Abstract
Background
BCL6 gene rearrangement is the most frequent chromosomal abnormality in diffuse large B-cell lymphoma, a malignancy characterized by genetic heterogeneity and wide variability in clinical outcome. The prognostic significance of BCL6 rearrangement has not been evaluated in the context of rituximab therapy for diffuse large B-cell lymphoma. We analyzed the effect of the BCL6 rearrangement on survival in patients with diffuse large B-cell lymphoma treated with CHOP and CHOP plus rituximab (R-CHOP).
Design and Methods
BCL6 rearrangement status was analyzed by fluorescence in situ hybridization with break-apart probes in 164 patients with diffuse large B-cell lymphoma treated with CHOP (n=65) or R-CHOP (n=99). Cell-of-origin immunophenotype including BCL6 protein expression were determined by immunohistochemistry on a tissue microarray.
Results
BCL6 rearrangement was detected in 19.5% of cases. The presence of the gene rearrangement was associated with a non-germinal center B-cell immunophenotype (P=0.006), and showed no correlation with BCL6 protein expression. A trend toward inferior overall survival was observed in association with the BCL6 rearrangement among patients treated with R-CHOP (P=0.08), but not among patients treated with CHOP (P=0.64). However, BCL6 rearrangement also correlated with a high International Prognostic Index score (P=0.02), and did not demonstrate independent prognostic value by multivariate analysis.
Conclusions
The introduction of rituximab may have altered the prognostic impact of BCL6 gene rearrangement in patients with diffuse large B-cell lymphoma. However, prospective analysis within large randomized clinical trials will be needed to clarify the prognostic significance of this biomarker in the rituximab era.
Keywords: diffuse large B-cell lymphoma, biomarkers, BCL6, FISH, rituximab
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin’s lymphoma, accounting for 30–40% of newly-diagnosed cases.1 Although curable in the majority of cases with anthracycline-based combination chemotherapy and the monoclonal antibody rituximab, approximately 30–40% of patients with DLBCL will relapse after standard first-line therapy.2,3 This variability in clinical outcome likely relates to genetic heterogeneity within DLBCL, reflected in a wide array of cytogenetic and molecular abnormalities. Recently, microarray gene expression studies have identified multiple genes of potential prognostic significance in DLBCL, and have led to the subdivision of DLBCL into two major biological categories based on their presumed cell of origin: germinal center B-cell (GCB), and activated B-cell (ABC).4 However, the prognostic value of genetic markers in DLBCL remains controversial, and these have not yet been incorporated into routine clinical practice.5
Rearrangement of the BCL6 proto-oncogene at chromosome band 3q27 is the most frequent cytogenetic abnormality in DLBCL, occurring in up to 35% of cases.6–8 The BCL6 gene, a zinc-finger transcription factor, may be translocated with diverse partners in DLBCL, including both immunoglobulin (IGH, IGK, IGL) and non-IG loci.9,10 Clinical studies investigating the prognostic impact of BCL6 rearrangement in DLBCL have yielded contradictory results, variably demonstrating favorable,6 intermediate,7,8,11 and adverse outcomes12,13 in association with this abnormality.
An association has recently been reported between BCL6 rearrangement and ABC phenotype.14 This cell-of-origin profile was initially demonstrated as an adverse biological marker in DLBCL patients treated with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), and has been shown to retain its predictive impact in patients treated with CHOP plus rituximab (R-CHOP).15
The introduction of rituximab to standard first-line therapy has significantly improved clinical outcome in DLBCL, and may alter the prognostic impact of both clinical and biological markers in this disease.2,3 The prognostic significance of BCL6 rearrangement has not been reevaluated since the introduction of rituximab into DLBCL therapy. In this study we used tissue microarray-based fluorescence in situ hybridization (FISH) to analyze BCL6 rearrangement status in a retrospective cohort of patients with DLBCL. The objectives of this study were to: (i) compare the effect of BCL6 rearrangement on survival in DLBCL patients treated with CHOP and R-CHOP, and (ii) evaluate the relationship between BCL6 rearrangement and other clinical and biological prognostic variables in this disease, including cell-of-origin phenotype.
