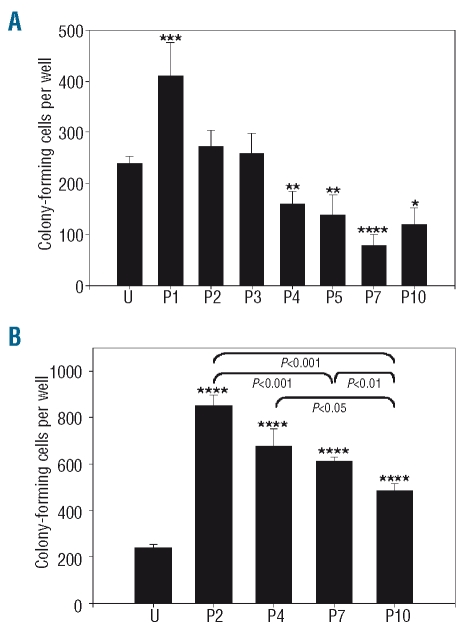
The ability of MSC to support hematopoiesis in vitro was evaluated by establishing long-term cultures in contact and non-contact conditions. In contact conditions, the output of secondary colony-forming cells was higher after co-culture with P1 MSC and lower after co-culture with MSC harvested after four or more passages, relative to uncultured cells (P<0.05; Figure 1A). The number of secondary colony-forming cells was higher in non-contact conditions (Figure 1B) than in contact conditions for all the MSC preparations tested. A significant decline of hematopoiesis supporting ability with the number of passages was also noted in non-contact conditions (P<0.05).
Figure 1.
Supportive activity of MSC in contact and non-contact conditions. (A) Absolute number of colony-forming cells after 3 weeks of co-culture initiated with 5000 cord blood CD34+ cells in contact with MSC harvested at indicated passage (P) or in 5000 uncultured CD34+ cells (U). (B) Cultures were conducted in non-contact conditions. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001 versus uncultured CD34+ cells, n≥4.
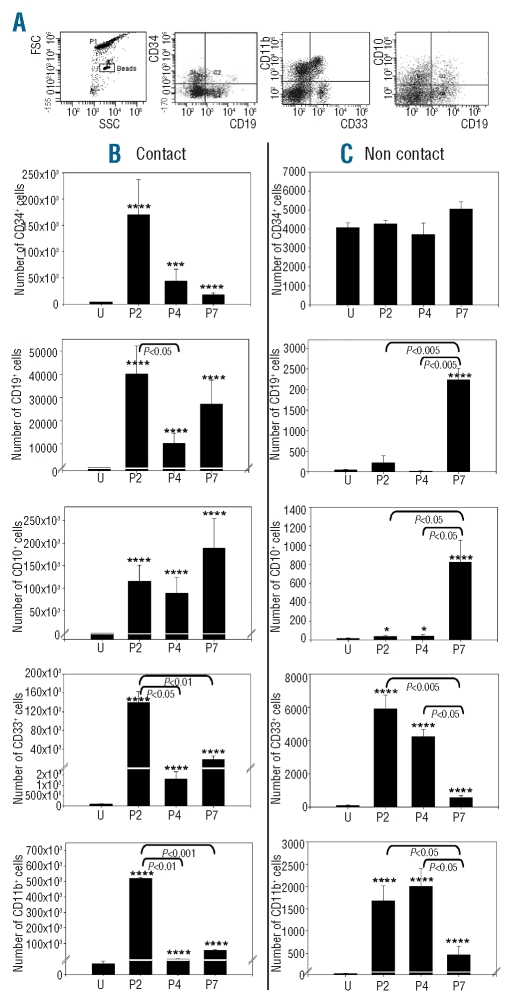
The phenotype of hematopoietic cells grown in cultures with MSC was established. The expression of CD34, CD33, CD11b, CD19 and CD10 was tested on cells harvested after 3 weeks in contact and non-contact conditions. Flow cytometric analysis was carried out in tubes containing a defined number of latex beads, in order to measure absolute numbers of cells with a given phenotype (Figure 2A). In contact conditions (Figure 2B), an expansion of CD34+ cells was noted after culture with P2 MSC. CD34+ cell expansion was less substantial after culture in contact with MSC harvested after four or more passages (P>0.05). Myeloid cells expressing CD33 and/or CD11b also increased substantially after culture with P2 MSC, and much less with MSC of further passages (P<0.05). In contrast, lymphoid cells expressing CD19 and/or CD10 were maintained or expanded with P2 MSC as well as with P4 and P7 MSC. In non-contact conditions (Figure 2C), CD34+ cells persisted after culture with P2, P4 or P7 MSC without net proliferation. Mature myeloid cells virtually disappeared after culture with P7 MSC (P<0.05), while lymphoid cell numbers were higher after culture with P7 MSC than with P2 or P4 MSC (P<0.05). Thus, in both contact and non-contact conditions, MSC support for myeloid progenitors decreased with the number of passages while B lymphoid support was maintained or even increased in late passage MSC.
