Abstract
Background
C-type lectin-like molecule-1 is a transmembrane receptor expressed on myeloid cells, acute myeloid leukemia blasts and leukemic stem cells. To validate the potential of this receptor as a therapeutic target in acute myeloid leukemia, we generated a series of monoclonal antibodies against the extracellular domain of C-type lectin-like molecule-1 and used them to extend the expression profile analysis of acute myeloid leukemia cells and to select cytotoxic monoclonal antibodies against acute myeloid leukemia cells in preclinical models.
Design and Methods
C-type lectin-like molecule-1 expression was analyzed in acute myeloid leukemia cell lines, and in myeloid derived cells from patients with acute myeloid leukemia and healthy donors. Anti-C-type lectin-like molecule-1 antibody-mediated in vitro cytotoxic activity against acute myeloid leukemia blasts/cell lines and in vivo anti-cancer activity in a mouse xenograft model were assessed. Internalization of C-type lectin-like molecule-1 monoclonal antibodies upon receptor ligation was also investigated.
Results
C-type lectin-like molecule-1 was expressed in 86.5% (45/52) of cases of acute myeloid leukemia, in 54.5% (12/22) of acute myeloid leukemia CD34+/CD38− stem cells, but not in acute lymphoblastic leukemia blasts (n=5). Selected anti-C-type lectin-like molecule-1 monoclonal antibodies mediated dose-dependent complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity specifically against acute myeloid leukemia-derived cell lines. Exogenous expression of the transmembrane receptor in HEK293 cells rendered the cells susceptible to antibody-mediated killing by monoclonal antibodies to the receptor. Furthermore, these monoclonal antibodies demonstrated strong complement-dependent cytotoxicity against freshly isolated acute myeloid leukemia blasts (15/16 cases; 94%). The monoclonal antibodies were efficiently internalized upon binding to C-type lectin-like molecule-1 in HL-60 cells. Moreover, a lead chimeric C-type lectin-like molecule-1 monoclonal antibody reduced the tumor size in xenograft mice implanted with HL-60 cells.
Conclusions
Our results demonstrate that targeting C-type lectin-like molecule-1 with specific cytotoxic monoclonal antibodies is an attractive approach which could lead to novel therapies for acute myeloid leukemia.
Keywords: C-type, lectin-like molecule-1, immunotherapy, acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia affecting adults and its incidence increases with age. The prognosis of AML is poor, primarily because of the relapses occurring on conventional chemotherapy regimens. The overall 5-year leukemia-free survival rate is only 25–35% and even lower in patients over 60 years old.1–3 Alternative therapeutic strategies, such as using FLT3 tyrosine kinase inhibitors in combination with chemotherapy in patients positive for the FLT3 mutation, are currently being evaluated.4,5
Therapies using monoclonal antibodies to eliminate leukemic cells provide a promising targeted approach for the treatment of AML. Gemtuzumab ozogamicin is a toxin-conjugated monoclonal antibody that binds to CD33 on myeloid leukemia blasts and is approved for treatment of patients over 60 years old with relapsed AML. The overall response rate to this product is about 30% in clinical studies.6,7 Better treatments are clearly still needed.3,4
Human C-type lectin-like molecule-1 (CLL-1), also known as MICL or CLEC12A, is a type II transmembrane glycoprotein and member of the large family of C-type lectin-like receptors involved in immune regulation. CLL-1 has previously been identified from myeloid-derived cells by four different groups.8–11 The intracellular domain of CLL-1 contains an immunotyrosine-based inhibition motif (ITIM) and a YXXM motif. Phosphorylation of ITIM-containing receptors on a variety of cells results in inhibition of activation pathways through recruitment of protein tyrosine phosphatases SHP-1, SHP-2 and SHIP.12 The YXXM motif has a potential SH2 domain-binding site for the p85 subunit of PI-3 kinase,13 which has been implicated in cellular activation pathways, revealing a potential dual role of CLL-1 as an inhibitory and activating molecule on myeloid cells. Indeed, association of CLL-1 with SHP-1 and SHP-2 has been demonstrated experimentally in transfected8 and myeloid-derived10 cell lines.
The pattern of expression of CLL-1 in hematopoietic cells is restricted; it is found in particular in myeloid cells derived from peripheral blood and bone marrow, as well as in the majority of AML blasts.9 A recent study indicated that CLL-1 is also present on the majority of leukemic stem cells in the CD34+/CD38− compartment in AML but absent from CD34+/CD38− cells in normal and in regenerating bone marrow controls, which aids the discrimination between normal and leukemic stem cells.14
Here we describe the generation of specific monoclonal antibodies against CLL-1 and the use of these antibodies in detailed expression analyses of normal and AML samples as well as in functional studies of this target for potential immunotherapy in in vitro and in vivo assays.
Design and Methods
Antibody generation
Hybridomas were generated using standard protocols.15 In brief, 6-week old Balb/c mice were immunized with 100 μg of the purified recombinant protein in Freund’s adjuvant. After three bi-weekly boosts in incomplete Freund’s adjuvant, titers were assessed and spleen cells fused 3 days after a last boost in saline. Sp2/0 cells were used as the fusion partner. Hybridomas were selected and supernatants from the resulting clones were screened by enzyme linked immunosorbent assay (ELISA) and fluorescent activated cell sorting (FACS). Monoclonal antibodies were purified using standard protein A columns (GE Healthcare, Piscataway, NJ, USA).