Design and Methods
Patients’ samples
We included all patients identified through the Centre for Lymphoid Cancer database of the British Columbia Cancer Agency (BCCA) who met the following criteria: (i) confirmed diagnosis of DLBCL (excluding primary mediastinal B-cell lymphoma) following a pathology review; (ii) treated with either CHOP alone or in combination with rituximab immunotherapy (R-CHOP); (iii) available diagnostic paraffin material on a tissue microarray; (iv) negative for human immunodeficiency virus; (v) treated at the BCCA between 1999 and 2007. Ethical approval for this study was obtained from the University of British Columbia – BCCA Research Ethics Board.
Fluorescence in situ hybridization and immunohistochemistry on tissue microarrays
Archived, formalin-fixed, paraffin-embedded, diagnostic biopsy specimens were selected for construction of the tissue microarrays and 0.6 mm duplicate cores were obtained from representative areas containing large B cells with typical morphology. FISH was performed according to a standard protocol for paraffin-embedded material, described elsewhere,14,16 using commercially available Vysis LSI BCL6 Dual Color, Break-Apart Rearrangement Probes (Abbott Molecular, IL, USA). Cases were recorded as having BCL6 rearrangement (BCL6+) if break aparts occurred in at least one signal of more than 5% of nuclei. All other signal constellations were regarded as negative (BCL6−). Immunohistochemical analysis was performed using monoclonal antibodies for BCL2 (Dako, clone 124, Denmark), BCL6 (Ventana Medical Systems, Tucson, Arizona), CD10 (Ventana Medical Systems, Tucson, Arizona), and MUM1 (Dako, Denmark), following routine protocols for automated immunohistochemistry on the Ventana Benchmark XT (Ventana Medical Systems, Tucson, Arizona). Cases were then categorized as GCB-like or non-GCB using a standard algorithm.17 Accordingly, for each case, the core with the highest percentage of tumor cells stained was used for analysis. Cases were considered positive if 30% or more of the tumor cells were stained with an antibody.
Statistical analysis
Group comparisons were performed by means of χ2 and Student’s t tests. For time to event analyses we used SPSS software version 11.0.0, applying Kaplan-Meier survival estimates and univariate and multivariate Cox proportional hazard models with the end-point of overall survival, defined as the time from initial diagnosis to death from any cause. P values less than 0.05 were considered statistically significant.
Results
Patients’ characteristics
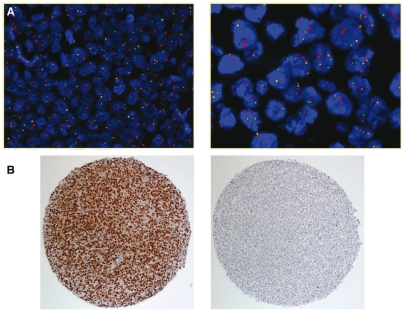
A total of 174 patients with DLBCL who met all inclusion criteria were identified. FISH was successfully performed in 164 out of these 174 cases (94.3%). BCL6 rearrangement was detected in 32 out of the 164 cases (19.5%). Representative BCL6+ FISH images of the tissue microarray sections are shown in Figure 1A.
Figure 1.
(A) Fluorescence in situ hybridization on formalin-fixed, paraffin-embedded tissue sections on a tissue microarray. Left panel: interphase nuclei with BCL6 break apart (BCL6+). The most common signal pattern is one fusion signal (yellow) and two split signals (1 red, 1 green) visualized at 200× magnification. Right panel: BCL6+ interphase nuclei with break apart (split signals) and polyploidy (multiple fused signals) of the BCL6 locus (high power field). (B) Immunohistochemistry for BCL-6 on tissue microarray cores. Left panel: positive staining in virtually all large B cells. Right panel: negative staining.
