Abstract
Background
The aims of the present study were to ascertain the activation status of Akt in the primary cells of chronic lymphocytic leukemia and to investigate the effects of specific Akt inhibition on chronic lymphocytic leukemia-cell survival.
Design and Methods
Anti-phospho-Akt (Ser473 or Thr308) antibodies and western blotting were used to establish the activation status of Akt. The effects of two different, specific small-molecule inhibitors (A-443654 or Akti-1/2) or small interfering RNA on cell survival and downstream targets of Akt were assessed. Apoptosis was determined by fluorescence-activated cell sorting analysis of phosphatidylserine exposure and by measurement of PARP cleavage. The phosphorylation status of GSK-3 and MDM2, two immediate downstream substrates of Akt, levels of the anti-apoptotic proteins BCL2 and MCL1, and expression of p53 and p21 were all measured by western blotting.
Results
Fully activated Akt was demonstrable in all chronic lymphocytic leukemia clones examined (n=26). These results were validated with extensive controls and it was shown that a harsh method of cell extraction is needed for detection of the active enzyme. Specific inhibition of Akt induced extensive apoptosis of chronic lymphocytic leukemia cells, which was associated with both a rapid loss of MCL1 through proteasomal degradation and increased expression of p53. Moreover, the Akt inhibitors, at concentrations that induced extensive apoptosis in chronic lymphocytic leukemia cells, had little or no effect on normal peripheral blood mononuclear cells.
Conclusions
Chronic lymphocytic leukemia clones consistently contain activated Akt which plays a pivotal role in maintaining cell survival. Inhibition of the Akt pathway may be of potential value as a novel therapeutic strategy in chronic lymphocytic leukemia.
Keywords: Akt, Akt inhibitors, apoptosis, chronic lymphocytic leukemia, MCL1
Introduction
Chronic lymphocytic leukemia (CLL) is a malignancy of CD5+ B cells that have been linked to memory cells.1 Although the pathogenesis of the disease is still not fully understood, chronic (auto)antigenic stimulation via the B-cell antigen receptor is thought to be involved.2 B-cell antigen receptor stimulation activates a number of downstream pathways and probably contributes to expansion of the malignant cells and, at least in certain clones, to cell survival.3 In addition, a number of other mechanisms are important in CLL-cell survival. These include over-expression of anti-apoptotic proteins such as BCL2,4 aberration of the ATM/p53 tumor suppressor pathway,5 deregulation of autocrine cytokines6 and interaction with microenvironmental factors such as paracrine cytokines and accessory cells.7 Many of these mechanisms involve the activation of the phosphatidylinositol-3 kinase (PI-3K) pathway which is thought to play a vital role in the survival of CLL cells.8–10
Following cell stimulation, PI-3K is recruited to the inner surface of the plasma membrane where it becomes activated and phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to become phosphatidylinositol 3,4,5,-triphosphate (PIP3).11 The PIP3 then recruits to the cellular membrane a set of proteins that contain a pleckstrin homology domain; such proteins include phosphatidylinositol-dependent kinase 1 (PDK1) and the serine-threonine kinase Akt (also known as PKB, protein kinase B).12 PDK1 phosphorylates Akt1 at threonine 308, but full activation of Akt requires additional phosphorylation at serine 473 by other kinases.13,14
Activated Akt is the principal mediator of pro-survival signaling regulated by PI-3K.14 This pro-survival effect can be mediated by transcriptional, translational and post-translational regulation of a number of apoptosis-related molecules.15–20 Furthermore, there is compelling evidence from animal models linking Akt with the development of a number of malignant lymphoid disorders. For example, transgenic mice expressing myristoylated Akt1 (Myr-Akt1) (which is membrane bound and hence constitutively active) rapidly develop aggressive B-cell lymphomas with leukemic involvement.21 More recently, a separate study with a similar experimental approach has also shown that mice expressing human Myr-Akt1 develop a mature B-cell leukemia.22 In addition, expression of human Akt1 containing an E17K mutation in the pleckstrin homology domain (which facilitates Akt activation by aiding its plasma-membrane localization) induces a B-cell leukemia in mice.22 Also, transgenic mice over-expressing Tcl1 (T cell leukemia-1) develop a CLL-like disorder associated with TCL1-stimulated Akt activation.23,24
The role of Akt in the pathogenesis of CLL in humans is, however, still controversial. Previous studies have given contradictory results concerning the presence of phosphorylated Akt in unstimulated CLL cells. For example, some studies have reported the presence of phosphorylated enzyme,25–27 while others have not, despite demonstrating active PI-3K kinase in CLL cells.8–10 In particular, a very recent study did not detect phosphorylated Akt in any of 21 CLL samples studied.28 These conflicting data make it difficult to know whether pharmacological inhibitors of Akt29,30 might be of potential therapeutic value in CLL.
Here, we studied the activation status of Akt in CLL, examining the effect of Akt inhibition on selective killing of CLL cells and the mechanisms involved.
Design and Methods
Patients
All samples were obtained with informed consent and with the approval of the Liverpool Research Ethics Committee. The diagnosis of CLL was based on standard morphological, and immunophenotypic criteria, as described elsewhere.31 The clinical details of the CLL patients are shown in Online Supplementary Table S1. The diagnosis of mantle-cell lymphoma (MCL) with leukemic involvement was made on the basis of the presence in the blood of cells expressing typical surface markers (clonal CD5+ B cells expressing strong surface immunoglobulin, but lacking CD23). In MCL#1, more than 30% of all the cells had a blastic morphology and this case, therefore, had the blastoid variant of MCL.
