Abstract
Glomerular capillary hemorrhage (GCH) in rat kidney provided a model for assessing in vivo gas body efficacy in diagnostic or therapeutic applications of ultrasound. Two diagnostic ultrasound machines were utilized: one monitored the harmonic B-mode contrast-enhancement of the left kidney and the other exposed the right kidney for GCH production. Definity contrast agent was infused at 1, 2, 5 or 10 μl/kg/min and infusion durations were 30, 60, 120 or 300 s. Exposure of the right kidney was at a peak rarefactional pressure amplitude of 2.3 MPa at 1.5 MHz. The circulating dose was estimated with a simple model of agent dilution and gas body loss. For 300 s infusion at 5 μl/kg/min, the left kidney image brightness increased to a plateau with an estimated 6.4±1.3 μl/kg circulating dose with no GCH in histological sections. Exposure of the right kidney with a 1 s image interval reduced the estimated circulating dose to 1.3±0.3 μl/kg and induced 68.4 % GCH. Dose and duration increases gave rapidly diminishing treatment effectiveness per gas body. The effective in vivo agent dose in rats can be reduced greatly due to high gas body destruction in the small animal, complicating predictions for similar conditions of human treatment.
Keywords: ultrasound contrast agent, ultrasonic cavitation biology, non-thermal ultrasound therapy, diagnostic ultrasound adverse effects, glomerular hemorrhage, flash-echo imaging
Introduction
The study of ultrasonic cavitation biology has been aided by the availability of contrast agents for diagnostic ultrasound imaging. These agents are injectable suspensions of gas bodies (stabilized microbubbles), which circulate with the blood and yield enhanced ultrasound image brightness. The strong echoes result from pulsation of the gas bodies in response to the pulses of diagnostic ultrasound, for which peak rarefactional pressure amplitudes (PRPAs) exceeding 1.9 MPa (giving a tension 19 times atmospheric pressure) are common. Gas bodies in commercial contrast agents can be destabilized at PRPAs within the range of diagnostic ultrasound and rapidly disappear from the image. The gas bodies can also serve as individual nuclei for acoustic cavitation, which is of value for in vivo cavitation biology research. For this purpose, the contrast agent can be considered to be a cavitation-nucleation agent for adding nuclei to the circulation. The cavitation phenomenon can induce micro-scale bioeffects in tissue. This bioeffect mechanism poses some risk of harm in contrast enhanced diagnostic ultrasound [1]. Conversely, cavitation bioeffects provide new means for therapeutic applications of ultrasound using controlled nucleation with image-guided treatment. Several bioeffects have been associated with use of contrast-agent enhanced ultrasound, particularly in certain intermittent modes, which utilize the gas body destabilization and destruction for perfusion imaging [1]. An important bioeffect is rupture of capillaries. For example, in the kidney, glomerular capillary hemorrhage (GCH) flows into Bowman's urinary space and the associated tubules [2, 3]. Prospective therapeutic applications include directed drug delivery and gene therapy [4], among others [5]. However, the basic dosimetric understanding of the circulation and persistence of gas bodies remains limited and the efficiency of the gas body dose for cavitation nucleation and microscale bioeffects is uncertain.
Contrast agent gas bodies can nucleate inertial cavitation for exposure above the inertial cavitation threshold [6]. However, the destabilization and loss of contrast is not directly connected to inertial cavitation nucleation. This can be demonstrated in physical testing [7]. In particular, the destabilization is not directly associated with ultrasound exposure in terms of the on-screen mechanical index [8]. In biological testing, the contrast agent gas bodies can be destroyed without inducing bioeffects such as glomerular capillary rupture, even when the PRPA of the imaging pulses are sufficient for this bioeffect [9]. Continuous imaging at high PRPA produces minimal effect, while widely spaced intermittent scans with a fast sweep (brief image pulse sequences at a point) produce maximal effect. On the other hand, the agents can nucleate cavitation leading to bioeffects even after loss of image contrast [10]. This confusing situation complicates safety considerations for contrast enhanced diagnostic ultrasound and development of optimal efficacy strategies for therapeutic applications.