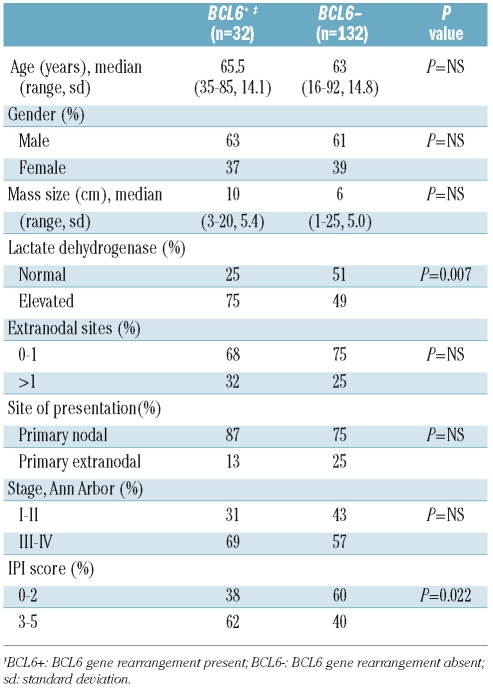
The baseline clinical characteristics of the patients, divided according to BCL6 rearrangement status, are listed in Table 1. There were no significant differences in initial treatment regimen between the two subgroups, with similar proportions of BCL6+ (15/32) and BCL6− (84/132) patients receiving rituximab as part of their initial therapy. A higher proportion of patients in the BCL6+ subgroup had a high-intermediate or high-risk International Prognostic Index (IPI) score (IPI 3–5) (62.5% versus 40.2%, P=0.02). Stage IE/IIE cases were designated as ‘primary extranodal disease’, while all other cases were considered as ‘primary nodal disease’. The frequency of primary extranodal disease was not significantly different between the BCL6+ and BCL6− subgroups (13% versus 25%, P=NS).
Table 1.
Clinical characteristics of 164 DLBCL patients, by BCL6 rearrangement status.
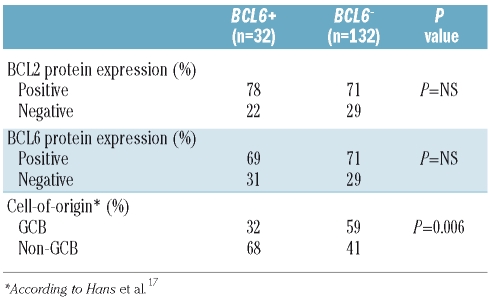
Table 2 shows the patients’ immunohistochemical characteristics according to BCL6 rearrangement status. BCL6 staining and cell-of-origin classification17 was successfully performed in 162 of the 164 patients. Representative BCL6 staining results are shown in Figure 1B. Results of BCL2 staining were available for 115 of the 164 (70%) patients. The presence of BCL6 rearrangement was significantly correlated with cell-of-origin; 68% of BCL6+ patients had a non-GCB subtype, as compared with 48% of BCL6− patients (P=0.006). No association was found between BCL6 rearrangement and either BCL6 (P=0.78) or BCL2 (P=0.95) protein expression.
Table 2.
Immunophenotypic characteristics of 164 DLBCL patients, by BCL6 rearrangement status.
Survival
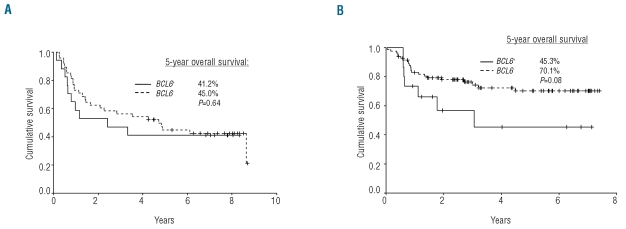
The median duration of follow-up was 2.9 years (range, 0.3–8.7). Rituximab use was associated with a significant survival improvement among BCL6− patients (5-year overall survival, 70.1% versus 45.0%, P=0.009), but not among BCL6+ patients (5-year overall survival, 45.3% versus 41.2%, P=0.70). Kaplan-Meier analysis of the entire cohort revealed a trend toward decreased overall survival in patients with a BCL6 rearrangement, though this did not reach statistical significance (5-year overall survival, 59.4% versus 43.4%, P=0.07). Within treatment groups, BCL6 rearrangement was associated with a trend toward inferior overall survival among patients treated with R-CHOP (5-year overall survival, 45.3% versus 70.1%; P=0.08), but not among patients treated with CHOP (5-year overall survival, 41.2% versus 45.0%; P=0.64) (Figure 2). No significant association was found between BCL6 protein expression and overall survival in the whole cohort (5-year overall survival, BCL6+ versus BCL6−, 52.2% versus 31.8%, P=0.11).18
Figure 2.
