Nonsense-mediated mRNA decay (NMD) is an intron-dependent RNA-degradation pathway responsible for depleting transcripts containing premature termination codons (PTCs), presumably to control the synthesis of truncated proteins, potentially deleterious to cells. PTC-bearing (PTC+) mRNAs are unstable only when the PTC is located more than 50–55 nucleotides upstream of the last intron.1 However, not all genes undergo NMD. Among coagulation genes, NMD was demonstrated for factors V, XI, and XIII, whereas it was shown to be inactive for fibrinogen (FGA, FGG) and factor VIII (FVIII) genes (Online Supplementary Table S1).
Von Willebrand factor (VWF) is a multimeric glycoprotein, synthesized by endothelial cells and megakaryocytes, promoting both platelet adhesion to the subendothelium at sites of vascular injury, and platelet-platelet interactions in high shear-rate conditions. It also binds and stabilizes FVIII.2
Quantitative VWF deficiency can be classified as partial (VWD1) or complete (VWD3), whereas qualitative defects (VWD2) are subdivided into four main types: VWD2A, VWD2B, VWD2M, VWD2N.2
The aim of this study was to investigate whether PTC-introducing mutations in the VWF gene are associated with NMD.
To this purpose, three unrelated Italian probands (P1–P3), heterozygous for at least one truncating mutation, were studied (Figure 1). Their main clinical characteristics are listed in the Online Supplementary Table S2.
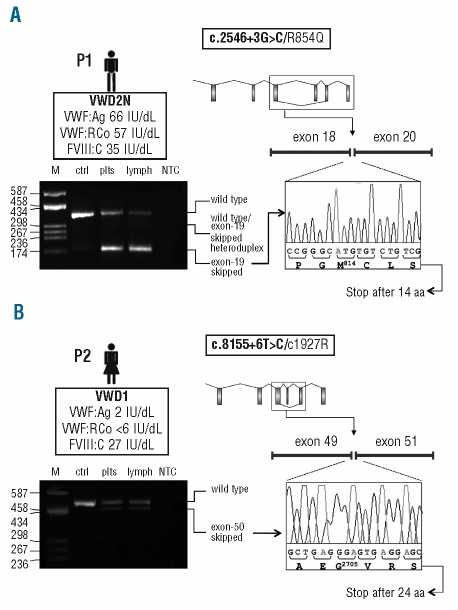
Figure 1.
Functional analysis of the effect of the c.2546+3G>C and c.8155+6T>C splicing mutations on the VWF mRNA. (A, B) Top left: In the box underneath the patient’s ideogram: patient’s VWD type (in bold), VWF:Ag, VWF:RCo, and FVIII:C levels are listed. Top right: Schematic representation of the VWF gene. Exons (boxes) and introns (lines) are drawn to scale. The aberrant splicing event observed in each patient (i.e. exon-19 and exon-50 skipping for patients carrying the c.2546+3G>C or the c.8155+6T>C mutations, respectively) is represented. The patient’s mutations are indicated in the box above the gene scheme, with the splicing mutation in bold. Mutation numbering is according to GenBank accession numbers NM_000552.3 (according to cDNA position) and NP_000543.2 (native protein). Bottom left: RT-PCR products separated on a 2% agarose gel. Lane M: molecular weight marker (pUC8-HaeIII); other lanes (from left to right): RT-PCR products amplified from total RNA extracted from patients’ platelets (plts) and lymphocytes (lymph), and from platelet-derived RNA of a wild-type individual (ctrl); lane NTC: no template control. Note: the exon-19 skipped transcript was detectable only after a semi-nested PCR, performed starting from 1 μL of the original RT-PCR product using a specific primer spanning exons 18–20. Traces of the aberrantly-spliced product were evidenced also in the wild-type sample, indicating that this splicing is present also under physiological conditions. Bottom right: Sequence electropherograms of RT-PCR products confirming the aberrant junction between exons 18/20 (c.2546+3G>C), panel A, and exons 49/51 (c.8155+6T>C), panel B.