The well-defined GCH bioeffect in kidney represents a model system for evaluating the relation between gas body infusion, cavitation nuclei dose and capillary hemorrhage. Simple estimates of gas body efficacy, which neglect gas body loss, for glomerular capillary hemorrhage seem to indicate a rather low probability for a gas body to nucleate cavitation and rupture a capillary. Definity ultrasound contrast agent (perflutren lipid microsphere injectable suspension, Lantheus Medical Imaging, Inc., N. Billerica, MA USA) contains up to 1.2×1010 ml-1 gas bodies (package insert) with a recommended dose of 10 μl/kg. Rats have a blood volume of 64 ml/kg of body weight [11]. Therefore, the recommended dose would provide 0.156 μl of Definity, or 1.9×106 gas bodies per ml of blood in the circulation. The volume of the capillaries in a rat glomerulus is about 0.58×10-6 ml [12]. Thus each glomerulus might contain an average of 1.1 gas bodies at any given time after IV injection and dilution of the recommended dose in the circulating blood. From this estimate, the exposure of the kidney to a single ultrasound scan in excess of the cavitation threshold might be expected to yield GCH approaching 100 percent of the glomeruli. In fact, diagnostic ultrasound scanning only produced 37 % GCH after 60 scans at 1 sec intervals (to allow refill of the kidney tissue in the scan plane with blood containing contrast agent between images) during infusion of the recommended dose [3]. This suggests a very low efficiency of cavitation nucleation yielding induction of the bioeffect for circulating gas bodies, perhaps less than 1 %, which does not seem reasonable given the ideal size range of the gas bodies. A more accurate accounting of the gas bodies in relation to the resulting bioeffects is clearly needed. Such an accounting is particularly important for a meaningful extrapolation of bioeffect risk or therapeutic efficacy in animal models to potential human examinations or treatment.
The purpose of this study was to examine the GCH bioeffect in relation to the in vivo gas body dose. A method for assessing the concentration of circulating gas bodies in real time was needed. Ultrasound contrast agent imaging methods are suitable for this purpose, including harmonic image flash replenishment [13,14], Doppler [15] and other methods [16]. For this study, the left kidney was observed using an ultrasound machine with second harmonic B mode imaging and used to generate time intensity curves (TICs) of image enhancement. These curves are indicative of the increase in circulating contrast agent, as it is infused, and gradual decline after cessation of the infusion. In addition, the rats were scanned with a second ultrasound machine from the right side with intermittent diagnostic ultrasound image-exposures of the right kidney at high PRPA to induce GCH as in previous research [3]. The GCH was characterized by counting glomeruli with capillary hemorrhage in histological sections. The contrast agent dosage and exposure duration were varied. Simple modeling was used to interpret the TIC data in terms of circulating dose and to estimate the gas body nucleation efficiency for GCH. These experiments revealed a complicated dose-effect process, which involves substantial gas body loss for the small animals.
Methods
Animal preparation
This investigation was conducted with the approval of the University Committee on the Use and Care of Animals, University of Michigan. A total of 50 rats (CD hairless, Charles River Laboratories, Wilmington MA) weighing 310 ± 37 gm were used in this study. The rats were anesthetized with ketamine (87 mg/kg) plus xylazine (13 mg/kg) injected into the lower abdomen. A 24 gauge cannula was inserted into a tail vein for intravenous injections. The anesthetized rats were mounted with their ventral surface against a plastic holder, which then was fixed in a vertical position in a water bath filled with degassed water heated to 37°C.
Definity® (perflutren lipid microsphere injectable suspension, Lantheus Medical Imaging, Inc., N. Billerica, MA USA) was prepared each day according to the manufacturer's instructions. This contrast agent was diluted in sterile saline to the desired concentration and then the dilution was infused at 0.5 ml/kg/min. In different groups of rats, the infusion contained 1, 2, 5 or 10 μl/kg/min of Definity.
Ultrasound Exposure and GCH Measurement
The ultrasound exposure of the right kidney was provided by a diagnostic ultrasound machine, as described previously [3]. Briefly, a phased array probe (FPA2.5, GE Vingmed System V, General Electric Co., Cincinnati OH) was clamped in the water bath and operated at 1.5 MHz with the right kidney located 3.5-4.5 cm from the transducer face. At this position, the peak rarefactional pressure amplitude (PRPA) was maximal and the -6 dB scan plane thickness (beam width) was 4.6 mm. The kidney image was a cross section at the pelvis of the kidney. The PRPA at the kidney was determined by calibrated hydrophone measurement with correction for the attenuation between the skin and kidney (-0.45 dB) and was 2.3 MPa. This in situ PRPA corresponds to the PRPA expected for a Mechanical Index of MI= 1.9, which is essentially the upper limit for diagnostic ultrasound. Images were triggered intermittently with a 1 s or 10 s interval to allow contrast refill between images.
Glomerular capillary hemorrhage was evaluated by histology as described previously [3]. Kidney samples were removed, trimmed and immersion fixed in neutral buffered formalin. Histological processing was performed at the Research Histology and Immunoperoxidase Laboratory of the University of Michigan Comprehensive Cancer Center Tissue Core. The slides were examined by an observer blinded to the test conditions. Glomeruli were scored positive for GCH when red blood cells were seen in Bowman's urinary space. The percent GCH was calculated as 100 times the count, divided by the total number of glomeruli in the section.