Overall survival according to BCL6 rearrangement status. (A) CHOP-treated patients (n=65). (B) R-CHOP-treated patients (n=99).
Subgroup analysis of overall survival was performed according to the site of presentation of disease and the patients’ age. Survival analysis limited to patients with primary nodal disease showed that the 5-year overall survival among BCL6+ patients was 39.7% compared with 54.7% in BCL6− patients (P=0.09). Among patients who presented with primary extranodal disease, no difference in overall survival was found between BCL6+ and BCL6− patients (P=0.95). Subgroup analysis by patients’ age did not reveal statistically significant associations between gene rearrangement status and overall survival in patients under 65 (P=0.38) or those over 65 years of age (P=0.24).
In R-CHOP-treated patients, high IPI score (IPI 3–5) predicted a significantly worse overall survival (5-year overall survival, 46.1% versus 77.8%, P=0.002), while non-GCB immunohistochemical profile was associated with a trend toward inferior outcome (5-year overall survival, 50.8% versus 76.8%, P=0.07). A Cox regression analysis was performed for the R-CHOP-treated cohort using the following four variables: IPI score, cell-of-origin immunophenotype, BCL2 protein expression, and BCL6 gene rearrangement status. In multivariate analysis, only high IPI score was found to be an independent adverse prognostic factor for overall survival (P=0.002).
Discussion
This study analyzed the clinical impact of BCL6 gene rearrangement in a retrospective cohort of DLBCL patients, treated in both the pre- and post-rituximab eras. Our data suggest an association between BCL6 rearrangement and inferior outcome in patients treated with R-CHOP, but not in patients treated with CHOP. However, BCL6 rearrangement also correlated with the presence of high-risk clinical features and was not demonstrated to have a prognostic significance independent of the IPI score.
The effect of the BCL6 rearrangement on outcome in DLBCL has remained uncertain despite previous clinical analyses. In a seminal study by Offit et al., BCL6 rearrangement was found to be a favorable prognostic marker in DLBCL, possibly due to an association with limited extranodal disease.6 Most subsequent investigators failed to demonstrate any influence of BCL6 rearrangement on prognosis in DLBCL, and similarly our analysis did not find that this marker had predictive value in patients treated with CHOP alone.7,8,11,14 A more recent analysis, restricted to DLBCL patients with primary nodal disease, reported inferior survival in patients with BCL6 rearrangement, as well as a correlation between this marker and non-GCB immunophenotype.12 In agreement with our results, Vitolo et al. reported a correlation between BCL6 rearrangement and adverse clinical features,11 although this has not been a uniform finding in previous studies. These discrepancies may be explained in part by heterogeneity between study populations, due to concomitant genetic alterations, selection biases, and differences in molecular diagnostic techniques. Specifically, rearrangement of the MYC oncogene has been found by several authors to impart an inferior prognosis in CHOP-treated DLBCL patients, although this abnormality occurs in only 5–10% of patients19,20 and MYC and BCL6 rearrangement rarely coexist.6,8,11
The addition of rituximab to first-line combination therapy has led to a significant improvement in survival in patients with DLBCL, requiring a re-evaluation of established prognostic markers.3 Biological prognostic markers in particular may be influenced by changes in standard therapy, because of the differential effects of new agents within biological subgroups. It has recently been reported that BCL2 protein expression, an adverse prognostic marker in CHOP-treated patients, loses its prognostic effect in the rituximab era, due to a disproportionate benefit from rituximab in BCL2+ cases.21 In our analysis, the addition of rituximab appeared primarily to benefit patients without BCL6 rearrangement, and was associated with significantly prolonged overall survival in this subgroup. In contrast, little improvement in outcome was observed with the addition of rituximab among BCL6+ patients, in whom long-term survival remained less than 50% even with R-CHOP. These data suggest a potential value for BCL6 rearrangement as an adverse predictive biomarker in DLBCL in the rituximab era.