Cell preparation and culture
Peripheral blood mononuclear cells (PBMC) were isolated from patients and healthy volunteers by centrifugation of blood over Lymphoprep (Axis-Shield PoC AS, Oslo, Norway); in most experiments, PBMC were stored in liquid nitrogen prior to use. After thawing, PBMC were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Paisley, UK). To ensure that the phospho-Akt detected in the CLL-cell samples was not derived in any significant way from contaminating cells, some CLL clones (n=8) were purified after thawing by depleting mononuclear preparations of contaminating T, NK and monocytic cells. This was done with fluorescein thiocyanate-conjugated anti-CD3, -CD16 and -CD14 antibodies (BD Biosciences, Oxford, UK), respectively, and with subsequent removal of reactive cells using magnetic microbeads coated with anti-fluorescein thiocyanate mouse antibody (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of CLL cells was determined by fluorescence activated cell sorting (FACS) measurement of cells displaying both CD5 and CD19.
Akt inhibitors
Two different inhibitors were employed (A-443654 and Akti-1/2). A-443654 was provided by Dr Vincent Giranda at Abbott Laboratories (Abbott Park, IL, USA). A-443654 is a relatively specific inhibitor of Akt32–34 and, in cellular studies, inhibits the kinase with an EC50 of between 0.3 μM32 and 1 μM.33 The other inhibitor (Akti-1/2) ( Calbiochem/Merck Biosciences, Nottingham, UK) is highly selective34,35 and completely inhibits Akt1 and Akt2 in intact cells of established cell lines at concentrations of 1 μM36 to 5μM.37
Antibodies and other reagents
Three antibodies against phospho-Akt (all from Cell Signaling Technology, New England Biolabs, Herts, UK) were employed for western blotting: a rabbit polyclonal antibody specific for phospho-Akt (Ser473) and two mouse monoclonal antibodies against phospho-Akt (Ser473) (clone 587F11) and phospho-Akt (Thr308) (clone L32A4). Additional antibodies used were rabbit polyclonal antibodies against total Akt, phospho-GSK3α/β (Ser21/9), GSK3α, phospho-MDM2 (Ser166) and a mouse monoclonal antibody specific for the Akt1 (clone 2H10) (Cell Signaling Technology). Rabbit polyclonal antibodies to MCL1 and BCL2 and a mouse monoclonal anti-MDM2 (clone SMP14) were from Santa Cruz Biotechnology (Insight Biotechnology, Middlesex, UK). Mouse monoclonal antibodies against p53 (clone PAb1801) and p21 (clone EA10) (both from Oncogene/Merck Biosciences), PARP (clone C2–10) (R&D Systems, Minneapolis, MN), and β-actin (clone AC-74) (Sigma-Aldrich, Gillingham, UK) were also employed. Regarding inhibitors, SB216763 (BIOMOL Research Laboratories, Plymouth Meeting, PA, USA) was employed to block GSK-3 activity. The pan-caspase inhibitor Z-VAD.fmk, the proteasome inhibitor MG-132, and the PI-3K inhibitor LY296002 were all from Calbiochem/Merck Biosciences. Other chemicals, unless otherwise stated, were obtained from Sigma-Aldrich.
Western blotting
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were performed essentially as described elsewhere.38 Briefly, cell pellets were resuspended on ice in lysis buffer containing 10 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 1% Triton X-100, 100 mM NaCl, 10 mM NaF, 1 mM Na3VO4 and a protease inhibitor cocktail. After sonication, cellular proteins were separated on an SDS-polyacrylamide gel and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA, USA), which were probed with the appropriate primary antibodies. Immunoreactivity was detected with the relevant horseradish peroxidase-labeled secondary antibodies which, in turn, were visualized on an Image Reader LAS-1000 (Fujifilm, Tokyo, Japan) using an enhanced chemiluminescence kit (Amersham Biosciences, Buckinghamshire, UK). For quantification of the data, the images were further analyzed on the same instrument using 2D Densitometry Aida Image Analyzer software (Fujifilm). In selected experiments, membranes were reprobed after stripping in buffer containing 62.5 mM Tris-HCl (pH 6.7), 2% SDS and 100 mM 2-mercaptoethanol at 50°C for 30 min.
Flow cytometric analysis of apoptosis
CLL cells were seeded at 4×106 cells/mL in a multi-well plate precoated with polyHEMA (to prevent cell adhesion) in the presence of the Akt inhibitors. After incubation, apoptosis was determined in aliquots of cells using a FACS method employing dual-staining with annexin V and propidium iodide;38 the remaining cells were analyzed by western blotting.
Transfection of small interfering RNA in chronic lymphocytic leukemia cells
The transfection was performed using the Human B cell Nucleofector Kit (Amaxa AG, Cologne, Germany), and its efficiency was monitored by FACS analysis 24 h after transfecting 1×107 CLL cells with 2 μg of pMaxGFP (Amaxa AG) or with 2 μg of fluorescent non-specific control siRNA duplex (Integrated DNA Technologies (IDT), Coralville, IA, USA). As the transfection efficiency varied considerably between individual CLL samples, only cells with a transfection efficiency of more than 35% were used for knock-down experiments. To knock down Akt1, we used a mixture of equal amounts of three different siRNA duplexes (IDT). The detailed methodology is given in the Online Supplementary Methods.
Statistical analysis
A two-tailed Mann-Whitney U test was performed to determine the statistical significance of the difference between two groups of data using SPSS v15.0 software.
LC50 values (LC50 is defined as the concentration of the inhibitors which induced apoptosis in 50% of treated cells) were calculated from dose-response experiments using BioDataFit software v1.02 from Chang Biosciences Inc. (Castro Valley, CA, USA).