Screening of hybridoma supernatants for binding to C-type lectin-like molecule-1
ELISA for binding to CLL-1 was performed using standard techniques.16 The secondary goat anti-mouse immunoglobulin (Ig)-horseradish peroxidase antibody was from Bio-Rad (Hercules, CA, USA) (#170–6516) and tetramethylbenzidine substrate from KPL (Gaithersburg, VA, USA) (#50-76-03). Plates were read on a Spectramax plate reader (Molecular Devices, Sunnyvale, CA, USA). Flow cytometry was performed using standard protocols.17 Secondary antibody was from BD Pharmingen (San Diego, CA, USA) (goat anti-mouse phycoerythrin-conjugated, #550589). Cells were analyzed using an Automated Microsampler (Cytek, Fremont, CA, USA) attached to a FACScalibur (Becton Dickinson, San Jose, CA, USA).
Chimeric monoclonal antibody generation
RNA was isolated from hybridoma fusion cells expressing the anti-IREM-1 monoclonal antibody of interest. Using standard rapid amplification of cDNA ends (RACE)/reverse transcriptase polymerase chain reaction (RT-PCR) techniques, the heavy and light variable regions were cloned into two separate expression vectors in fusion with cDNA encoding for human IgG1 constant regions. The resulting plasmids were co-transfected into Chinese hamster ovary cells and stable cell lines were selected secreting full-length chimeric monoclonal antibodies.
Real-time binding analysis
Surface plasmon resonance was carried out on a Biacore (Piscataway, NJ, USA) system. Monoclonal antibodies were diluted to 2 μg/mL and then captured on the biosensor surface using an anti-mouse monoclonal antibody. Antigen was diluted to a starting concentration of 46 nM and tested for binding to the monoclonal antibody samples using a 3-fold dilution series. Each of five concentrations was tested twice except for the highest concentration which was tested five times in total: twice with a short dissociation of 300 s and then three times with a dissociation of 60 min. The data sets from the long dissociation experiments were fitted with those of the shorter association experiments to determine binding constants for the interactions. The analysis was carried out in HBS buffer, pH 7.4, at 25°C.18
Flow cytometric determination of expression of C-type lectin-like molecule-1
QIFIKIT (K0078 from Dako) was used, according to the manufacturer’s instruction, for the quantitative determination of the number of receptors per cell. Before use, cells were blocked for 10 min in 100 μL of 10% heat inactivated human serum.
Cell lines and western blotting
Cell lines were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and DSMZ (Braunschweig, Germany) and maintained in their recommended medium. Lysates were prepared and 25 μg of total lysate were analyzed by western blotting. Secondary antibody (goat anti-mouse Ig-horseradish peroxidase, BioRad #170–6516) was used and membranes were developed using Pierce’s ECL western blot substrate (#32209) (Rockford, IL, USA).
Flow cytometry staining of normal specimens and patients’ samples
Clinical samples were obtained from patients diagnosed at the Cleveland Clinic and the Stanford Cancer Center after informed consent. In order to determine the expression of CLL-1 on patients’ samples or normal bone marrow aspirate, four-color staining with CLL-1 Alexa488/CD34PE-CD45PerCP/CD38APC was performed. For normal blood specimens, immunophenotyping was performed with a CLL-1 Alexa488/CD14-16PE/CD45PerCP/CD33APC panel. IgG-Alexa488 was used as a control for all of the above tests. Expression of CD33 was analyzed with the CD33FITC/CD34PE/CD45PerCP/CD38APC panel and IgG-fluorescein isothiocyanate was used as a control. Details of the staining process have been described elsewhere.19 Blasts were gated based on low side scatter versus CD45dim expression. A sample was considered positive for CLL-1 or CD33 if the ratio of the geometric mean fluorescence intensity (MFI) of the stained sample and that of isoform IgG control was greater than two and more than 20% of the cells expressed the antigen compared with the control sample. All antibodies used for flow cytometry were from BD Biosciences except for CLL-1 Alexa488 and IgG Alexa488 which were prepared in house using Invitrogen’s Alexa Fluor 488 monoclonal antibody labeling kit according to the instructions.
Tissue microarray staining of C-type lectin-like molecule-1
Tissue microarray slides were stained using a Benchmark XT platform (Ventana Medical System, Inc., Tucson, AZ, USA), including online deparaffinization without antigen retrieval. Mouse monoclonal antibody to CLL-1 was diluted to 2.5 μg/mL and incubated on section for 1 h. An Ultraview DAB kit (Ventana) was used for the detection of the antibody.
Complement-dependent cytotoxicity assay
Fresh peripheral blood or bone marrow aspirate from AML patients was used to obtain blasts through Ficoll-Paque gradient separation. In most AML cases, enriched blasts accounted for more than 85% of the total cell population, according to flow cytometry assays. Cell lines were used during the log phase of growth: 105 cells were suspended in 50 μL complete RPMI media and plated in a 96-well plate, 50 μL of 2 × antibody/IgG isotype, made up in same medium, were added to each well and the plates were left at room temperature for 15 min. Next, 2.5 μL of freshly prepared baby rabbit complement (CL3441; Cedarlane labs, Burlington, NC, USA) was added to the wells followed by incubation at 37°C for 1 h. After equilibrating plates to room temperature, cell viability was analyzed using Cell Titer Glo (G7571; Promega, Madison, WI) and luminescence was measured with a Victor 1420 Multilabel Counter (Perkin Elmer Life Science). Tests were conducted in triplicate for each group and data were normalized to the results for complement plus isotype.