P1 has VWD2N and is compound heterozygous for the previously reported R854Q mutation (c.2561G>A, exon 20)2 and the novel c.2546+3G>C splicing defect (intron 19). P2 has VWD1 caused by compound heterozygosity for two novel mutations: C1927R (c.5779T>C, exon 34) and c.8155+6T>C (intron 50). P3 is heterozygous for the VWD3-causing c.6182delT mutation (exon 36).3
To evaluate the effect of the novel c.2546+3G>C and c.8155+6T>C splicing mutations on VWF pre-mRNA processing, cDNA regions spanning exons 18–21 and 49–52 were amplified by RT-PCR from platelet- and lymphocyte-derived mRNA of each patient. Sequencing of RT-PCR products demonstrated that c.8155+6T>C causes the skipping of exon 50 (Figure 1B), leading to a PTC in exon 51 (for details on methods, see Online Supplementary Appendix).
Concerning c.2546+3G>C, a product lacking exon 19 could be amplified and sequenced only after a second semi-nested PCR (Figure 1A); this mutation would lead to the introduction of a PTC in exon 20. A very low amount of the same skipped transcript could be detected also in the control individual, indicating the existence of a “physiological” aberrant splicing event.
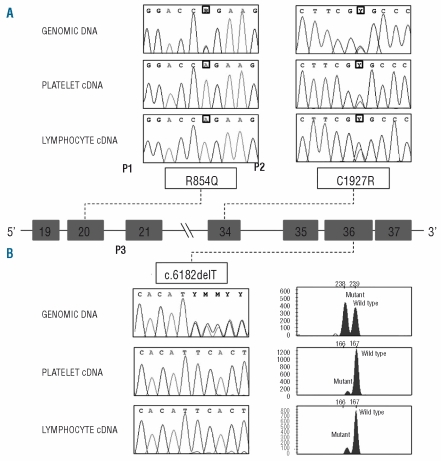
To investigate whether the two PTC-introducing splicing defects are associated with mRNA degradation, a fragment containing the relevant missense mutation was PCR amplified from genomic DNA and from platelet and lymphocyte cDNAs of P1 and P2, and sequenced.
Concerning P1, the product obtained from genomic DNA resulted heterozygous for R854Q, whereas platelet- and lymphocyte-derived RT-PCR products were homozygous for this missense substitution (Figure 2A), confirming that the PTC+ allele was degraded. As for patient P2, the C1927R mutation was detected in the heterozygous state both on genomic DNA and on cDNA (Figure 2A), suggesting that the PTC+ allele is not subjected to NMD, as expected for a PTC located 23 bp upstream of the last exon-exon junction.
Figure 2.
Missense mutations and 1-bp deletion analyses. The central panel shows a schematic representation of part of the VWF gene (introns 18–21 and introns 33–37; exons are represented by boxes, introns by lines, and are not drawn to scale). Panel (A) Electropherograms of the regions surrounding the missense mutations sequenced on the genomic DNA and on platelet and lymphocyte cDNAs of the 2 patients. The position of the mutations is boxed. Panel (B) left: Electropherograms of the region surrounding the 1-bp deletion obtained by sequencing PCR-amplified fragments from genomic DNA and on platelet and lymphocyte cDNAs of P3. Panel (B) right: GeneMapper windows displaying fluorescence peaks corresponding to wild-type and mutant fragments obtained from genomic DNA as well as platelet and lymphocyte cDNAs. The areas of the fluorescence peaks corresponding to the mutant and wild-type PCR fragments were measured by the GeneMapper v4.0 software. The X-axis represents GeneMapper data points and the Y-axis represents fluorescence units (FUs).