Results
Primary chronic lymphocytic leukemia cells contain phosphorylated Akt
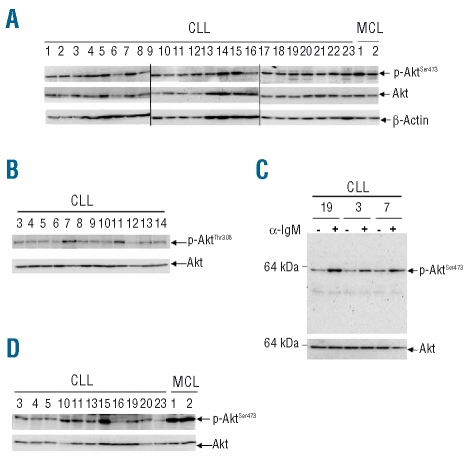
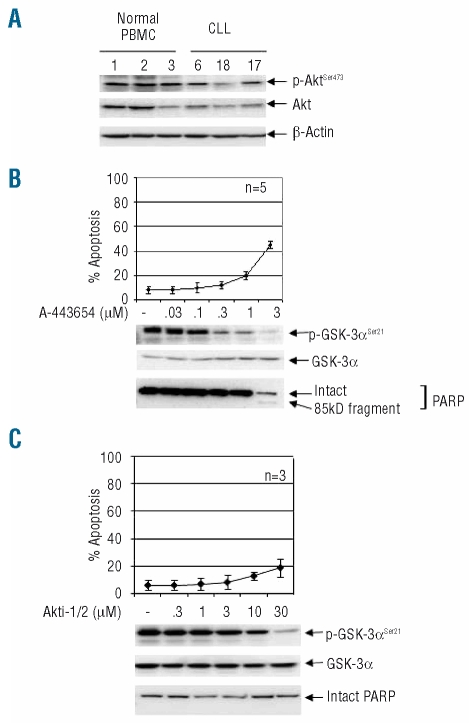
When whole CLL-cell lysates extracted by sonication in 1% Triton X-100 were western blotted with antibodies against phospho-Akt (p-Akt) on serine 473 or threonine 308, varying amounts of p-Akt were readily detected with both antibodies in all cases studied (n=26, Figure 1A and B cases 1–23 and Online Supplementary Figure S1 cases 24–26). For comparison, mononuclear cells from patients with MCL were also studied using the same extraction method and the anti-p-Akt (Ser473) antibody. MCL cells were chosen because they, like CLL cells, express CD5 and because they contain, especially in the blastoid variant of the disease, high levels of constitutively active Akt39 and hence served as positive controls. As expected, MCL cells exhibited high levels of p-Akt which were greater than those observed in most CLL clones (Figure 1A). Since most samples were obtained from patients with very high white blood cell counts (Online Supplementary Table S1), the purity of the CLL cells was high. However, to ensure that contaminating cells were not contributing significantly to the levels of p-Akt detected, samples from eight randomly chosen patients were examined before and after depletion of non-CLL cells. Levels of p-Akt (Ser473) were indeed similar after purification (Online Supplementary Figure S1A), confirming that CLL cells contain p-Akt. This also indicates that levels detected in CLL PBMC closely reflect those present in the malignant clones.
Figure 1.
CLL cells express fully activated Akt. (A) Western blotting with an anti-p-Akt (Ser473) rabbit polyclonal antibody was used as a measure of activated Akt. Whole-cell lysates were prepared immediately after thawing and, in these and all subsequent blots, material from 1×106 cells was loaded in each lane. Total Akt and β-actin were the protein loading controls. (B) Cell lysates of 12 of the clones studied above were probed with antibody against p-Akt (Thr308). (C) The specificity of the rabbit polyclonal anti-p-Akt (Ser473) was confirmed by demonstrating an increase in p-Akt after B-cell antigen receptor cross-linking (3 min at 37°C) with goat F(ab′)2 anti-human IgM antibody (10 μg/mL, Jackson Laboratories, West Grove, PA, USA). Cells similarly treated with F(ab′)2 fragments (10 μg/mL) of non-specific goat IgG (Jackson Laboratories) constituted a negative control. (D) Cell lysates were western blotted with an anti-p-Akt (Ser473) mouse monoclonal antibody (n=11 of the CLL clones studied in (A), together with the two of the same MCL samples).
In the light of the existing controversy concerning the activation status of Akt in CLL cells, we examined the specificity of the two anti-p-Akt antibodies employed. To do this, CLL cells were incubated with a PI-3K inhibitor (LY296002) and probed for p-Akt (Thr308) (PI-3K is the major upstream kinase responsible for phosphorylation of Akt at this site12). As expected, the inhibitor completely abolished reactivity with the anti-p-Akt (Thr308) antibody (Online Supplementary Figure S1B, right panel). In contrast, LY296002 reduced, but did not abolish, reactivity with the anti-p-Akt (Ser473) antibody (Online Supplementary Figure S1B, left panel). Again, this was expected since it is well known that several kinases can induce phosphorylation at this site.12,13 We then used two approaches to examine further the specificity of the p-Akt (Ser473) band. First, CLL cells were stimulated by B-cell antigen receptor cross-linking to see whether the band was increased by a stimulus known to induce Akt phosphorylation at serine 473; as expected, such stimulation produced marked enhancement of the p-Akt (Ser473) band (Figure 1C). Secondly, a different antibody (mouse monoclonal) against p-Akt (Ser473) was employed; a band at an identical position in the gel was obtained in all cases studied (Figure 1D). These studies, therefore, confirmed the specificity of the anti-p-Akt antibodies used.
In our experiments above, cells were examined immediately after thawing. Consequently, we investigated the possibility that freezing/thawing might activate Akt. Furthermore, it seemed plausible that the period of recovery/culture of cells before lysis could have affected the results. We, therefore, examined both fresh CLL cells and thawed cells cultured briefly to allow recovery. The p-Akt content was higher in fresh cells, but still readily detected in frozen cells after thawing (Online Supplementary Figure S1C). Also, levels of p-Akt remained unchanged in thawed cells cultured for up to 4 h (data not shown). It was, therefore, concluded that freezing/thawing and culture for short periods after thawing are not critical factors in the detection of p-Akt in CLL cells.