Figure 2.
Support of myeloid and lymphoid progenitors by MSC. Absolute numbers of CD34+, CD19+, CD10+, CD33+ and CD11b+ cells grown after 3 weeks in long-term cultures with MSC harvested at indicated passage (P) or uncultured CD34+ cells (U), n=3. (A) Representative data of flow cytometric analysis. (B) Cultures were conducted in contact with MSC. (C) Cultures were conducted in non-contact conditions. *P<0.05, **P<0.01, ***P<0.005, ****P<0.001 versus uncultured CD34+ cells.
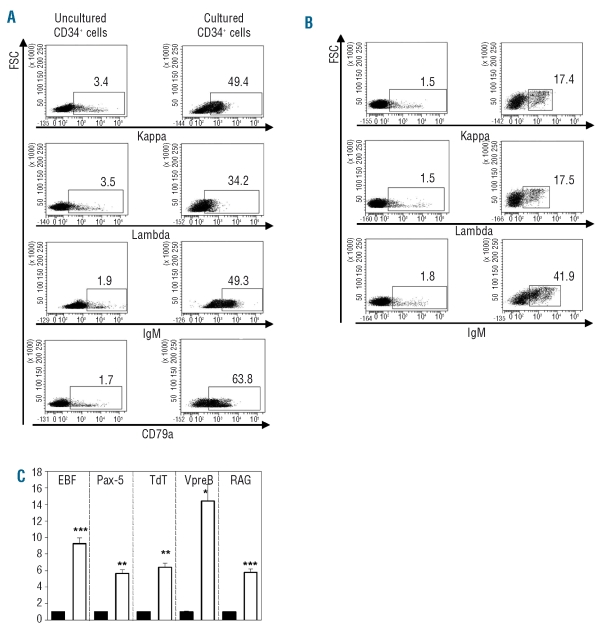
In vitro B-cell maturation of human CD34+ cells has been reported previously to be dependent on co-culture with specific murine stromal cell lines, such as MS-5 and S17.19,20 This prompted us to investigate whether recapitulation of B-cell ontogeny could be obtained with human MSC. Phenotypic and molecular analyses were carried out to define the stage of maturation stage of outgrown B cells. FACS analysis of cultured CD34+ cells showed the expression of cCD79a, cytoplasmic κ and λ light chains as well as cytoplasmic μ heavy chain on more than 30% of the cells. In comparison, less than 4% of uncultured CD34+ expressed the same B-cell markers (Figure 3A). Membrane expression of κ and λ light chains as well as complete IgM was demonstrated on cultured CD34+ cells but not on native CD34+ cells (Figure 3B). Real-time quantitative PCR analysis detected the expression of B-cell-specific transcripts, such as TdT, RAG, VpreB, EBF and Pax-5, in cells harvested after 3 weeks of co-culture with MSC, but not in uncultured CD34+ cells (Figure 3C). This indicates induction of the B-cell transcriptional program following contact with MSC. Thus, CD34+ cells undergo B-cell differentiation up to the stage of immature IgM+ B cells when in contact with human MSC, similarly to previously reported findings in models using murine stromal cell lines.
Figure 3.
B-cell generation stimulated by MSC. Flow cytometric analysis of cells grown after 3 weeks in long-term cultures with MSC (right) or uncultured CD34+ cells (left). (A) Intracellular staining. (B) Surface staining. Representative experiment out of three. (C) Relative quantitation of mRNA for B specific transcripts (EBF, Pax-5, TdT, VpreB and RAG) in uncultured CD34+ cells (black bars) or in cells grown after 3 weeks of co-culture with MSC (white bars). *P<0.05, **P<0.005, ***P<0.001 versus uncultured CD34+ cells, n=3.