Antibody-dependent cell cytotoxicity
The effector cells were human natural killer cells isolated from buffy coat (purchased from Stanford blood center, Palo Alto, CA, USA) by negative selection using Rosette Sep NK cell enrichment cocktail from Stem Cell Technologies, according to the manufacturer’s instructions. Specific lysis of target cells was determined by using a standard 4 h 51Cr release assay in a 96-well plate format as previously described.20 The target: effector ratio used was typically 1:40. No pre-incubation of effector cells and antibody was performed. Percentage lysis was calculated using the following standard equation:
where TEST is sample release of 51Cr, BGD is spontaneous release and Max. is Triton X mediated release.
The effector control was subtracted to obtain the percentage specific lysis.
Internalization assessed by immunofluorescence microscopy and flow cytometry
For immunofluorescence microscopy experiments, CLL-1-expressing HEK 293 cells were seeded onto 12-mm glass cover-slips coated with poly-L-lysine (Sigma-Aldrich, St. Louis, MO, USA). The next day, the cells were preincubated in phosphate-buffered saline (PBS)-10% human serum for 15 min before incubation for 1 h at 4°C with 10 μg/mL of Alexa-488-conjugated monoclonal antibody 1075.7 or Alexa-488 control isotype antibody in PBS-2.5% human serum. After three washes with ice-cold FACS buffer (PBS-1% bovine serum albumin), cells were incubated at 37°C (in 5% CO2 and air) in PBS-5% fetal bovine serum for up to 2 h to allow internalization. Cells were then washed with ice-cold PBS, fixed with 4% paraformaldehyde and mounted onto slides with Prolong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA, USA). The stained specimens were examined using a Zeiss Axiovert 200 microscope with X40 oil objective (Zeiss, Thornwood, NY, USA). For flow cytometry experiments, HL-60 cells were preincubated with ice-cold PBS-10% human serum, followed by incubation for 40 min at 4°C with saturating amounts of Alexa-488-conjugated monoclonal antibody 1075.7, phycoerythrin-labeled anti-CD44 antibody (negative control, clone 515, BD Biosiences, San Jose, CA, USA) or their corresponding isotype control antibodies. After three washes in ice-cold FACS buffer, cells were resuspended in 100 μL of RPMI-10% FBS-PS and incubated either at 4°C (no internalization) or at 37°C for different times. After washing, cell-surface-bound antibody complexes were stripped from the cells using 50 μL of Qiagen protease (QP, Qiagen #19155) at 5 AU/mL in cold PBS for 30 min at 4°C. Cells were washed three times with ice-cold FACS buffer with 0.02% NaN3, fixed in PBS-1% formaldehyde and analyzed by flow cytometry using the FACS Calibur system. The background mean fluorescence intensity (MFI) determined with cells incubated with the labeled isotype control antibodies was subtracted for each time point. The specific MFI at time 0 in the absence of protease treatment was normalized to 100% and the relative specific MFI for each time point was calculated using the formula: 100* (specific MFI antibody + QP Tx)/(specific MFI antibody − QP Tx).
HL-60 xenograft
The xenograft HL-60 model in BALB/c SCID mice has been described previously.21 The animal study protocols were reviewed and approved by the Institutional Animal Care and Use Committee according to governmental guidelines for animal welfare.
All of the mice were acclimated before use. Xenografts were allowed to establish to an average size of 50–100 mm3, after which mice were randomized into various groups (10 animals per group). Monoclonal antibodies, at the designated dose, were given to each mouse via intraperitoneal injection twice a week. Tumor size was measured by measuring the animal’s size with a caliper on alternate days. Animal body weight and any sign of morbidity were also closely monitored. The monoclonal antibody treatment lasted for 2 to 3 weeks at which point the mice were euthanized, the tumor xenografts extirpated and weighed, and the weight of the tumors correlated with the tumor size measurements. A log-rank (Mantel-Cox) test was used for the statistical analysis.
Results
Generation of monoclonal antibodies against C-type lectin-like molecule-1
A comprehensive analysis of the expression of putative cell surface proteins allowed us to identify several genes encoding for receptors that were differentially expressed in AML disease samples. Using this approach we identified a member of the C-type lectin-like receptor family, CLL-1, which showed a myeloid-restricted expression pattern. To further characterize the cell surface expression and function of CLL-1, we generated recombinant protein spanning the extracellular domain of CLL-1 and used it to immunize mice to generate monoclonal antibodies. We generated approximately 500 hybridoma clones recognizing CLL-1 protein by ELISA and 275 of these monoclonal antibodies recognized cell surface expression of CLL-1 on the myeloid cell line HL-60. To select lead monoclonal antibodies we determined affinity, isotype and cytotoxic activity. Lead antibodies (21.16 and 1075.7) were selected and further characterized and subsequently used in detailed expression analysis and functional studies.
Affinity of lead C-type lectin-like molecule-1 monoclonal antibodies
After confirming the binding of the lead monoclonal antibodies to CLL-1 on the surface of myeloid cells, the affinity of the antibodies for CLL-1 was measured using surface plasmon resonance. Anti-CLL-1 monoclonal antibodies were captured using anti-mouse Fc antibody and recombinant extra-cellular domain of CLL-1 was allowed to bind to the monoclonal antibody at various concentrations. The kinetic rate constants (ka and kd) of this reaction were determined and used to calculate the KD values of our lead monoclonal antibodies, 21.16 (15.3 nM) and 1075.7 (0.36 nM).