Having unequivocally demonstrated that unstimulated CLL cells contain fully activated Akt, we next examined the role of the activated Akt in CLL-cell survival.
Inhibition of Akt induces apoptosis in chronic lymphocytic leukemia cells
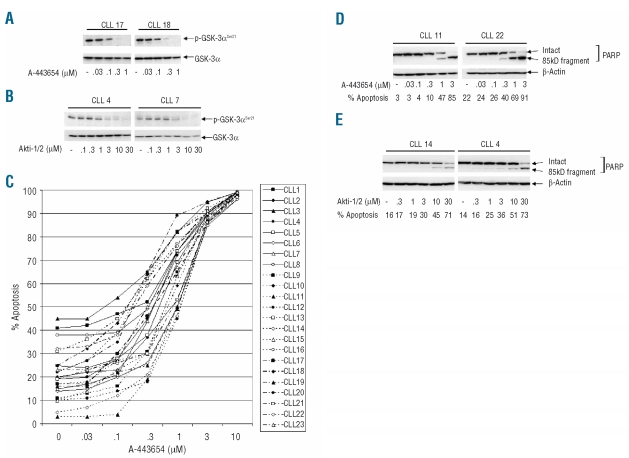
We first confirmed the activity of the two inhibitors in CLL cells by showing that both, at relevant concentrations (0.3–1μM for A-443654 and 10–30 μM for Akti-1/2), completely abolished the phosphorylation of GSK-3 (Figure 2A and B), an immediate downstream substrate of Akt.14
Figure 2.
Inhibition of Akt induces apoptosis in CLL cells. (A) and (B). The activity of the two Akt inhibitors was confirmed by demonstrating a loss of phosphorylation of GSK-3α in CLL cells treated with either of the inhibitors. (A) Dose-dependent loss of p-GSK-3α (Ser21) after culture of CLL cells (4×106/mL) for 24 h in the presence of A-443654 (two representative examples of western blots from six experiments involving six CLL clones). (B) Two representative results of similar experiments (n=6 clones examined) employing Akti-1/2. (C) CLL cells were cultured for 24 h in the presence of a range of concentrations of A-443654, and apoptosis was determined by a FACS method measuring phospatidylserine exposure. An individual dose-response curve is shown for 23 cases. In all 23 cases, apoptosis was also confirmed by western blotting for PARP cleavage, and the results for two representative clones are shown in (D). (E) Apoptosis is induced by a second Akt inhibitor (Akti-1/2). Two representative examples of the six cases studied are shown.
We then used A-443654 to perform conventional dose-response measurements of the induction of apoptosis (as measured by FACS analysis of phosphatidylserine exposure). In all cases studied, treatment of CLL cells with A-443654 resulted in the dose-dependent induction of apoptosis (Figure 2C). LC50 values for individual CLL clones were then calculated (Online Supplementary Table S1) and, when ranked, a median value of 0.71 μM (range: 0.23–1.62 μM) was obtained; this indicates that A-443654 is a potent inducer of apoptosis in primary CLL cells. We next confirmed the induction of apoptosis by western blotting for poly (ADP-ribose) polymerase (PARP) cleavage, a biochemical marker of apoptosis. As shown in Figure 2D, A-443654 induced PARP cleavage at concentrations of 0.3 μM to 1 μM.
Similar studies were then performed with Akti-1/2. Again, dose-dependent apoptosis was induced in all six cases studied, with a median LC50 of 16.51 μM (range: 8.46–32.15 μM). Induction of apoptosis was also confirmed by PARP cleavage (Figure 2E). It was, therefore, concluded that the activated Akt of unstimulated CLL cells plays a role in the survival of the malignant cells.
The apoptosis induced by Akt inhibition involves both the GSK-3/MCL1 and p53 pathways
We next examined the mechanism(s) by which inhibition of Akt induces cell death, using the more potent of the two chemical inhibitors, A-443654. We focused on the expression of anti-apoptotic BCL2-family proteins and the proapoptotic p53 because they are critically involved in the survival/death of CLL cells.5,40 In particular, abundant expression of BCL2 and MCL1,41,42 and TP53 deletion/mutation43,44 in CLL are all associated with rapid disease progression and reduced sensitivity to chemotherapeutic agents, both in vitro and in vivo.
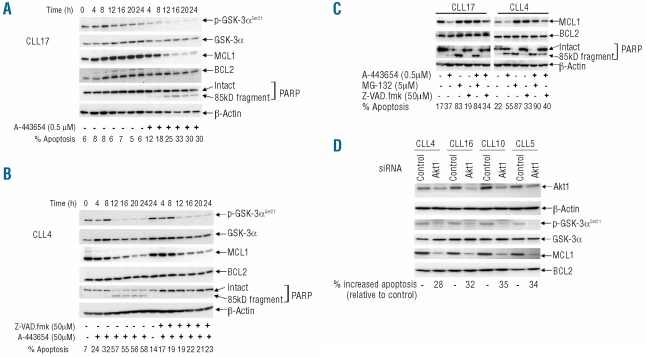
When expression levels of BCL2 and MCL1 were measured at various time points during continuous in vitro exposure to A-443654, MCL1 was found to decline progressively over 24 h, while BCL2 levels remained relatively constant (Figure 3A and B). As expected, the inhibitor also caused a progressive reduction of p-GSK-3α (Figure 3A and B) while, in untreated cells, levels of p-GSK-3α, BCL2 and MCL1 remained largely unchanged (Figure 3A).
Figure 3.