Maintenance of SCID repopulating cells in co-culture with mesenchymal stem cells
Two-month old NOD/SCID mice sublethally irradiated with 300 cGy were used in these experiments. Cells were transplanted by intravenous tail injection. After 6 weeks the mice were killed and bone marrow cells were harvested from femora and tibiae. Human chimerism was analyzed by flow cytometry (Figure 4A). Mice were considered positive when at least ten cells co-expressing hCD45 and either hCD19 or hCD33 were detected in 100×103 mouse bone marrow cells (detection limit: 0.01%).
Figure 4.
Human repopulating cells in NOD/SCID mice. (A) Representative flow cytometric analysis of recipient mice bone marrow mononuclear cells. (B) Experimental design. (C) Mice received uncultured CD34+ cells (U) or the expansion product of CD34+ cells co-cultured for 1 week in contact with confluent MSC harvested at P1 to P10. *P<0.05 versus other passages. Each open symbol represents one mouse. Solid symbols are the mean ± SEM. (D) Mice received the expansion product of CD34+ cells co-cultured in contact with P4 MSC, in non-contact conditions in transwell seeded with P4 MSC, in P4 MSC-conditioned medium (MSC-CM) or in unconditioned medium (UM). *P<0.05 versus UM (E) Uncultured CD34+ cells were injected alone (U) or co-injected with MSC harvested at P1 to P10 without prior culture. *P<0.01 versus other passages; **P<0.01 versus co-injection with MSC of passages P1-3, P4-6 and P7-8. The detection limit of human cell engraftment was set at 0.01% of recipient mice bone marrow mononuclear cells.
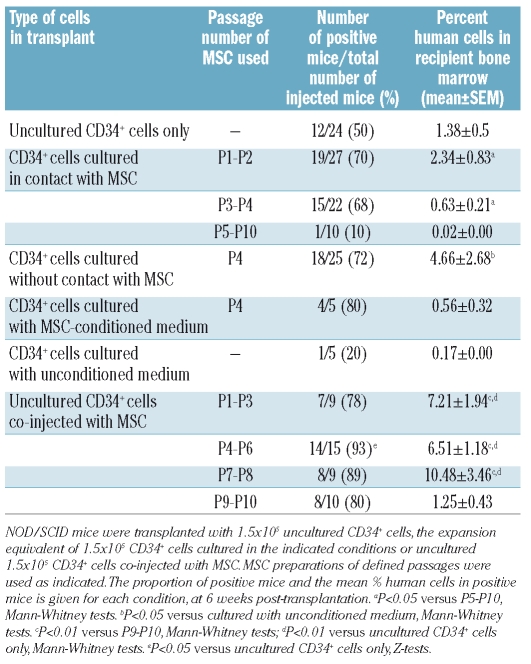
First, mice received 1.5×105 CD34+ uncultured cells or the expansion product of 1.5×103 CD34+ cells co-cultured for 1 week with confluent MSC harvested at P1 to P10 (Figure 4C, Table 1). Human CD45+/CD19+ or CD33+ cells were detected in the bone marrow of 50% of mice transplanted with uncultured CD34+ cells, with an average chimerism of 1.38±0.53%. After co-culture with early passage MSC, more than 50% of the mice were positive (P1-P2: 70%, P3-P4: 68%, P>0.05). However, there was no significant difference in the percentage of engrafted human cells between mice transplanted with uncultured CD34+ cells and mice receiving CD34+ cells co-cultured with early passage MSC (up to P4). After co-culture with late passage MSC (P5 to P10) only 10% of the mice showed reconstitution and the percentage of engrafted human cells was very low, at 0.02% (P<0.005 compared to co-culture with P1-P4 MSC). Thus maintenance of repopulating cells was higher in contact with early passage MSC than with late passage MSC.
Table 1.
Recovery of repopulating cells after co-culture or co-injection with MSC.
We next asked whether prolonged co-culture of CD34+ cells in contact with MSC could increase repopulating activity. Mice were injected with the product of 1.5×105 CD34+ cells co-cultured with confluent P2 MSC for 2 weeks and 3 weeks. After the 2-week culture, no bone marrow repopulation was observed (human chimerism <0.01% in 5/5 recipients), whereas after the 3-week culture, human engraftment was 0.09±0.04% (n=5) but consisted exclusively of CD19+ B cells without myeloid engraftment. Thus, co-culture of CD34+ cells in contact with MSC allows maintenance but not expansion of repopulating cells.