Expression of C-type lectin-like molecule-1 in myeloid cells
We first surveyed the expression of CLL-1 in a wide variety of AML cell lines derived from AML patients with different disease subtypes. CLL-1 was expressed at variable levels on myeloid cell lines, as detected by either flow cytometry or western blotting analysis. The number of receptors ranged between 0 and 100,000 CLL-1 molecules per cell as determined by quantitative flow cytometry (Online Supplementary Figure S1A). CLL-1 expression was not detected in cell lines from lymphoid origin (data not shown).
It was previously reported that CLL-1 is expressed on the surface of cells of myeloid lineages.9 We used our specific CLL-1 monoclonal antibodies to further analyze the expression of CLL-1 in peripheral blood leukocytes and bone marrow and extended the analysis to samples from patients with AML (Online Supplementary Figure S1).
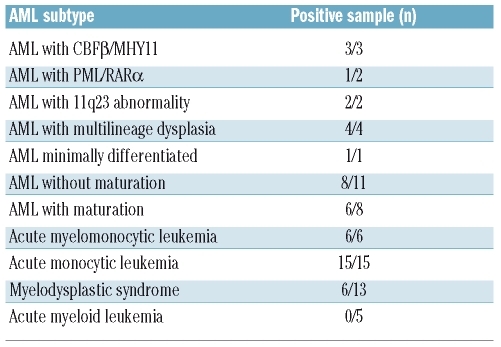
Peripheral blood and bone marrow samples from 52 AML patients with a variety of disease subtypes were analyzed (summarized in Table 1). CLL-1 expression was detected in 86.5% of AML cases (45/52) with the range of positive blasts being 23–99% (mean 69.6%); the incidence in myelomonocytic and monocytic AML was 100% (21/21). Immunohistochemical staining of bone marrow clots for CLL-1 in a tissue microarray AML panel showed positive staining in 37 out of 38 cases (Online Supplementary Figure S1C). In contrast, CLL-1 expression was not detected in blasts from five patients with acute lymphocytic leukemia (data not shown). Next, we analyzed CLL-1 expression in the CD34+/CD38− stem cell compartment. Sufficient CD34+/CD38− leukemia stem cells were collected from 22 AML cases and CLL-1 was detected on stem cells in 12 of these cases (54.5%, summarized in Online Supplementary Figure S1F). CD34+ progenitor cells bone marrow specimens that were negative for lymphoma (n=8) expressed CLL-1 in two cases (23% and 59% positive cells). We also tested CD34+/CD38− bone marrow derived stem cells from five morphologically normal samples and found that one case had more than 20% cells positive for CLL-1.
Table 1.
CLL-1 expression in blasts of patients with acute myeloid leukemia, myelodysplastic syndrome and acute lymphocytic leukemia.
Additionally, we surveyed CLL-1 expression in bone marrow aspirate or peripheral blood from 13 patients with myelodysplastic syndromes (MDS) and found that the receptor was expressed in six cases (46.2%) with the range of positive blasts being 21–85% (mean 49.8%) (data not shown). Among these 13 patients with MDS, one with refractory anemia and all five with refractory anemia with excess blasts-1 were negative for CLL-1 expression, while two of three with refractory anemia with excess blasts-2 were positive, and each of the one patients with MDS/myeloproliferative disease, therapy-related MDS, MDS disease with 5q(−) syndrome and MDS - unclassifiable were all positive. CD34+/CD38− cells from two of these patients (the ones with therapy-related MDS and 5q-syndrome) were negative for CLL-1.
We next compared the expression pattern of CLL-1 with that of the myeloid antigen CD33. As previously reported, monocytes expressed the highest levels of CD33, while lower expression was observed in granulocytes, and no expression could be detected in lymphocytes regardless of whether the specimens were obtained from healthy controls or AML patients.22 The pattern of expression of CLL-1 was similar to that of CD33 in normal fresh peripheral blood cells (Online Supplementary Figure S2A). Similarly to CLL-1, CD33 was detected in AML blasts of 44/47 (93.6%) cases tested in this study, while in the CD34+/CD38− cell population of the five cases tested, two and three cases exhibited more than 20% cells positive for CD33 and CLL-1 expression, respectively (Online Supplementary Figure S2B).
These studies confirm the restricted expression of CLL-1 in cells from myeloid origin, in the majority of AML blasts and in a subset of CD34+/CD38− leukemia stem cells, highlighting the potential of CLL-1 as a target for antibody-based therapeutics in the treatment of myeloid leukemia.