Patient P3 was similar to P1 in that the genomic sequence was heterozygous for the T deletion, whereas the cDNA sequences appeared wild type (Figure 2B), suggesting a selective degradation of the mutant transcript. To calculate the extent of the PTC+ mRNA degradation, the fragment containing the T deletion was also PCR amplified using a (6-Fam)-labeled primer. PCR reactions were separated on an ABI-3130XL sequencer and the peak areas measured by the GeneMapper v4.0 software. On genomic DNA, the wild-type/mutant ratio was equal to ~1, as expected. Conversely, a degradation of 91.2% and 86.1% of the PTC+ mRNA was calculated in platelets and lymphocytes (Figure 2B).
To summarize, our data consistently demonstrate that truncated VWF proteins are unlikely to be produced as a result of mRNA degradation, a topic on which conflicting data were reported in the literature.4–6 Moreover, we confirm that NMD susceptibility of VWF transcripts is PTC-position dependent. Last but not least, we were able to measure the extent of degradation of the c.6182delT transcript (~90%).
Given the effects of VWF inhibitors, i.e. ineffectiveness of replacement therapy and anaphylactic reactions to treatment,2 it would be important to establish if NMD might be a modulator of inhibitor development. Considering that some PTC-introducing mutations escape degradation even in genes known to be targets of NMD7,8 it would be interesting to analyze a larger group of VWD3 patients with truncating mutations, to verify if mRNA degradation represents a general rule for truncating mutations in the VWF gene.
Acknowledgments
the authors would like to thank Rosanna Garavaglia who followed up the patients, biologist Giovanna Cozzi for the assays performed to diagnose VWD, and Maria Teresa Canciani for her helpful thoughts and suggestions on discussion of the data.
Footnotes
Funding: this study was supported by research funding from Bayer HealthCare to LB.
The online version of this article has a supplementary appendix.
References
- 1.Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21(15):1833–56. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 2.Castaman G, Federici AB, Rodeghiero F, Mannucci PM. Von Willebrand’s disease in the year 2003: towards the complete identification of gene defects for correct diagnosis and treatment. Haematologica. 2003;88(1):94–108. [PubMed] [Google Scholar]
- 3.Baronciani L, Cozzi G, Canciani MT, Peyvandi F, Srivastava A, Federici AB, Mannucci PM. Molecular defects in type 3 von Willebrand disease: updated results from 40 multiethnic patients. Blood Cells Mol Dis. 2003;30(3):264–70. doi: 10.1016/s1079-9796(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Cumming A, Grundy P, Keeney S, Lester W, Enayat S, Guilliatt A, et al. An investigation of the von Willebrand factor genotype in UK patients diagnosed to have type 1 von Willebrand disease. Thromb Haemost. 2006;96(5):630–41. [PubMed] [Google Scholar]
- 5.Eikenboom JC, Ploos van Amstel HK, Reitsma PH, Briët E. Mutations in severe, type III von Willebrand’s disease in the Dutch population: candidate missense and nonsense mutations associated with reduced levels of von Willebrand factor messenger RNA. Thromb Haemost. 1992;68(4):448–54. [PubMed] [Google Scholar]
- 6.Mohlke KL, Nichols WC, Rehemtulla A, Kaufman RJ, Fagerström HM, Ritvanen KL, et al. A common frameshift mutation in von Willebrand factor does not alter mRNA stability but interferes with normal propeptide processing. Br J Haematol. 1996;95(1):184–91. doi: 10.1046/j.1365-2141.1996.7572377.x. [DOI] [PubMed] [Google Scholar]
- 7.Danckwardt S, Neu-Yilik G, Thermann R, Frede U, Hentze MW, Kulozik AE. Abnormally spliced beta-globin mRNAs: a single point mutation generates transcripts sensitive and insensitive to nonsense-mediated mRNA decay. Blood. 2002;99(5):1811–6. doi: 10.1182/blood.v99.5.1811. [DOI] [PubMed] [Google Scholar]
- 8.Dreumont N, Maresca A, Boisclair-Lachance JF, Bergeron A, Tanguay RM. A minor alternative transcript of the fumarylacetoacetate hydrolase gene produces a protein despite being likely subjected to nonsense-mediated mRNA decay. BMC Mol Biol. 2005;6(1):1. doi: 10.1186/1471-2199-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]