Loss of MCL1 through proteasomal degradation is involved in the apoptosis induced by Akt inhibition. (A) CLL cells were cultured in the presence or absence of A-443654. Phospho-GSK-3α constituted a marker of Akt activity, while MCL1 and BCL2 were measured as relevant pro-survival proteins for CLL. Again, PARP cleavage and FACS analysis were used to examine apoptosis, while total GSK-3α and β-actin were used as loading controls. This is a representative example of five experiments on cells from five different CLL cases. (B) Cells were treated as in (A), except that the result of incubation with the pan-caspase inhibitor Z-VAD.fmk in combination with A-443654 was determined. This is a representative example of three experiments involving three different CLL clones. (C) The effect of the proteasome inhibitor, MG-132, was also analyzed. These are representative findings from four separate experiments involving four different CLL clones. In all the above experiments, the inhibitors were added to the cells 1 h prior to treatment with the Akt inhibitor. (D) The effects of knockdown of Akt1 on cell survival and levels of Mcl-1. Here, 1×107 CLL cells were mixed with 100 μL transfection solution (Amaxa) containing a total of 2 μg of siRNA duplexes or 2 μg of non-specific control siRNA before nucleofection using program X-01. Cells (5×106/mL) were subsequently cultured for 72 h, after which levels of Akt1 were measured by western blotting using an Akt1-isoform-specific antibody, while apoptosis was assessed by the FACS method. β-actin constituted a protein loading control. Reduction of Akt1 was associated with loss of p-GSK-3α and MCL1, while total GSK-3α and BCL2 were unaffected.
As MCL1 can be cleaved by caspases during apoptosis in CLL cells,45 we incubated cells with the Akt inhibitor in the presence or absence of the pan-caspase inhibitor Z-VAD.fmk. Although co-incubation with Z-VAD.fmk effectively blocked A-443654-induced PARP cleavage and apoptosis, the inhibitor failed to prevent the losses of MCL1 and p-GSK-3α (Figure 3B). These results clearly show that the loss of MCL1 following treatment with the Akt inhibitor is caspase-independent.
To explore the possibility that the Akt-inhibitor-induced loss of MCL1 is mediated by the proteasome pathway, we incubated CLL cells with A-443654 in the presence or absence of the proteasome inhibitor, MG-132. Treatment with MG-132 alone induced extensive apoptosis, which was associated with increased levels of MCL1 (Figure 3C). This observation supports previous work by others showing that proteasomal inhibitors cause apoptosis of CLL cells by MCL1-independent mechanisms.46,47 When CLL cells were incubated with A-443654 and MG-132 together, the proteasome inhibitor effectively prevented the degradation of MCL1 induced by the Akt inhibitor (Figure 3C). The above results indicate that the pro-apoptotic effect of Akt inhibition is associated with proteasome-mediated degradation of MCL1. However, as shown and discussed below, loss of MCL1 is not the only mechanism by which Akt inhibitors induce CLL-cell apoptosis.
To further prove that inhibition of Akt has an adverse effect on the survival of CLL cells, we next employed siRNA to knock-down Akt1 expression. To do this, CLL cells were transfected with a combination of siRNA duplexes targeting three different regions of human Akt1 mRNA. Although the knock-down of Akt1 was variable and incomplete, the Akt1 siRNA consistently induced increased apoptosis as compared with the apoptosis in cells treated with non-specific siRNA (Figure 3D); furthermore, the extent of the increased apoptosis correlated with the extent of Akt1 knock-down (Figure 3D). Also, knock-down of Akt1 with specific siRNA reduced the expression of p-GSK-3α and MCL1 proteins, without affecting that of total GSK-3α or BCL2 (Figure 3D).
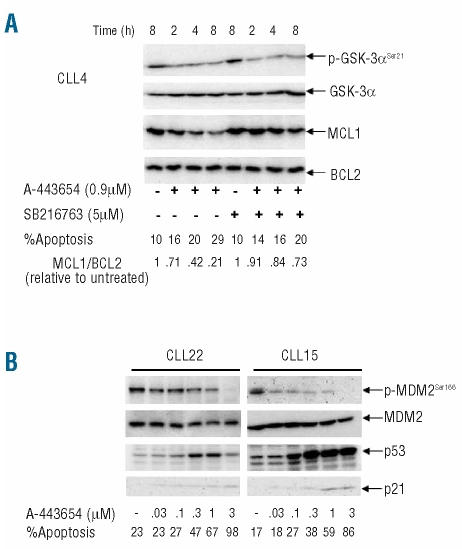
We next investigated how Akt inhibition leads to the degradation of MCL1. We focused on GSK-3 because this kinase has been shown in other cell types to be a direct link between Akt and MCL1.48 Active Akt inhibits GSK-3 through inhibitory phosphorylation. Consequently, inhibition of Akt releases GSK-3 from this inhibition and allows the kinase to phosphorylate MCL1 (at serine 159), resulting in increased ubiquitination and proteasomal degradation of MCL1.19 We, therefore, hypothesized that inhibition of GSK-3 activity would stabilize MCL1 in CLL cells treated with the Akt inhibitor. To test this idea, we used SB216763, a relatively selective inhibitor of GSK-3.49 When cells were co-incubated with the Akt and GSK-3 inhibitors, the latter clearly reduced the loss of MCL1, without affecting levels of p-GSK-3· or BCL2 (Figure 4A). These results indicate that active GSK-3 does indeed contribute to the degradation of MCL1 in CLL cells.
Figure 4.
The pro-apoptotic effect of Akt inhibition involves both the GSK-3/MCL1 and p53 pathways. (A) Western blotting analysis was used to determine the effects on MCL1 and BCL2 of prior incubation of CLL cells for 1 h with a GSK-3 inhibitor (SB216763) followed by culture in the presence or absence of A-443654. This is a representative example of three experiments on cells from three different CLL cases. (B) The effects of incubation (24 h) with A-443654 on p53, phospho-MDM2 and p21 are shown. These two panels are representative of five experiments on cells from five different CLL patients.