The IPI remains the most successful clinical prognostic instrument in DLBCL.22 However, the IPI score appears to lose discriminatory power in R-CHOP-treated patients, and in a recent analysis was unable to identify a subgroup with a less than 50% chance of long-term survival.3 Biological markers may help to refine risk-stratification in DLBCL, and to identify high-risk subgroups of patients who might benefit from intensified or novel therapies. However, to be clinically useful these markers must provide prognostic information complementary to that provided by the IPI. In our study, the presence of a BCL6 gene rearrangement correlated with high IPI score, and in multivariate analysis this biomarker did not show independent prognostic significance. BCL6 rearrangement has also shown an association with non-GCB immunophenotype, previously demonstrated to be an unfavorable prognostic marker in CHOP-treated patients with DLBCL.17 However, in our cohort of R-CHOP-treated patients, cell-of-origin phenotype did not emerge as an independent prognostic variable in multivariate analysis.
The clinical impact of oncogene rearrangements may also depend on the site of disease presentation. DLBCL arising from extranodal sites differs from nodal DLBCL in terms of pathogenesis and cell of origin, and several distinct subtypes of extranodal DLBCL are recognized in the 2008 revision of the WHO classification of lymphoid malignancies.1 Unlike previous groups, we found no correlation between BCL6 rearrangement and extranodal presentation,6,8 and no statistically significant difference in overall survival between BCL6+ and BCL6− patients was demonstrated in either the primary nodal or extranodal subgroup, although this analysis was limited by the small numbers in the two groups.
The BCL6 proto-oncogene encodes a transcriptional repressor essential for normal germinal center formation. Its deregulated expression has been implicated as an important pathway in lymphomagenesis.23 BCL6 protein expression has been identified as a hallmark of GCB derivation in DLBCL, and has been associated with a favorable clinical outcome.17,24 However, in keeping with prior studies, we did not find a correlation between BCL6 rearrangement and BCL6 protein expression, but demonstrated a relationship between BCL6 rearrangement and non-GCB phenotype.12,14 This seemingly paradoxical finding may be related to several factors. First, mechanisms other than gene rearrangement have been shown to deregulate BCL6 expression in DLBCL, including targeted mutations which may interrupt negative gene autoregulation.23 Second, the functional effect of BCL6 rearrangement may vary depending on other biological factors, including the chromosomal translocation partner and cellular context. One group of investigators demonstrated significantly lower BCL6 expression with non-IGH/BCL6 than with IGH/BCL6 rearrangements, and also reported inferior survival in DLBCL patients with non-IGH/BCL6 translocations.13,25 A more recent study by the Leukemia/Lymphoma Molecular Profiling Project examined the effect of BCL6 rearrangement on gene expression in patients with both GCB- and ABC-subtype DLBCL.14 In this analysis, BCL6 rearrangement was associated with increased BCL6 mRNA levels in patients with ABC-subtype DLBCL, but not in patients with GCB-subtype DLBCL. The clinical significance of these biological subdivisions remains to be clarified.
In summary, the introduction of rituximab may have altered the prognostic impact of BCL6 gene rearrangement in patients with DLBCL. However, to be incorporated into clinical practice as a useful prognostic biomarker, BCL6 rearrangement must be shown to provide prognostic information additional to that afforded by the IPI. BCL6 rearrangement also correlates strongly with non-GCB immunophenotype, and the interdependence of these two prognostic variables requires further study. Correlative biomarker analysis within prospective randomized clinical trials will be needed to determine the prognostic value of BCL6 rearrangement in the rituximab era.
Acknowledgments
we thank Chris Salski and the Center for Translational and Applied Genomics (CTAG) laboratory for excellent technical support.
Footnotes
Funding: this work was supported by postdoctoral fellowships of the Deutsche Forschungsgemeinschaft (DFG), the Canadian Research Society (CRS), the Michael Smith Foundation for Health Research (MSFHR), and the Lymphoma Research Foundation (LRF) to CS. RDG, DEH and JMC are supported by a Terry Fox Program Project grant from the National Cancer Institute of Canada (# 016003).
Authorship and Disclosures
JS wrote the manuscript, GH and PF performed experiments and analyzed results, NAJ, JMC, LHS and DEH designed the research and approved the manuscript, SB analyzed and reviewed results, RDG and CS analyzed and reviewed results, designed the research and wrote the paper.