Collection and Interpretation of Time-Intensity Curves
Another ultrasound machine (Powervision 8000, model SSA-390A, Toshiba Corporation, Tochigi-Ken, JPN) with curved linear array (PVN-375AT) was used to interrogate the left kidney. Flash echo imaging in harmonic B-mode was used (nominally 2.3 MHz transmit and 4.6 MHz receive frequencies) to enhance the gas body detection relative to tissue. This interrogation probe was aimed at the left kidney, and provided an assessment of the circulating dose of gas bodies specifically appearing in kidney tissue. The left kidney was 3-4 cm away and was magnified somewhat in size in the cross sectional images of 5 cm depth. The line of aim was about 90 degrees from the line of aim of the exposure transducer toward the right kidney on the other side of the spine, but the cross-sectional scan plane was about 5 mm lower for the left than for the right kidney. The dynamic range was set to the minimum of 30 dB with the gain adjusted for a nearly dark kidney image in the absence of contrast agent. As an optimum balance between minimizing gas body loss while maximizing detection sensitivity, the PRPA was set to 1.4 MPa PRPA, only one “flash” image scan was used without monitor images, and the scan interval was normally 6 s. This PRPA essentially avoided induction of GCH, because it was approximately at the threshold for GCH found previously at this frequency [17].
The measurement of the time-intensity curve (TIC) was performed using the machine's measurement functions on an oval-shaped region of interest. The region was placed over the left kidney image to include as much of the renal cortex as possible while excluding other bright structures (e. g. intestine) which moved slightly from image to image (due to breathing motion). The TIC function generated a mean illuminance value of the selected region for each image, yielding a curve with up to 101 points which was saved for off line analysis. The raw values of the TICs, compressed for image display, were indicative of the time varying numbers of gas bodies in the tissue [13,14]. The TICs included the period of infusion (wash in) and 300 s post infusion (wash out). For analysis, the values in the TIC were converted to dB (i. e. within the 30 dB dynamic range of the image) and adjusted to zero at the baseline (no contrast agent). The illuminance intensity values I were extracted from the compressed dB image values by the anti-log formula
| (1) |
This decompressed illuminance intensity of ultrasound backscatter was considered to be approximately proportional to contrast agent concentration.
In a simple closed loop circulation with rapid mixing, the circulating dose Ci(t) would increase linearly with time t at an infusion rate of ci, reaching Co = ciTi at the end of infusion duration Ti, which was assumed in the rough calculation outlined in the Introduction. However, the circulating gas bodies are lost from the blood through natural processes and ultrasound exposure during infusion at a rate of Ci(t)/τi, in which the loss time constant is τi. The change in the circulating dose Ci(t) of gas bodies with time (the differential d/dt) including gas body loss can be expressed as:
| (2) |
The solution to this equation is
| (3) |
For small t, Ci(t) is approximately equal to cit. For sufficient infusion duration (Ti≫τi), Ci(Ti) reaches a plateau equal to ciτi. The decaying circulationg dose Cd(t) of the gas bodies after the end of the infusion is calculated similarly:
| (4) |
This begins at the concentration existing at the end of infusion and decays to zero for t ≫τd. These two functions (Eqs. 3 and 4) are complementary for τd = τi, so that the integral over the two parts of the wash in and wash out yields the same result (area under the curve) as a rectangular curve of length Ti at the plateau level. However, τi and τd are not assumed to be equal, because the values of the constants are determined separately, usingthe TIC data as described below.
For characterization of the TICs in terms of the model functions (Eqs. 3 and 4), nonlinear curve fitting of the TIC data with exponential functions was performed (SigmaPlot 11, Systat Software Inc., San Jose, CA USA). The mathematical function of Eq. 3 was fitted to the TIC data during infusion and Eq. 4 was fitted to the TIC data beginning at the cessation of infusion. This returned the value of the time constants and the plateau level in terms of the illuminance intensity. Since the value of the infusion rate ci was known (set by the syringe pump assuming patency of the tail vein connection), the illuminance intensity could be calibrated approximately in terms of the circulating dose. However, it should be noted that the gas body dose contained in the infused volume dose can be altered by several factors (see Discussion).
This model of the infusion and gas body concentration oversimplifies the actual in vivo situation. The dilution of contrast agents (or other drugs) can be described by several models with varying complexity [18-22]. The simple model essentially assumed that there was only one diluting volume (the total blood volume). In fact, the initial infusion dilutes into the limited circulation of the veins, right ventricle, lungs and left ventricle before reaching the kidney. After passing through the kidney, the infusion is diluted into the entire blood pool before recirculation back into the kidney after several seconds. The recirculated concentration then builds with the continued infusion. However, for this study using slow infusions, for which the circulating dose reaches a plateau, the simple model above was useful for estimating the circulating dose achieved in vivo. The infusion time constant τi as determined was smaller than the true loss (decay) time constant, because of the initial dilution effect of the infusion during wash-in. The decay time constant τd was not influenced by the infusion, and was assumed to approximate the true loss time constant better than τi. Therefore, the fitted illuminance intensity plateau value was calibrated as Cp = ciτd, with the correction (1-e-Ti/τd) for smaller values of Ti. An overall reduction fraction for the gas body loss was calculated as the estimated circulating dose at the plateau divided by the total dose, which is given by
| (5) |
The value of τd depends on the test conditions, with a larger value expected for the sham tests and a smaller value expected for the exposure with added gas body destruction in the right kidney.