Specific complement-dependent cytotoxicity of antibodies against C-type lectin-like molecule-1
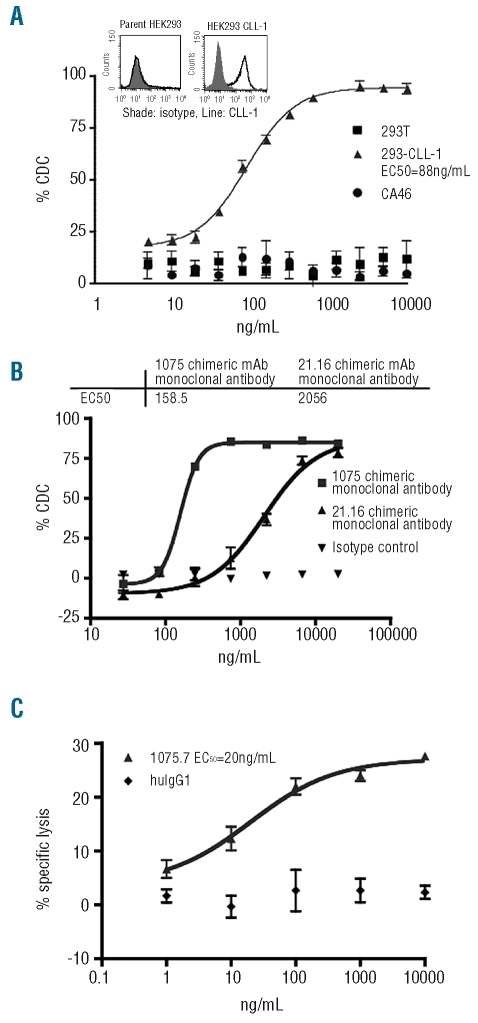
To assess the therapeutic potential of anti-CLL-1 monoclonal antibodies we measured their cytotoxic activity. We first characterized complement-dependent cytotoxicity (CDC) against the AML-derived cell line OCI-AML-5 which expresses CLL-1. Lead monoclonal antibodies against CLL-1 displayed dose-dependent CDC and eliminated over 80% of target cells at doses of 100 ng/mL and higher (results not shown). To determine whether the cytotoxic activity of the anti-CLL-1 monoclonal antibodies was mediated through a direct interaction with CLL-1 on the cell membrane, CDC assays were performed against a surrogate cell line. The CLL-1-negative HEK293 cell line was transfected to express CLL-1 stably. The CLL-1 monoclonal antibody showed CDC against the HEK293-CLL-1 cells but not against the HEK293 parental line (Figure 1A), indicating that this CDC is mediated specifically through CLL-1. In addition, the Burkitt’s lymphoma B-cell line CA46, which does not express CLL-1, was insensitive to cytotoxicity induced by anti-CLL-1 monoclonal antibodies (Figure 1A).
Figure 1.
Complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) of CLL-1 monoclonal antibodies. (A) CDC assay using human embryonic kidney (HEK) 293 cells stably expressing CLL-1 compared to wild-type HEK293 cells and lymphoma CA46 cells. Expression of CLL-1 on 293 parental and transfectants is represented by the histograms in the top left corner. (B) Ex vivo CDC of chimeric monoclonal antibody 1075.7 against freshly isolated AML blasts. (C) ADCC activity of anti-CLL-1 chimeric monoclonal antibody 1075.7 against HL-60 cells.
To further explore the therapeutic potential of CLL-1 monoclonal antibodies, we performed CDC assays on fresh AML blasts obtained from AML patients. In these ex vivo experiments, the mouse monoclonal antibody showed cytotoxic activity against human CLL-1 in 15 of 16 (94%) CLL-1-positive samples tested, with the effect being dose-dependent; the proportion of lysed cells ranged between 25 and 85%. In contrast, acute lymphocytic leukemia blasts lacking CLL-1 were insensitive to CDC (data not shown). To further study the lead CLL-1 monoclonal antibodies for AML therapeutics, two chimeric monoclonal antibody constructs were generated by fusing the 1075.7 and 21.16 variable sequences to human IgG1 constant region sequences. Recombinant chimeric monoclonal antibodies were expressed in and purified from Chinese hamster ovary cells and the activities of the mouse and chimeric antibodies were compared. Similarly to their mouse counterparts, both chimeric monoclonal antibodies were capable of mediating dose-dependent CDC against freshly isolated AML blasts, as determined in five samples expressing CLL-1. The EC50 from a representative experiment with the chimeric monoclonal antibodies 1075.7 and 21.16 were 158.5 ng/mL and 2056 ng/mL, respectively (Figure 1B).
Antibody-dependent cellular cytotoxicity of C-type lectin-like molecule-1 monoclonal antibody
Antibody-dependent cellular cytotoxicity (ADCC) is an important mechanism of killing cancer cells in vivo. To evaluate the ability of anti-CLL-1 chimeric antibody to elicit ADCC, HL-60 cells were labeled with 51Cr and incubated with freshly isolated human natural killer cells in the presence of chimeric monoclonal antibody. A dose-dependent ADCC-mediated lysis was observed when using the chimeric monoclonal antibody 1075.7, whereas an isotype control monoclonal antibody showed no detectable activity (Figure 1C). Nearly 30% of cells were lysed with an EC50 of 20 ng/mL.
These results indicate that CLL-1 monoclonal antibodies are potent and selective cytotoxic agents in vitro and ex vivo against CLL-1-expressing cells and exhibit potential as targeted anti-cancer agents, possibly through a combination of multiple mechanisms.