Having demonstrated that the Akt/GSK-3/MCL1 pathway is involved in the survival of CLL cells, we next examined the involvement of the p53 pathway in the cell death induced by Akt inhibition. As shown in Figure 4B, A-443654 increased expression of p53 protein, and at the same time inhibited activation of MDM2 (as measured by the loss of phorphorylated serine 166). As Akt has been shown to phosphorylate MDM2 specifically at serine 166, resulting in the increased nuclear translocation of this ubiquitin ligase specific for p53,20 the above result suggests that activated Akt in CLL cells stimulates loss of p53 via MDM2-mediated ubiquitination and degradation. The increased p53 induced by the Akt inhibitor was also associated with the induction of p21 (Figure 4B), a major transcriptional target of p53. This suggests that the up-regulated p53 is transcriptionally active. Taken together, our data show that the apoptosis induced by Akt inhibition is associated with activation of p53, as well as loss of MCL1.
Chronic lymphocytic leukemia cells are more sensitive to the pro-apoptotic effect of Akt inhibitors than are normal peripheral blood mononuclear cells
In order to establish the potential value of Akt inhibitors as a therapeutic strategy in CLL, it was important to determine whether the cytotoxic effect of Akt inhibitors showed selectivity towards CLL cells. To address this question, we examined their effect on normal PBMC which were also shown to contain significant amounts of phosphorylated Akt (Figure 5A). At concentrations of 0.3 to 1 μM, which induced extensive apoptosis in CLL cells (Figure 2C and D), A-443654 produced minimal apoptosis as measured by phosphatidylserine exposure and PARP cleavage (Figure 5B). Likewise, Akti-1/2, at concentrations (10–30 μM) that induced substantial apoptosis in CLL cells (Figure 2E), did not cause apoptosis of normal PBMC (Figure 5C). This indicates that normal PBMC are less dependent on Akt for survival than are CLL cells, and supports the notion that Akt inhibitors may have therapeutic potential in CLL.
Figure 5.
The pro-apoptotic effect of Akt inhibitors is less potent in normal PBMC. (A) p-Akt is readily demonstrable in normal PBMC by western blotting (methods described in Figure 1). (B) Normal PBMC were cultured for 24 h in the presence or absence of different concentrations of A-443654, and apoptosis and p-GSK-3 measured as before. The three lower panels are representative western blots from the same experiments. (C) The results of similar experiments with the second inhibitor Akti-1/2. In both (B) and (C), each data point in the graph represents the mean ± SD of at least three experiments involving the indicated number of different normal volunteers.
Sensitivity of chronic lymphocytic leukemia cells to Akt inhibition correlates inversely with p53 dysfunction and losses of 17p/11q, but not with other prognostic indicators
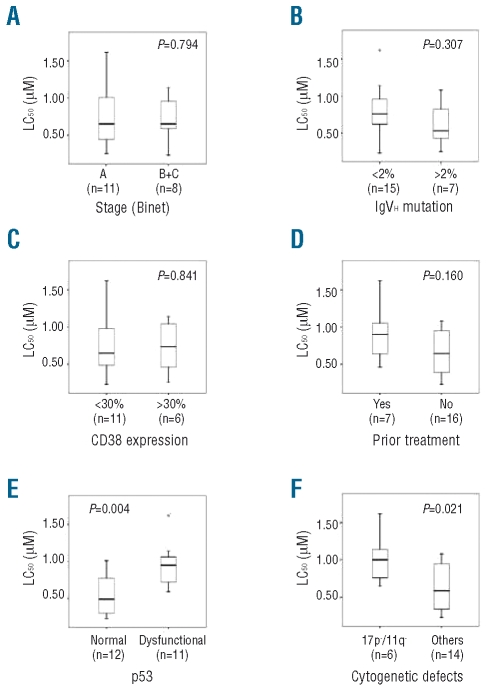
Having established that the apoptotic effect of A-443654 is relatively selective for CLL cells, we next investigated whether or not the Akt inhibitor induces apoptosis equally in cells from sub-groups of the disease with different prognoses. Subgroups were identified by differences in clinical stage, IgVH mutation, and CD38 expression, by whether or not patients had been previously treated, and by whether CLL cells displayed p53/ATM dysfunction or loss of 17p/11q. Only for the group with p53/ATM dysfunction or loss was any correlation observed (Figure 6). p53 dysfunctional clones were significantly less sensitive to the induction of apoptosis by A-443654 [median LC50 =0.95 μM (range, 0.59–1.62 μM) versus 0.49 μM (range, 0.23–1.01 μM) for CLL clones displaying normal p53 function, P<0.01] (Figure 6E). Similarly, the 17p−/11q− CLL samples were less sensitive to A-443654 than were cells without these chromosomal defects [median LC50 =1.0 μM (range, 0.65–1.62 μM) versus 0.58 μM (range, 0.23–1.08 μM) respectively, P<0.05] (Figure 6F).
Figure 6.
Sensitivity of CLL cells to Akt inhibition correlates inversely with p53 dysfunction and loss of 17p/11q, but not with other prognostic indicators. Clinical stage (A), IgVH mutation (B), CD38 expression (C) and p53 dysfunction (E) were determined as described elsewhere.5,31,52 (D) Prior treatment consisted of therapeutic administration of various combinations of steroid, chlorambucil, fludarabine, or fludarabine plus cyclophosphamide. (F) The sensitivity to A-443654 of clones with deletion of 17p or 11q was compared with that of all other CLL clones for which cytogenetic data were available.