Experimental plan and statistics
The interrogation method provided a means to monitor the actual in vivo gas body dosage and account for destruction of gas bodies during exposure. Nine separate test groups were planned in order to examine the relation of the TIC curves to GCH, as listed in Table 1. Four different agent infusion dose-rates were used; 1, 2, 5, and 10 μl/kg/min for 2 min of infusion. In addition, infusion at 5 μl/kg/min was timed for durations of 0.5, 1, 2, and 5 min. An intermittent interval of 1 s was used for all exposures except for one group with a 10 s interval. For each test, the infusion wash-in and wash-out was run twice, both times with the interrogation probe and TIC determination on the left kidney. An additional 5 min delay was taken between the two runs to allow complete wash out. The first run was a sham exposure (i. e. a no-exposure control) of the right kidney. The second run added the exposure of the right kidney. This procedure was intended to provide a sham TIC for each individual animal together with an exposure TIC. For one group (A), both runs used a sham exposure, which is referred to as the sham group.
Table 1.
Infusion and durations for each of the 9 test groups. Two infusions with monitoring of the left kidney (sh) were performed for each animal with exposure of the right kidney (ex) during the second infusion for most groups.
| TEST | Number of rats | Infusion rate μl/kg/min | Total dose μl/kg | Inf. Duration minutes | Interval seconds | Kidney Samples |
|---|---|---|---|---|---|---|
| A sh/sh | 5 | 5 | 25 | 5 | 1 | left+right |
| B sh/ex | 4 | 1 | 2 | 2 | 1 | right |
| C sh/ex | 5 | 2 | 4 | 2 | 1 | right |
| D sh/ex | 4 | 5 | 10 | 2 | 1 | right |
| E sh/ex | 5 | 10 | 20 | 2 | 1 | right |
| F sh/ex | 5 | 5 | 2.5 | 0.5 | 1 | right |
| G sh/ex | 5 | 5 | 5 | 1 | 1 | right |
| H sh/ex | 5 | 5 | 25 | 5 | 1 | left+right |
| I sh/ex | 5 | 5 | 25 | 5 | 10 | right |
Numerical results for GCH and circulating dose estimates are presented as the mean plus/minus the standard deviation for groups of 4 or 5 samples, or plotted as the mean with standard error bars. Seven of the total of 50 rats were lost or excluded from the study. Two rats died, apparently from the anesthesia. Two rats were excluded from the study because a TIC curve for each rat inadvertently was not retained for analysis. Three rats were excluded due to poor wash-in results indicative of problems with patency of the tail vein infusion set up. Additional animals were used to replace 5 of these, so that 7 test groups had 5 rats and 2 groups had four rats, as listed in Table 1. For statistical analysis, Student's t-tests or Mann-Whitney rank sum tests were used to compare means of the measured parameters, with statistical significance assumed at P=0.05.
Results
TIC results
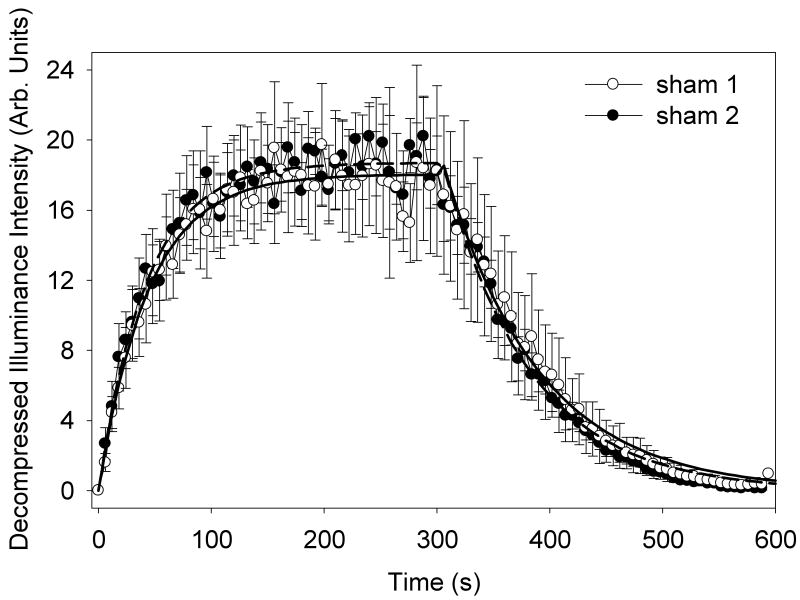
The TIC data was collected for each group and analyzed as described above. For group A, a 5 μl/kg/min infusion for 5 min, followed by a 5 min washout, produced the averaged TIC data shown in Fig. 1. The infusion and decay curves (Eqs. 3 and 4) were used to fit the data, and are shown as the solid lines in Fig. 1. These curves represent the trends in the data well. Average parameters for the curve fitting of individual results are listed in Table 2. The plateau in the data indicates a 12.6 dB contrast enhancement of the image for this infusion. The repeated sham was very similar with a 12.7 dB contrast enhancement and the reduction fractions were not significantly different (P=0.72). That is, the repetition of the sham test repeated the first result. This confirmed that the double infusion method in the same rat provided a second independent test for exposures, which was not effected by the first sham test. The reduction fractions of 0.26± 0.05 and 0.25 ± 0.02 indicate that a substantial fraction of the contrast agent gas bodies are lost during the 5 min infusions even for the sham tests.