Internalization of C-type lectin-like molecule-1
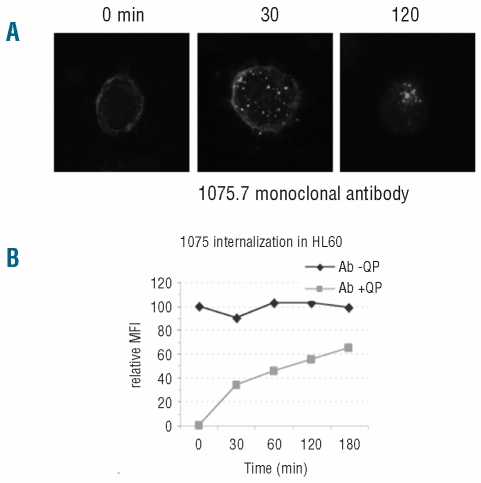
While the CLL-1 chimeric monoclonal antibodies were effective in cytotoxic assays, we also considered a toxin conjugation approach to inflict cell death upon antigen-mediated toxin uptake. To investigate the potential of a CLL-1 antibody-drug conjugate we first determined whether CLL-1 monoclonal antibodies trigger receptor internalization. HEK293 cells stably expressing CLL-1 were stained with fluorescent labeled mouse antibody 1075.7 and then incubated at 37°C for up to 2 h, fixed and visualized by fluorescence microscopy. As shown in the left panel of Figure 2A, incubation of the cells with Alexa488-1075.7 monoclonal antibody resulted in a clear membrane-bound staining pattern. However, distinct intracellular punctate staining was detected after 30 min of incubation at 37°C, indicating rapid internalization of the receptor-antibody complex (Figure 2A, middle panel). The staining pattern became increasingly intracellular over time, with most of the antibody detected inside the cells after 2 h (Figure 2A, right panel).
Figure 2.
Internalization of CLL-1 upon monoclonal antibody binding. (A) HEK293 cells stably expressing CLL-1 were stained with fluorescently labeled CLL-1 mouse monoclonal antibody 1075.7, incubated for the indicated times at 37°C, fixed and examined using fluorescence microscopy. (B) CLL-1 internalization in HL-60 cells. After staining with monoclonal antibody 1075.7, cells were incubated for the indicated times at 37°C after which they were (squares) or were not (diamonds) subjected to protease treatment prior to flow cytometry analysis. The staining observed in the protease-treated cells represents only intracellular staining.
Flow cytometry-based assays were performed to measure internalization rates in AML-derived cell lines. After staining HL-60 cells with fluorescent-labeled 1075.7 antibody and incubating them at 37°C, the cells were treated with protease and the signal associated with a protease-resistant compartment was measured over time by flow cytometry. Approximately 60% of anti-CLL-1 monoclonal antibody 1075.7 became internalized within 3 h of incubation, while an anti-CD44 control monoclonal antibody was not (Figure 2B and results not shown). These data confirm the internalization of CLL-1 upon engagement with anti-CLL-1 monoclonal antibodies9 and the potential to design an antibody-drug conjugate for this target.
Anti-tumor activity in a mouse xenograft model
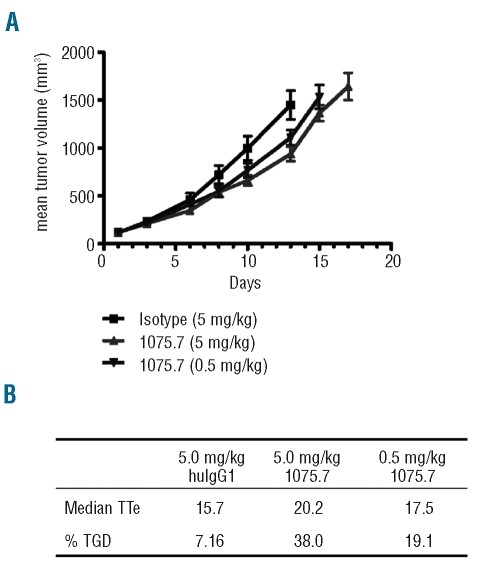
To further evaluate the potential in vivo anti-tumor effect of the 1075.7 chimeric anti-CLL-1 monoclonal antibody, it was tested in immunodeficient mice bearing HL-60 xenografts. HL-60 cells were inoculated into the flank of SCID mice and treated with the 1075.7 antibody after tumors had been established. These rapidly growing tumors were measured twice weekly and a dose-dependent delay in tumor growth was observed in mice treated with the chimeric CLL-1 monoclonal antibody compared to the growth in mice treated with an isotype control chimeric antibody (Figure 3A). Tumor growth delay was calculated using an end-point of tumor size reaching 2000 mm3. The time to end-point in animals given control chimeric human IgG1 (5.0 mg/kg) was 15.7 days while that in animals treated with the chimeric 1075.7 antibody was significantly increased to 20.2 days (P<0.05). At a dose of 0.5 mg/kg, chimeric 1075.7 also produced a detectable prolongation in the time to end-point (17.5 days). In terms of tumor growth delay as a percentage, compared to a saline-treated control group, chimeric 1075.7 delayed tumor growth by 38% and 19.1% at the doses of 5 and 0.5 mg/kg, respectively (Figure 3B). These data validate the efficacy of anti-CLL-1 monoclonal antibody 1075.7 as an inhibitor of myeloid leukemia cell growth in vivo.
Figure 3.
In vivo anti-tumor activity of anti-CLL-1 chimeric monoclonal antibody 1075.7 in HL-60 cell-inoculated SCID mice. Animals were dosed twice weekly with the indicated amount of 1075.7 monoclonal antibody upon establishment of subcutaneous tumors. (A) Growth curves of control and experimental treatments as measured 3 times/week. (B) Summary of HL-60 xenograft tumors treated with chimeric monoclonal antibody 1075.7 at 0.5 and 5 mg/kg. TTE=time to end-point in days, TGD=tumor growth delay as percentage compared to growth in animals administered saline control treatment.