Discussion
This study was conducted to define the role of Akt as a potential therapeutic target in CLL in the light of previous contradictory data concerning the activation status of the enzyme in unstimulated CLL cells. Using cell lysates extracted by sonication in 1% Triton X-100, we readily detected p-Akt in the cells of all 26 cases examined. Furthermore, the Akt was fully activated since the kinase was phosphorylated on both serine 473 and threonine 308. Also, treatment of CLL cells with two different Akt inhibitors consistently resulted in dose-dependent inhibition of Akt activity, as measured by the loss of phosphorylated GSK-3 and MDM2, two well-characterized direct downstream substrates of Akt.14
Our findings, therefore, support those of previous studies suggesting that CLL cells express phosphorylated Akt,25–27 but disagree with others failing to detect the activated enzyme in unstimulated CLL cells.8–10,28 Consequently, we went to considerable lengths to validate our positive findings. In particular, we showed that freezing/thawing and short periods of culture after thawing have little effect on the amounts of p-Akt detected. Also, the specificity of the anti-p-Akt antibodies employed was confirmed with extensive controls.
Having conclusively shown that unstimulated CLL cells contain activated Akt – a finding fully in accord with the current notion that all CLL cells have been variably activated in vivo1–3 – we investigated the role of the kinase in CLL-cell survival. Surprisingly, although it is established that PI-3 kinase is important in CLL-cell survival,8–10 there have been no studies of the effects on CLL cells of direct Akt inhibition. We, therefore, inhibited Akt activity with two different specific chemical inhibitors and with siRNA knock-down of the kinase. All three methods of inhibition induced apoptosis. Mechanistically, this apoptosis was associated with loss of MCL1, but not BCL2. Furthermore, a caspase inhibitor had no effect on the loss of MCL1, while a proteasome inhibitor prevented this loss. In addition, we demonstrated that this proteasomal degradation of MCL1 is mediated by GSK-3. However, this Akt/GSK-3/MCL1 pathway is unlikely to be the only mechanism by which Akt inhibition leads to MCL1 loss. For example, Akt indirectly activates mTORC1,14 thereby promoting translation of MCL1 through a GSK-3-independent mechanism.18 This may explain why, in the present study, the GSK-3 inhibitor employed did not completely abrogate the loss of MCL1 induced by Akt inhibition (Figure 4A).
In addition, Akt is known to favor cell survival by inhibiting the pro-apoptotic p53 pathway through activating MDM2, the ubiquitin ligase targeting p53 for degradation.20 We, therefore, also examined involvement of this pathway in the apoptosis of CLL cells induced by Akt inhibition. We found that such inhibition resulted in the loss of phorphorylated MDM2, with consequent increased expression of p53, and associated cell death. This increased p53 was associated with the induction of p21, suggesting that the p53 induced by the Akt inhibitor is transcriptionally functional. However, it is likely that the p53 induced by the Akt inhibition acts at post-transcriptional level since it has recently been reported that, in CLL cells, p53 induces apoptosis mainly through a non-transcriptional mechanism involving direct binding to, and inhibition of, mitochondrial anti-apoptotic proteins including BCL2.50 We, therefore, conclude that inhibition of Akt is strongly pro-apoptotic via at least two mechanisms involving the loss of MCL1 and activation of the p53 pathway.
Our demonstration that activated Akt is important in the survival of unstimulated CLL cells is in accord with previous studies implicating Akt activation in the pro-survival effect of various stimuli.25,26 In particular, our results complement the recent work of Longo et al.51 who introduced activated Akt into CLL cells and observed increased cell survival associated with upregulation of MCL1. However, by showing that Akt is activated in primary CLL cells, our work raises the possibility that inhibitors of Akt may prove useful in treatment. This led us to examine the effect of Akt inhibition on the survival of normal PBMC as compared with CLL cells. In fact, normal PBMC, despite containing substantial amounts of p-Akt, were largely insensitive to the Akt inhibitors at concentrations that produced extensive apoptosis of CLL cells. The pro-apoptotic effect of Akt inhibitors in CLL cells is, therefore, relatively selective. Consequently, we suggest that these agents may have therapeutic potential in CLL.
Supplementary Material
Footnotes
Funding: this work was supported by the Leukemia Research UK.
The online version of this article has a supplementary appendix.
Authorship and Disclosures
JZ designed the study, performed most of the experimental work, analyzed data and wrote the paper, while SFH and MAG performed some of the experiments. KL carried out the statistical analysis of the data and, together with GGJ, performed the IgVH mutational analysis. AC did the FACS analysis of CD38 expression and p53 function. JCC and ARP provided clinical samples and information, and contributed to the design of the study, analysis of the data, and writing the paper.
The authors declare no potential conflicts of interests.