Figure 1.
A plot of the mean illuminance time intensity curves (TICs) in arbitrary units for 5 rats with standard error bars showing the wash-in and wash-out of a 5 min contrast agent infusion. For this sham group, the sham exposure TIC was expected to duplicate the initial sham TIC, and the second set of data did have a very similar result. The two data sets are well represented by the fitted exponential functions (sham 1, solid curve and sham 2, dashed curve).
Table 2.
Curve fitting parameters with the average correlation coefficient for the sham and exposure TICs in tests with 5 min durations (groups A, H and I listed in Table 1). The gas body reduction fraction relative to no loss was calculated as τd/300 for these 300 s infusions. There was no significant difference between the different sham TIC sums, but the exposures greatly reduced the circulating dose, based on the TIC sums.
| Test | Infusion (wash-in) | Decay (wash-out) | Calibration μl/kg/(au) | Reduction Fraction | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| max a. u. | τi s | r2 | max. a. u | τd s | r2 | ||||
| A sham1 | 18 ± 8 | 45 ± 7 | 0.88 | 18 ± 11 | 77 ± 7 | 0.97 | 0.38 ± 0.11 | 0.26 ± 0.05 | 0.72 |
| A sham2 | 19 ± 4 | 49 ± 23 | 0.83 | 18 ± 4 | 74 ± 7 | 0.96 | 0.33 ± 0.06 | 0.25 ± 0.02 | |
| H sham | 15 ± 6 | 47 ± 15 | 0.86 | 14 ± 7 | 94 ± 20 | 0.96 | 0.63 ± 0.33 | 0.31 ± 0.07 | <0.01 |
| H expose | 3.3 ± 1.2 | 9.8 ± 2.9 | 0.52 | 1.6 ± 0.6 | 16 ± 3 | 0.93 | 0.46 ± 0.21 | 0.054 ± 0.010 | |
| I sham | 229 ± 4 | 67 ± 25 | 0.92 | 22 ± 6 | 101 ± 16 | 0.97 | 0.40 ± 0.13 | 0.34 ± 0.05 | <0.01 |
| I expose | 13.3 ± 2.6 | 38 ± 17 | 0.86 | 12 ± 4 | 43 ± 7 | 0.98 | 0.28 ± 0.06 | 0.15 ± 0.02 | |
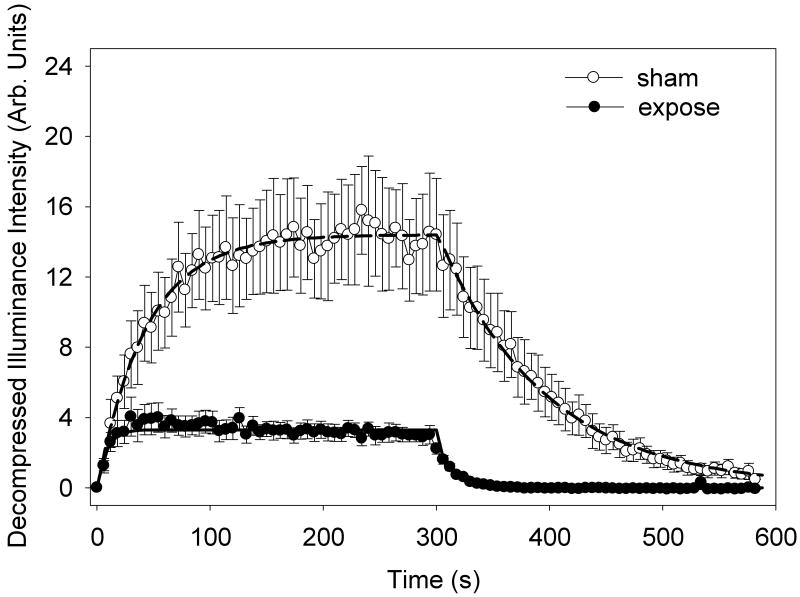
Group H received diagnostic ultrasound exposure to the right kidney during the expose test (second infusion). The TIC data are plotted in Fig. 2 for the sham and expose tests. The exposure produced a significant and substantial reduction in the TIC data, see Table 2. The mean image enhancement of the left kidney was 11.6 dB without exposure and 5.2 dB with exposure of the right kidney. In addition, there were a faster rise for the infusion and a faster decline for the decay part of the curves, with a decay time constant of 94.2 ± 19.8 s for the sham but only 16.0 ±3.1 s for the exposure. This implies that the concentration of contrast agent in the circulation was decreased by about 80 % due to gas body destruction by the exposure of the right kidney and other tissues in the scan plane (e. g., possibly including muscle, stomach, intestine, liver and large blood vessels). Furthermore, the gas body reduction fraction was 0.054 ± 0.01 in the expose test, which indicates substantial gas body destruction (P<0.01) above that in the sham test (reduction fraction 0.31± 0.07).