Discussion
Selective expression of a cell surface antigen on target cells provides the opportunity for antibody-based therapy for both leukemias and solid tumors. Here, we identified CLL-1 as a potential receptor expressed on normal myeloid and leukemic blast cells. We generated a series of monoclonal antibodies against CLL-1 and selected two lead antibodies with high affinity and strong cytotoxic activity. Flow cytometry and western blot analysis of CLL-1 in normal and patients’ samples revealed that the expression of CLL-1 is restricted to cells from myeloid origin. We generated murine and chimeric monoclonal antibodies against CLL-1, demonstrated their specificity and evaluated their anti-cancer activity against AML cell lines and primary blasts in various in vitro, ex vivo and in vivo models.
Consistent with previously published work,9,14 we confirmed that CLL-1 is expressed on the cell surface of the majority of malignant AML blasts (86.5%) as well as in the majority of CD34+/CD38− leukemic stem cells (54.5%). Based on our flow cytometry assays, there is a very high incidence of CLL-1 expression in more mature acute myelomonocytic/monocytic leukemia (100%, Table 1). The immunohistochemical staining of bone marrow clots in a tissue microarray AML panel further demonstrated that CLL-1 is an abundant cell surface protein in AML blasts (97.3%). Our preliminary studies also indicated that CLL-1 is expressed in blasts of a subset of cases of MDS (46.2%), a disease with few effective treatment options, although six cases of refractory anemia and refractory anemia with excess blasts-1 (<9% blasts) were all negative for CLL-1. Further studies are needed to address whether the pattern of expression of CLL-1 differs in lower risk MDS (< 5% marrow blasts) compared to higher risk MDS and whether its expression changes with the progression of MDS to AML. A further analysis of a large number of MDS samples will be required to fully elucidate CLL-1 expression levels in MDS stem cells.
The expression profile of CLL-1 as presented here and in previous reports,9,14 highlights the potential of CLL-1 as a target for antibody-mediated therapy against AML blasts and possibly CD34+/CD38− leukemic stem cells. We demonstrate for the first time direct cytotoxic and anti-cancer activity of anti-CLL-1 monoclonal antibodies in functional studies using in vitro, ex vivo and in vivo models. Therefore, CLL-1 has potential as a novel therapeutic target for antibody-mediated immunotherapy. Multiple mechanisms, including CDC and ADCC, may be involved in antibody-mediated targeted immunotherapy in vivo.23 One report described the density of CD33 as a limiting factor prohibiting significant induction of ADCC and CDC.24 While anti-CD33-induced cytotoxicity has been demonstrated,25 our novel chimeric anti-CLL-1 monoclonal antibodies described here appeared capable of mediating dose-dependent ADCC and CDC against blasts freshly isolated from all five AML cases tested. Our mouse and chimeric CLL-1 monoclonal antibodies showed CDC in 15 of 16 (94%) AML blasts derived from patients with various subtypes of AML.
Antigen-antibody interaction may cause altered downstream signaling and induce target cells to undergo apoptosis. This has been described for rituximab26 and anti-CD33 monoclonal antibodies27 as an alternative mechanism contributing to the efficacy of antibody-mediated therapy. Although anti-CLL-1-mediated CDC and ADCC were reported here, it is currently unclear whether engagement of CLL-1 with antibody could alter cell signaling in a way that may further contribute to anti-cancer activity against malignant blasts. Since the intracellular domain of CLL-1 contains an immunotyrosine-based inhibition motif (ITIM) and a YXXM motif, it is reasonable to assume that CLL-1 could serve as an inhibitory signaling molecule. The impact of CLL-1/antibody binding and consequent signaling alterations in AML blasts remains to be addressed.
In addition to CDC, ADCC and other potential direct cytotoxic activities, conjugation of CLL-1 monoclonal antibodies to a toxin or drug may represent an alternative strategy for therapeutic intervention in AML. We observed internalization within 30 min of exposure of cells to CLL-1 monoclonal antibodies. Intracellular signaling motifs in CLL-1 include an ITIM, which has been identified as required for internalization of CD3328 and a YXXM motif, which has been shown to be involved in lysosomal targeting of receptors.29 These motifs could be important for the internalization of CLL-1 that we observed in AML cells.
In the in vivo xenograft model described here, we treated established HL-60 tumors with anti-CLL-1 chimeric monoclonal antibodies and observed a significant tumor growth delay of up to 38%. Further work is needed to fully understand the potential of anti-leukemic monoclonal antibodies against CLL-1. Other animal models using cells with higher CLL-1 levels may better represent the situation in patients with (myelo)monocytic leukemia and lead to greater efficacy of anti-CLL-1 monoclonal antibodies. Combination treatment with chemotherapeutic agents or a toxin-conjugated antibody could also enhance the anti-cancer activity.
Taken together, we have shown that the CLL-1 receptor is expressed in AML blasts and the majority of leukemia stem cells and have selected CLL-1 cytotoxic monoclonal antibodies that could be developed for AML therapeutics.
Footnotes
The online version of this article has a supplementary appendix.
Authorship and Disclosures
XZ, SS, CP, JZ and WK performed experiments; XZ, EDH, AA and WK designed the study and wrote and reviewed the manuscript; XZ and EDH received honoraria from Nuvelo, Inc. SS, CP, JZ, AA and WK are employed by Nuvelo, Inc, and declare stock ownership of Nuvelo, Inc.