References
- 1.Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogenous phenotype related to memory B cells. J Exp Med. 2001;194(11):1625–38. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Caligaris-Cappio F, Ghia P. Novel insights in chronic lymphocytic leukemia: are we getting closer to understanding the pathogenesis of the disease? J Clin Oncol. 2008;26 (27):4497–503. doi: 10.1200/JCO.2007.15.4393. [DOI] [PubMed] [Google Scholar]
- 4.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820–8. [PubMed] [Google Scholar]
- 5.Pettitt AR, Sherrington PD, Stewart G, Cawley JC, Taylor MR, Stankovic T. p53 dysfunction in B-cell chronic lymphocytic leukemia: inactivation of ATM as an alternative to TP53 mutation. Blood. 2001;98(3):814–22. doi: 10.1182/blood.v98.3.814. [DOI] [PubMed] [Google Scholar]
- 6.di Celle PF, Mariani S, Riera L, Stacchini A, Reato G, Foa R. Interleukin-8 induces the accumulation of B-cell chronic lymphocytic leukemia cells by prolonging survival in an autocrine fashion. Blood. 1996;87(10):4382–9. [PubMed] [Google Scholar]
- 7.Pedersen IM, Reed JC. Microenvironmental interactions and survival of CLL B-cells. Leuk Lymphoma. 2004;45(12):2365–72. doi: 10.1080/10428190412331272703. [DOI] [PubMed] [Google Scholar]
- 8.Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98(10):3050–7. doi: 10.1182/blood.v98.10.3050. [DOI] [PubMed] [Google Scholar]
- 9.Barragàn M, Bellosillo B, Campàs C, Colomer D, Pons G, Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99(8):2969–76. doi: 10.1182/blood.v99.8.2969. [DOI] [PubMed] [Google Scholar]
- 10.Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100(10):3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 12.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 13.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–42. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Manning BD, Cantley LC. AKT/PKB signalling: navigating downstream. Cell. 2007;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 16.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10(19):1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 17.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203(7):1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, et al. mTORC1 promotes survival through translational control of MCL-1. Proc Natl Acad Sci USA. 2008;105(31):10853–8. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21(6):749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98(20):11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signaling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428(6980):332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 22.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448 (7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 23.Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA. 2002;99(10):6955–60. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pekarsky Y, Zanesi N, Aqeilan RI, Croce CM. Animal models for chronic lymphocytic leukemia. J Cell Biochem. 2007;100(5):1109–18. doi: 10.1002/jcb.21147. [DOI] [PubMed] [Google Scholar]
- 25.Cunì S, Pérez-Aciego P, Pérez-Chacòn G, Vargas JA, Sànchez A, Martìn-Saavedra FM, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18(8):1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 26.Petlickovski A, Laurenti L, Li X, Marietti S, Chiusolo P, Sica S, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):4820–7. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 27.Ticchioni M, Essafi M, Jeandel PY, Davi F, Cassuto JP, Deckert M, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26(50):7081–91. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–95. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68(7):2366–74. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 30.Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, et al. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22(1):147–60. doi: 10.1038/sj.leu.2404980. [DOI] [PubMed] [Google Scholar]
- 31.Lin K, Sherrington PD, Dennis M, Matrai Z, Cawley JC, Pettitt AR. Relationship between p53 dysfunction, CD38 expression, and IgV(H) mutation in chronic lymphocytic leukemia. Blood. 2002;100(4):1404–9. doi: 10.1182/blood-2001-11-0066. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Liu X, Han EK, Guan R, Shoemaker AR, Oleksijew A, et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of akt in vitro and in vivo. Neoplasia. 2005;7(11):992–1000. doi: 10.1593/neo.05355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, et al. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4(6):977–86. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- 34.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem J. 2005;385(Pt 2):399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logie L, Ruiz-Alcaraz AJ, Keane M, Woods YL, Bain J, Marquez R, et al. Characterization of a protein kinase B inhibitor in vitro and in insulin-treated liver cells. Diabetes. 2007;56(9):2218–27. doi: 10.2337/db07-0343. [DOI] [PubMed] [Google Scholar]
- 37.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4(2):271–9. [PubMed] [Google Scholar]
- 38.Zhuang J, Ren Y, Snowden RT, Zhu H, Gogvadze V, Savill JS, et al. Dissociation of phagocyte recognition of cells undergoing apoptosis from other features of the apoptotic program. J Biol Chem. 1998;273(25):15628–32. doi: 10.1074/jbc.273.25.15628. [DOI] [PubMed] [Google Scholar]
- 39.Rudelius M, Pittaluga S, Nishizuka S, Pham TH, Fend F, Jaffe ES, et al. Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma. Blood. 2006;108(5):1668–76. doi: 10.1182/blood-2006-04-015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114 (4):441–9. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood. 1998;91(9):3379–89. [PubMed] [Google Scholar]
- 42.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112(9):3807–17. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 43.Döhner H, Fischer K, Bentz M, Hansen K, Benner A, Cabot G, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85(6):1580–9. [PubMed] [Google Scholar]
- 44.el Rouby S, Thomas A, Costin D, Rosenberg CR, Potmesil M, Silber R, et al. p53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood. 1993;82(11):3452–9. [PubMed] [Google Scholar]
- 45.Snowden RT, Sun XM, Dyer MJ, Cohen GM. Bisindolylmaleimide IX is a potent inducer of apoptosis in chronic lymphocytic leukaemic cells and activates cleavage of Mcl-1. Leukemia. 2003;17(10):1981–9. doi: 10.1038/sj.leu.2403088. [DOI] [PubMed] [Google Scholar]
- 46.Jantus-Lewintre E, Sarsotti E, Terol MJ, Benet I, Garcìa-Conde J. Bortezomib induces different apoptotic rates in B-CLL cells according to IgVH and BCL-6 mutations. Clin Transl Oncol. 2006;8(11):805–11. doi: 10.1007/s12094-006-0136-3. [DOI] [PubMed] [Google Scholar]
- 47.Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, et al. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111(5):2797–805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 48.Ding Q, He X, Xia W, Hsu JM, Chen CT, Li LY, et al. Myeloid cell leukemia-1 inversely correlates with gkycogen synthase kinase-3beta activity and associates with poor prognosis in human breast cencer. Cancer Res. 2007;67(10):4564–71. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 49.Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurons from death. J Neurochem. 2001;77(1):94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 50.Steele AJ, Prentice AG, Hoffbrand AV, Yogashangary BC, Hart SM, Nacheva EP, et al. p53-mediated apoptosis of CLL cells: evidence for a transcription-independent mechanism. Blood. 2008;112(9):3827–34. doi: 10.1182/blood-2008-05-156380. [DOI] [PubMed] [Google Scholar]
- 51.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111(2):846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 52.Carter A, Lin K, Sherrington PD, Pettitt AR. Detection of p53 dysfunction by flow cytometry in chronic lymphocytic leukaemia. Br J Haematol. 2004;127(4):425–8. doi: 10.1111/j.1365-2141.2004.05223.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.