Figure 2.
A plot of the mean time intensity curves for 5 rats with standard error bars showing the wash-in and wash-out of a 5 min contrast agent infusion. The second infusion included exposure with a scan interval of 1 s of the opposite kidney, resulting in a substantial destruction of the circulating gas bodies and faster decay. The two data sets are well represented by the fitted exponential functions (solid curve for the exposure and dashed curve for the sham).
The sham tests (group A) had a substantial gas body reduction fraction of 0.25-0.26. This may have been a greater loss than the natural loss without ultrasound, because the interrogation probe exposed the left kidney to ultrasound. To assess the additional loss from the interrogation-exposure, two of the group A rats also had two infusion runs each using a 30 s interval between flash-echo frames (in addition to the two infusion with the normal 6 s interval). These four tests with reduced interrogation exposure had a τd value of 115 ± 11 s, with a reduction fraction of 0.38. The interrogation-exposure therefore appeared to have only a small impact on the gas body loss (relative to the right kidney exposures).
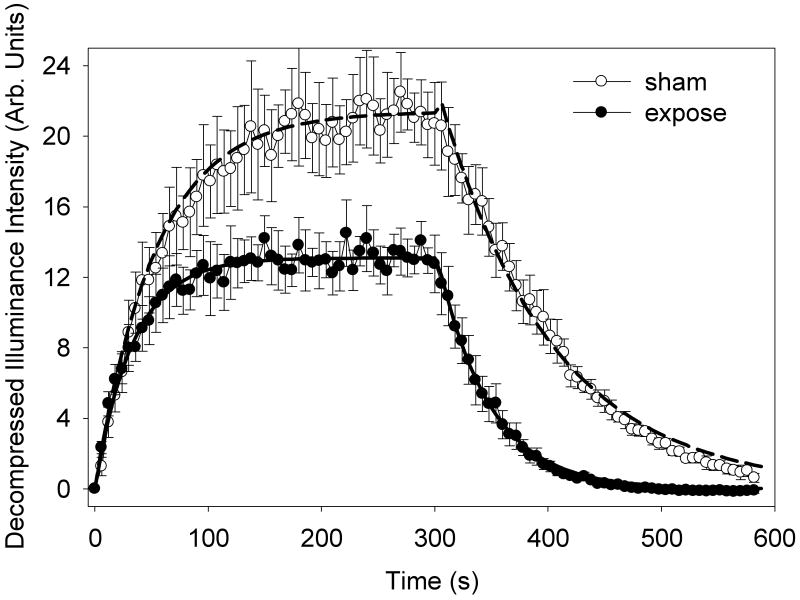
To confirm and clarify the apparent reduction in circulating gas bodies by the exposure, group I was exposed with a slower intermittent interval of 10 s (compared to 1 s in group H). The TIC data is shown in Fig. 3, and the characterization parameters are listed in Table 2. For this group, the left kidney contrast enhancement was 13.3 dB without and 11.2 dB with exposure of the right kidney. The gas body destruction from exposure was less than for group H (P<0.001) with a reduction fraction of 0.15 ± 0.02 in the expose test (0.31 ± 0.05 in the sham test). This indicated a reduction in gas body destruction, which was expected for the reduced number of exposure scans during infusion (600 for group H versus 60 for group I).
Figure 3.
A plot of the mean time intensity curves for 5 rats with standard error bars showing the wash-in and wash-out of a 5 min contrast agent infusion. The second infusion included exposure with a scan interval of 10 s of the opposite kidney, which significantly reduced the destruction of the circulating gas bodies seen in Fig. 2. The two data sets are well represented by the fitted exponential functions (sham, dashed curve and expose, solid curve).