References
- 1.Laubach J, Rao AV. Current and emerging strategies for the management of acute myeloid leukemia in the elderly. Oncologist. 2008;13(10):1097–108. doi: 10.1634/theoncologist.2008-0100. [DOI] [PubMed] [Google Scholar]
- 2.Leopold LH, Willemze R. The treatment of acute myeloid leukemia in first relapse: a comprehensive review of the literature. Leuk Lymphoma. 2002;43(9):1715–27. doi: 10.1080/1042819021000006529. [DOI] [PubMed] [Google Scholar]
- 3.Ravandi F, Burnett AK, Agura ED, Kantarjian HM. Progress in the treatment of acute myeloid leukemia. Cancer. 2007;110(9):1900–10. doi: 10.1002/cncr.23000. [DOI] [PubMed] [Google Scholar]
- 4.Doepfner KT, Boller D, Arcaro A. Targeting receptor tyrosine kinase signaling in acute myeloid leukemia. Crit Rev Oncol Hematol. 2007;63(3):215–30. doi: 10.1016/j.critrevonc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leuk Lymphoma. 2008;49(5):852–63. doi: 10.1080/10428190801895352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giles F, Estey E, O’Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98(10):2095–104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- 7.Tsimberidou AM, Giles FJ, Estey E, O’Brien S, Keating MJ, Kantarjian HM. The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br J Haematol. 2006;132(4):398–409. doi: 10.1111/j.1365-2141.2005.05872.x. [DOI] [PubMed] [Google Scholar]
- 8.Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem. 2004;279(15):14792–802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- 9.Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64(22):8443–50. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Zhang M, Li N, Chen T, Zhang Y, Wan T, et al. KLRL1, a novel killer cell lectinlike receptor, inhibits natural killer cell cytotoxicity. Blood. 2004;104(9):2858–66. doi: 10.1182/blood-2004-03-0878. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Floyd H, Olson NE, Magaletti D, Li C, Draves K, et al. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood. 2006;107(4):1459–67. doi: 10.1182/blood-2005-08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9(3):338–43. doi: 10.1016/s0952-7915(97)80079-9. [DOI] [PubMed] [Google Scholar]
- 13.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72(5):767–78. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 14.van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659–66. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 15.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J Immunol. 2005;174(5):2453–5. [PubMed] [Google Scholar]
- 16.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons; 2005. [Google Scholar]
- 17.Robinson JP, Darzynkiewicz Z, Dobrucki J, Hyun W, Nolan J, Orfao A, et al. Current Protocols in Cytometry. John Wiley & Sons; 2006. [Google Scholar]
- 18.Canziani GA, Klakamp S, Myszka DG. Kinetic screening of antibodies from crude hybridoma samples using Biacore. Anal Biochem. 2004;325(2):301–7. doi: 10.1016/j.ab.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Korver W, Singh S, Liu S, Zhao X, Yonkovich S, Sweeney A, et al. The lymphoid cell surface receptor NTB-A: a novel monoclonal antibody target for leukaemia and lymphoma therapeutics. Br J Haematol. 2007;137(4):307–18. doi: 10.1111/j.1365-2141.2007.06569.x. [DOI] [PubMed] [Google Scholar]
- 20.Coleman EJ, Brooks KJ, Smallshaw JE, Vitetta ES. The Fc portion of UV3, an anti-CD54 monoclonal antibody, is critical for its antitumor activity in SCID mice with human multiple myeloma or lymphoma cell lines. J Immunother. 2006;29(5):489–98. doi: 10.1097/01.cji.0000210079.52554.c3. [DOI] [PubMed] [Google Scholar]
- 21.Naumovski L, Ramos J, Sirisawad M, Chen J, Thiemann P, Lecane P, et al. Sapphyrins induce apoptosis in hematopoietic tumor-derived cell lines and show in vivo antitumor activity. Mol Cancer Ther. 2005;4(6):968–76. doi: 10.1158/1535-7163.MCT-04-0339. [DOI] [PubMed] [Google Scholar]
- 22.Korver W, Zhao X, Singh S, Pardoux C, Zhao J, Guzman ML, et al. Monoclonal antibodies against IREM-1: potential for targeted therapy of AML. Leukemia. 2009;23(9):1587–97. doi: 10.1038/leu.2009.99. [DOI] [PubMed] [Google Scholar]
- 23.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26(25):3629–36. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 24.Legrand O, Perrot JY, Baudard M, Cordier A, Lautier R, Simonin G, et al. The immunophenotype of 177 adults with acute myeloid leukemia: proposal of a prognostic score. Blood. 2000;96(3):870–7. [PubMed] [Google Scholar]
- 25.Caron PC, Co MS, Bull MK, Avdalovic NM, Queen C, Scheinberg DA. Biological and immunological features of humanized M195 (anti-CD33) monoclonal antibodies. Cancer Res. 1992;52(24):6761–7. [PubMed] [Google Scholar]
- 26.Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24(13):2121–43. doi: 10.1038/sj.onc.1208349. [DOI] [PubMed] [Google Scholar]
- 27.Vitale C, Romagnani C, Puccetti A, Olive D, Costello R, Chiossone L, et al. Surface expression and function of p75/AIRM-1 or CD33 in acute myeloid leukemias: engagement of CD33 induces apoptosis of leukemic cells. Proc Natl Acad Sci USA. 2001;98(10):5764–9. doi: 10.1073/pnas.091097198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105(3):1295–302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 29.Wu H, Windmiller DA, Wang L, Backer JM. YXXM motifs in the PDGF-β receptor serve dual roles as phosphoinositide 3-kinase binding motifs and tyrosine-based endocytic sorting signals. J Biol Chem. 2003;278(42):40425–8. doi: 10.1074/jbc.C300225200. [DOI] [PubMed] [Google Scholar]