Next, mice were transplanted with the expansion product of 1.5×105 CD34+ cells co-cultured for 1 week in non-contact conditions, in transwells seeded with confluent P4 MSC. An additional group of mice was injected with CD34+ cells simply incubated for 1 week in P4 MSC-conditioned medium. A third group was transplanted with CD34+ cells kept for 1 week in unconditioned medium (Figure 4D, Table 1). The percentages of positive mice were similar when the animals were transplanted with CD34+ cells cultured in non-contact conditions (72%) and in conditioned medium (80%), as well as in contact conditions (68%). There was no significant difference in the percentage of chimeric human cells between mice transplanted with CD34+ cells cultured in contact with MSC (P3-P4: 0.63±0.21%) and with MSC conditioned medium (0.56±0.32%). The percentage of human cells was slightly higher after transplantation of the expansion product of CD34+ cells co-cultured in transwells (4.66±2.68%) but the difference did not reach statistical significance. Transplantation of mice with CD34+ cells cultured in unconditioned medium yielded only one positive mouse out of five, with 0.17% human chimerism.
Next, we investigated the potential activity of MSC in improving bone marrow engraftment of uncultured CD34+ cells. For this purpose, 1.5×105 fresh cord blood CD34+ cells were directly co-injected with 1.5×105 MSC harvested after P1 to P10 (Figure 4E, Table 1). With this strategy, more than 50% of the mice showed human hematopoietic reconstitution. Compared to engraftment following infusion of CD34+ cells only, the percentage of engrafted human cells was dramatically increased after co-injection with MSC harvested at P1 to P8 (P<0.006). Conversely, MSC harvested at P9 and 10 were ineffective in providing enhanced engraftment of uncultured CD34+ cells (P<0.05).
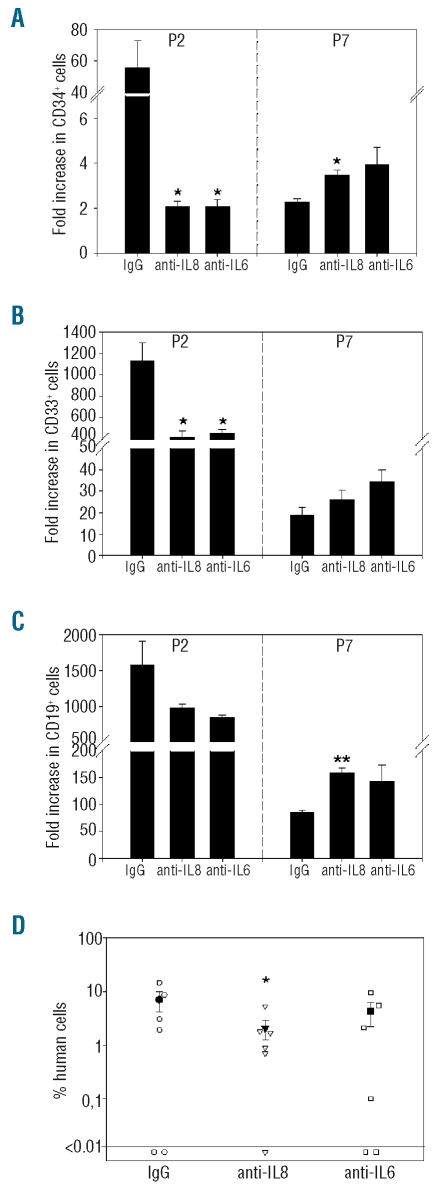
Regulation of the hematopoiesis-supporting activity of mesenchymal stem cells by interleukins 6 and 8
We attempted to define critical cytokines contributing to the pro-hematopoietic activity of MSC. CD34+ cells were cultured in contact with MSC in medium supplemented with antibodies neutralizing IL-6 or IL-8, or with control IgG. First long-term cultures were established with P2 or P7 MSC as feeder cells. A decrease of CD34+ (P<0.05, Figure 5A), CD33+ (P<0.05, Figure 5B) and CD19+ (P>0.005, Figure 5C) outgrown cells was observed after neutralization of IL-6 or IL-8 in 3-week cultures in contact with P2 MSC. Conversely, when cultures were set on P7 MSC, IL-6 neutralization had no effect while increases of CD34+ and CD19+ cell outgrowth were noted after inhibition of IL-8 (Figure 5A, B and C for CD34+ cells, CD33+ cells and CD19+ cells, respectively).