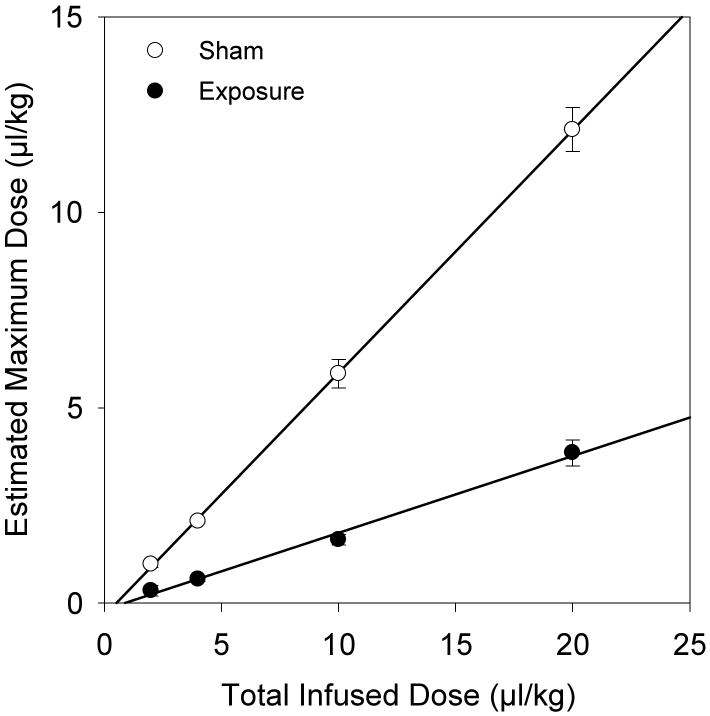
The dose series of 2 min infusions provide a means to confirm the proportionality of the TICs (illuminance intensity) to circulating gas body dose. The estimated maximum values for the TIC curves are plotted against total infused dose in Fig. 4 for both the sham and the exposure runs (groups B, C, D and E in Table 1). The linear regressions show proportionality with a correlation coefficient of r2 = 0.999 for the shams and 0.994 for the exposures. The slope of the line was 0.62 for the shams, and 0.21 for the exposures, indicating that only about 20% of the total infused dose was circulating during exposure at the end of infusion. The intercepts were -0.32 and - 0.17, for the shams and exposures, respectively, which suggests that the second harmonic TIC method cannot follow the concentration of agent for very low circulating doses.
Figure 4.
Results for the estimated circulating dose at the maxima of the time-intensity curves for two minute infusions with linear regression (solid lines). The detected in vivo doses were proportional to the actual total infused doses (r2 = 0.999 for shams and 0.994 for exposures), but reduced for the exposures by about 80% due to gas body destruction and loss.
GCH results
The GCH for group A was zero for both kidneys. For group H, the left kidney GCH was also zero in histology, apparently showing no bioeffect from the interrogation probe nor from the exposure of the right kidney. However, the left kidney did have a few petechiae grossly visible on the surface of 3 of 5 rats in group A (2, 4 and 5 petechiae) and also in group H (8, 4 and 5 petechiae), which indicated that the exposures related to the interrogation scans were near-threshold. The expose test in group H produced 68.4 % ± 12.1 % GCH in the right kidney, which was significantly different from the null Group A result (P<0.001).
The GCH from the exposures of group H and I are listed in Table 3. The decreased exposure with 10 s interval ameliorated gas body destruction with a plateau circulating dose of 3.6 ±0.6 μl/kg. In addition, this reduced right kidney GCH (P<0.001) but only to 30.3 %. Apparently, the increased circulating dose compensated substantially for the ten fold reduction in exposure. This illustrates the potential for relative gas body destruction to impact treatment outcomes, particularly for longer duration infusions, which might be designed to enhance results.
Table 3.
Selected measurements and estimated dose parameters for the second infusion test in each of the 9 groups (see Table 1). The exposures (ex) induced glomerular capillary hemorrhage (GHC) in the right kidney, while the sham-exposure (sh) did not.
| TEST | Infusion ci μl/kg/min | Infusion Ti min | GCH percent | Decay τd s | Maximum dose μl/kg | Reduction fraction |
|---|---|---|---|---|---|---|
| A sh | 5 | 5 | 0 | 76.9 ± 15.5 | 6.4 ± 1.3 | 0.26 ± 0.05 |
| B ex | 1 | 2 | 19.2 ± 11.3 | 19.2 ± 14.3 | 0.32 ± 0.24 | 0.16 ± 0.12 |
| C ex | 2 | 2 | 45.9 ± 18.2 | 18.5 ± 3.8 | 0.62 ± 0.13 | 0.15 ± 0.03 |
| D ex | 5 | 2 | 56.8 ± 23.1 | 19.4 ± 3.2 | 1.7 ± 0.3 | 0.16 ± 0.03 |
| E ex | 10 | 2 | 53.4 ± 17.6 | 23.1 ± 4.4 | 3.9 ± 0.7 | 0.19 ± 0.04 |
| F ex | 5 | 0.5 | 35.3 ± 27.2 | 16.7 ± 0.3 | 1.2 ± 0.1 | 0.46 ± 0.05 |
| G ex | 5 | 1 | 38.8 ± 15.3 | 19.6 ± 3.6 | 1.6 ± 0.2 | 0.31 ± 0.05 |
| H ex | 5 | 5 | 68.4 ± 12.1 | 16.0 ± 3.1 | 1.3 ± 0.3 | 0.054 ± 0.01 |
| I ex | 5 | 5 | 30.3 ± 7.3 | 43.4 ± 6.9 | 3.6 ± 0.6 | 0.15 ± 0.23 |
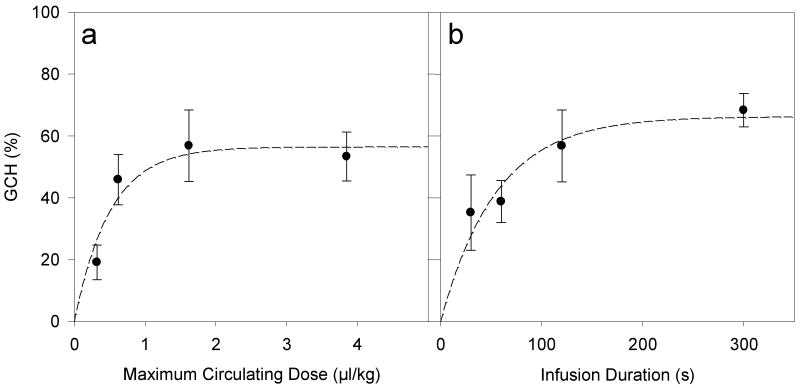