The authors reported no potential conflicts of interest.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 2.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 3.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 5.Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(6):995–1007. doi: 10.1200/JCO.2005.02.4786. [DOI] [PubMed] [Google Scholar]
- 6.Offit K, Lo Coco F, Louie DC, Parsa NZ, Leung D, Portlock, et al. Rearrangement of the bcl-6 gene as a prognostic marker in diffuse large-cell lymphoma. N Engl J Med. 1994;331(2):74–80. doi: 10.1056/NEJM199407143310202. [DOI] [PubMed] [Google Scholar]
- 7.Bastard C, Deweindt C, Kerckaert JP, Lenormand B, Rossi A, Pezzella F, et al. LAZ3 rearrangements in non-Hodgkin’s lymphoma: correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood. 1994;83(9):2423–7. [PubMed] [Google Scholar]
- 8.Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998;92(9):3152–62. [PubMed] [Google Scholar]
- 9.Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262 (5134):747–50. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 10.Kerckaert JP, Deweindt C, Tilly H, Quief S, Lecocq G, Bastard C. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nat Genet. 1993;5(1):66–70. doi: 10.1038/ng0993-66. [DOI] [PubMed] [Google Scholar]
- 11.Vitolo U, Gaidano G, Botto B, Volpe G, Audisio E, Bertini M, et al. Rearrangements of bcl-6, bcl-2, c-myc and 6q deletion in B-diffuse large-cell lymphoma: clinical relevance in 71 patients. Ann Oncol. 1998;9(1):55–61. doi: 10.1023/a:1008201729596. [DOI] [PubMed] [Google Scholar]
- 12.Barrans SL, O’Connor SJ, Evans PA, Davies FE, Owen RG, Haynes AP, et al. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br J Haematol. 2002;117(2):322–32. doi: 10.1046/j.1365-2141.2002.03435.x. [DOI] [PubMed] [Google Scholar]
- 13.Akasaka T, Ueda C, Kurata M, Akasaka H, Yamabe H, Uchiyama T, et al. Nonimmunoglobulin (non-Ig)/BCL6 gene fusion in diffuse large B-cell lymphoma results in worse prognosis than Ig/BCL6. Blood. 2000;96(8):2907–9. [PubMed] [Google Scholar]
- 14.Iqbal J, Greiner TC, Patel K, Dave BJ, Smith L, Ji J, Wright G, et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. 2007;21(11):2332–43. doi: 10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. Lymphoma/Leukemia Molecular Profiling Project. N Engl J Med. 2008;359(22):2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham AD, Faratian D, Rae F, Thomas JS. Tissue microarray technology in the routine assessment of HER-2 status in invasive breast cancer: a prospective study of the use of immunohistochemistry and fluorescence in situ hybridization. Histopathology. 2008;52(7):847–55. doi: 10.1111/j.1365-2559.2008.03047.x. [DOI] [PubMed] [Google Scholar]
- 17.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 18.Winter JN, Weller EA, Horning SJ, Krajewska M, Variakojis D, Habermann TM, et al. Prognostic significance of Bcl-6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107(11):4207–13. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 20.Klapper W, Stoecklein H, Zeynalova S, Ott G, Kosari F, Rosenwald A, et al. Structural aberrations affecting the MYC locus indicate a poor prognosis independent of clinical risk factors in diffuse large B-cell lymphomas treated within randomized trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). German High-Grade Non-Hodgkin’s Lymphoma Study Group. Leukemia. 2008;22(12):2226–9. doi: 10.1038/leu.2008.230. [DOI] [PubMed] [Google Scholar]
- 21.Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Sebban C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101(11):4279–84. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 22.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 23.Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RS, Dalla-Favera R. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003;101(8):2914–23. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 24.Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Zehnder JL, et al. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98(4):945–51. doi: 10.1182/blood.v98.4.945. [DOI] [PubMed] [Google Scholar]
- 25.Ueda C, Uchiyama T, Ohno H. Immunoglobulin (Ig)/BCL6 versus non-Ig/BCL6 gene fusion in diffuse large B-cell lymphoma corresponds to a high- versus low-level expression of BCL6 mRNA. Blood. 2002;99(7):2624–5. doi: 10.1182/blood-2001-11-0117. [DOI] [PubMed] [Google Scholar]