Figure 5.
Regulation of MSC supportive activity by IL-6 and IL-8. Outgrowth of CD34+ (A), CD33+ (B) and CD19+ (C) cells after 3 weeks in long-term cultures supplemented with anti-human IL-8, anti-human IL-6 or control IgG, n=3. Results are expressed as fold increase relative to input cells. *P<0.05, **P<0.005 versus cultures supplemented with control IgG. (D) Analysis of human cells in the bone marrow of recipient NOD/SCID mice that received the expansion product of CD34+ cells co-cultured for 1 week with confluent P2 MSC. Cultures were supplemented with anti-human IL-6, anti-human IL-8 or control IgG. Each open symbol represents one mouse, closed symbols are mean ± SEM. *P<0.05 versus IgG control.
Next, NOD/SCID mice were transplanted with CD34+ cells cultured for 1 week with P2 MSC, in medium supplemented with IL-6 or IL-8 function-blocking antibodies. A slight but statistically significant decrease in repopulating activity was noted after IL-8 neutralization, while IL-6 neutralization had no effect (Figure 5D). Thus, in contact with early passage (P2) MSC, both IL-6 and IL-8 contribute to in vitro differentiation of myeloid and lymphoid cells, while only IL-8 is involved in the maintenance of repopulating cells. Neither IL-6 nor IL-8 mediated hematopoietic support in contact with late passage (P7) MSC.
Homing of mesenchymal stem cells
Finally, the homing capacity of MSC in several tissues was tested after P3 and P7. NOD/SCID mice sublethally irradiated with 300 cGy received 2×05 MSC from P3 or P7 by intravenous tail injection. After 24 hours and 5 weeks, mice were killed and blood, bone marrow, spleen, lung, liver, kidney and small intestine were harvested for DNA analysis by real-time PCR of human albumin. Twenty-four hours after injection of P3 MSC, bone marrow, small intestine, liver and lung tested positive for human albumin in at least one mouse in each group of four (Online Supplementary Table S1). Spleen and kidney were negative in all mice. All mice tested positive in the lungs and at least 10-fold more MSC were detected in the lungs as compared to in other tissues. After 5 weeks bone marrow was positive in all mice, whereas the other organs were all negative. Late passage (P7) MSC showed a similar tissue distribution. Overall, these results suggest that MSC, irrespective of passage number, were first trapped in the lungs before reaching other organs such as small intestine or bone marrow. Only in the bone marrow could MSC survive up to 5 weeks.
Discussion
Recent clinical trials indicate that MSC might be useful to prevent and/or treat graft-versus-host disease and promote donor cell engraftment after hematopoietic stem cell transplantation.21,22 The procedures used to prepare MSC for clinical applications are based on enrichment of MSC present in bone marrow mononuclear cells by plastic adherence, followed by ex vivo expansion in selected serum-containing media.23 The duration of the ex vivo expansion step is highly variable and depends on the ratio between the number of MSC in the initial bone marrow inoculum and the target number of MSC to infuse to the patient, i.e., on the number of doublings. It has been described that the capacity of MSC to engraft in recipient bone marrow declines with increasing passage number.13 Whether the hematopoiesis-supporting activity of MSC is modified with culture duration had not been studied before.
In the present study, cultures of cord blood CD34+ cells were set up in contact with feeder cells consisting of MSC harvested after two to ten passages. Cells were plated in the absence of exogenous cytokines which might otherwise blunt changes in the hematopoiesis-supporting properties of MSC. Our main results indicate a progressive decline in the output of colony-forming cells by CD34+ cells cultured with MSC of increasing passage. Likewise, the outgrowth of differentiated myeloid CD33+ and CD11b+ cells was reduced when MSC of late passages were used. In addition, ex vivo maintenance of SCID repopulating cells (SRC) was reduced when MSC had already undergone more than five passages, which represents approximately 10 to 12 doublings. In contrast, MSC support of lymphoid CD19+ and CD10+ cell outgrowth was not dependent on the number of passages that the mesenchymal cells had undergone. When the engraftment-promoting activity of MSC was assayed by co-transplantation with uncultured CD34+ cells, it was also observed that late passage MSC (>9 passages or 25 doublings) were inferior compared to early passage MSC.
A detailed characterization of MSC preparations revealed only minor changes with passage number. The phenotype of MSC preparations was stable up until P10, as was the rate of doublings. Differentiation into fat, bone and cartilage was also conserved. The types of cytokines, adhesion molecules and matrix proteases detected in MSC-conditioned medium were similar in all tested passages. However, quantitative measurements of the two most abundant cytokines produced by MSC, i.e. IL-6 and IL-8, revealed a progressive decline of IL-8 secretion during culture while IL-6 secretion was increased. Inhibition experiments showed that both cytokines contributed to the outgrowth of CD34+, CD19+ and CD33+ cells in cultures supported by low passage MSC, but not by late passage MSC. Interestingly, inhibition of IL-8, but not of IL-6, was also associated with decreased maintenance of SRC in cultures with P2 MSC. This suggests that the level of IL-8 may represent a surrogate marker of the hematopoiesis-supporting activity of MSC, while high levels of IL-6, as observed in medium conditioned with late passage MSC, do not contribute to better support of hematopoietic cells. The mechanism by which IL-8 promotes recovery of SRC from in vitro cultures remains to be determined. IL-8 has been reported to stimulate metastasis and angiogenesis in various tumor models.24 Our hypothesis is that IL-8 enhances engraftment of repopulating cells by increasing expression of metalloprotease MMP-2 and consequently migration and infiltration within the bone marrow.25
Our data also indicate the influence of direct contact between MSC and CD34+ cells in co-cultures. Cultures of CD34+ cells and MSC separated in transwells by a semi-permeable filter did not reduce the output of colony-forming cells and SRC, in comparison with output of contact cultures. In contrast, outgrowth of differentiated myeloid and lymphoid cells was massively reduced in non-contact conditions. Thus, direct contact with MSC appears to be important for proliferation, late commitment and differentiation of hematopoietic cells but at the expense of a depletion of the progenitor cell compartment. From a practical standpoint for the clinical use of CD34+ cells/MSC co-cultures, non-contact conditions might allow for effective colony-forming cell expansion while limiting the generation of mature cells.
Our study also reveals the strong pro-lymphopoietic activity of MSC. In vitro B-cell generation was previously reported to depend on specific murine stromal cell lines.20 In our study and as recently reported by Ichii et al.,4 MSC were able to support the production of B-cell progenitors and immature IgM+ B cells, in serum-containing medium, in the absence of exogenous cytokines. We extend these findings by showing, by quantitative PCR analysis, the expression of TdT, RAG-1, VpreB, Pax-5 and EBF in outgrown B cells, but not in input CD34+ cells. Thus, this co-culture system recapitulates B-cell ontogeny up to immature B cells and could be further used to delineate extrinsic signals implicated in human B-cell development. The pro-lymphopoietic activity of MSC is not affected by prolonged ex vivo culture, up to 30 doublings. The generation of B cells by co-culture of CD34+ cells and MSC opens up new therapeutic possibilities. Whether MSC co-transplantation in patients undergoing hematopoietic stem cell transplantation can be used to hasten B-cell regeneration could be studied in future clinical trials. Enhancement of in vitro maturation of CD34+ cells to B lymphocytes by MSC could also have implications in the design of adoptive immunotherapy procedures.26
In conclusion, our study shows that prolonged ex vivo culture of bone marrow MSC is associated with decreased support of repopulating stem cells and myeloid progenitors, while the supportive activity toward B lymphoid progenitors is maintained. These changes are not accompanied by alterations in the phenotype or the differentiating capacity of MSC. An important implication is that current quality control procedures used in clinical cell therapy laboratories and based on phenotype analysis do not accurately reflect the biological properties of MSC preparations. Our data also imply that MSC prepared for clinical trials should be passaged as little as necessary.
Acknowledgments
flow cytometric data were acquired on the Cell Imaging and Flow Cytometry facility at GIGA-Research, University of Liège, under the supervision of Dr Sandra Ormenese.
Footnotes
Funding: this work was supported by grants from the Fonds National de la Recherche Scientifique (F.N.R.S., Belgium), the Centre Anticancéreux près l’Université de Liège and the Fédération Belge contre le Cancer (a non-profit organization). A.B. was supported by a Télévie fellowship. Y.B. is Research Director of the F.N.R.S.
The online version of this article has a supplementary appendix.
Authorship and Disclosures
AG was the principal investigator and takes primary responsibility for the paper. AB, SD, SB and MD collected the data. AB and AG analyzed the data and wrote the paper. YB provided study materials. AG and YB coordinated the research.
The authors reported no conflict of interest.
References
- 1.Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107(5):1878–87. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 2.Wagner W, Wein F, Roderburg C, Saffrich R, Faber A, Krause U, et al. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp Hematol. 2007;35(2):314–25. doi: 10.1016/j.exphem.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Huang GP, Pan ZJ, Jia BB, Zheng Q, Xie CG, Gu JH, et al. Ex vivo expansion and transplantation of hematopoietic stem/progenitor cells supported by mesenchymal stem cells from human umbilical cord blood. Cell Transplant. 2007;16(6):579–85. doi: 10.3727/000000007783465073. [DOI] [PubMed] [Google Scholar]
- 4.Ichii M, Oritani K, Yokota T, Nishida M, Takahashi I, Shirogane T, et al. Regulation of human B lymphopoiesis by the transforming growth factor- b-superfamily in a newly established coculture system using human mesenchymal stem cells as a supportive microenvironment. Exp Hematol. 2008;36(5):587–97. doi: 10.1016/j.exphem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 5.da Silva CL, Goncalves R, Crapnell KB, Cabral JMS, Zanjani ED, Almeida-Porada G. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005;33(7):828–35. doi: 10.1016/j.exphem.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Fei XM, Wu YJ, Chang Z, Miao KR, Tang YH, Zhou XY, et al. Co-culture of cord blood CD34(+) cells with human BM mesenchymal stromal cells enhances short-term engraftment of cord blood cells in NOD/SCID mice. Cytotherapy. 2007;9(4):338–47. doi: 10.1080/14653240701291638. [DOI] [PubMed] [Google Scholar]
- 7.Chan SL, Choi M, Wnendt S, Kraus M, Teng E, Leong HF, et al. Enhanced in vivo homing of uncultured and selectively amplified cord blood CD34+ cells by cotransplantation with cord blood-derived unrestricted somatic stem cells. Stem Cells. 2007;25(2):529–36. doi: 10.1634/stemcells.2005-0639. [DOI] [PubMed] [Google Scholar]
- 8.Van Overstraeten-Schlogel N, Beguin Y, Gothot A. Role of stromal-derived factor-1 in the hematopoiesis-supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76(6):488–93. doi: 10.1111/j.1600-0609.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 9.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, et al. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31(5):413–20. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 10.Noort WA, Kruisselbrink AB, in’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, et al. Mesenchymal stem cells promote engraftment of human umbilical cord bloodderived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30(8):870–8. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 11.in ‘t Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, et al. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31(10):881–9. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 12.Shi M, Li J, Liao L, Chen B, Li B, Chen L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):872–7. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakou C, Rabin N, Pizzey A, Nathwani A, Yong K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93(10):1457–65. doi: 10.3324/haematol.12553. [DOI] [PubMed] [Google Scholar]
- 14.Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68(11):4229–38. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 17.Jones EA, English A, Kinsey SE, Straszynski L, Emery P, Ponchel F, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391–9. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 18.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt9):1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 19.Berardi AC, Meffre E, Pflumio F, Katz A, Vainchenker W, Schiff C, et al. Individual CD34+CD38lowCD19-CD10- progenitor cells from human cord blood generate B lymphocytes and granulocytes. Blood. 1997;89(10):3554–64. [PubMed] [Google Scholar]
- 20.Fluckiger AC, Sanz E, Garcia-Lloret M, Su T, Hao QL, Kato R, et al. In vitro reconstitution of human B-cell ontogeny: from CD34(+) multipotent progenitors to Ig-secreting cells. Blood. 1998;92(12):4509–20. [PubMed] [Google Scholar]
- 21.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 22.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 23.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 24.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8(1):63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 25.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 26.Luo XM, Maarschalk E, O’Connell RM, Wang P, Yang L, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human Blymphocytes. Blood. 2009;113(7):1422–31. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]