The use of an interrogation probe on the opposite kidney appeared to be a useful means to monitor the in vivo dose of contrast agent. Further GCH results were determined using this methodology with 1, 2, 5, and 10 μl/kg/min infusion for 120 s (groups B, C, D and E in Table 1), and 5 μl/kg/min infusions for 30, 60, 120 and 300 s (groups F, G, D and H in Table 1) all including a 5 min washout with results listed in Table 3. The GCH results for varied dose are plotted in Fig. 5a against the estimated circulating dose for the TIC plateau. The GCH increases rapidly for low doses but becomes nearly constant for higher doses, a phenomenon noted previously [3]. In Fig. 5b, the GCH results for varied duration are plotted against the infusion duration. In this range of parameters, the GCH is not proportional to either agent dose or duration but instead rises to a maximum, which further indicates that high doses and long infusion times do not lead to commensurate increases in this bioeffect. A two parameter exponential rise to plateau (the same mathematical form as Eq. 3) was fitted to both data sets. For Fig. 5a, the initial rate of rise was 112 %/(μl/kg) with a plateau of 56.5 % GCH (r2=0.81), while for Fig. 5b the initial rate of rise was 1.2 %/s with a plateau of 66.3 % GCH (r2=0.88).
Figure 5.
Plots of the mean GCH with standard error bars (a) for different contrast agent doses and (b) for different agent infusion durations. The results saturated for higher doses and durations, which was characterized by fitting the 2 parameter exponential curves (dashed curves).
Discussion
The goal of this study was to explore the gas body dosimetry for the glomerular capillary hemorrhage (GCH) effect in rat kidney. A fixed maximum PRPA was used for exposure scans of the right kidney, with variations in the dose and duration of contrast agent infusion. The left kidney was interrogated with a second ultrasound machine operating in second-harmonic imaging mode developed for characterizing the contrast enhancement in tissue. This interrogation mode provided time-intensity curves (TIC) which tracked the circulating dose of gas bodies present in kidney tissue. During infusion, the TIC rose to a plateau and then decayed after the infusion ended. The GCH results were approximately as expected from previous work [3], but in this study, the TIC results were available for estimating the in vivo gas body dose. Three important dosimetric factors where noted: the large gas body destruction in the small animals, the declining response for increasing doses or durations, and the efficiency of gas body activation for bioeffects.
Simplified theory for the wash in and wash-out TIC curves allowed the estimation of the reduction of the circulating dose relative to the infused dose and of the calibration of the TIC curves (Table 2). For 5 min shams, the circulating gas body dose at the plateau of the infusion was estimated to be only 0.25-0.26 of the total infused dose (Fig. 1), a reduction which appeared to be mostly the natural gas body attrition in the circulation (plus a small amount of gas body destruction from the interrogation probe ultrasound). For 5 min exposures (Fig. 2 and 3), the TIC plateaus were reduced greatly with reduction fractions of 0.054 for 1 s exposure interval. For 2 min exposures and varying infusion dose, the plateau circulating dose was proportional, but reduced to about 20 % of the infused dose (Fig. 4). Gas body destruction within the scan plane in small animals represents a substantial factor for estimating bioeffects risk or therapeutic efficacy. The gas body destruction likely would be much less in humans, because a much smaller fraction of the perfused bodily tissue would fall into the ultrasound scan plane. These considerations indicate that the effect achieved in humans due to diagnostic or therapeutic treatment with gas body enhanced ultrasound may be much greater than expected based on research protocols developed using rats.
The gas body loss influenced the response to changes in intermittent image interval. 5 min exposure with 5 μl/kg/min infusion and 1 s scan interval induced GCH in 68.4 % of glomeruli in the exposure scan plane. The gas body reduction fraction was 0.054 for this treatment. The GCH from exposure with 10 s scan interval was significantly reduced (P<0.001) to about 30% (Table 3), but not as much as expected from the 10-fold reduction in exposure. For this reduced exposure, the gas body reduction fractions was 0.15. Apparently the increase in circulating dose, owing to the reduced number of image-exposures, allowed increased effect from each image-exposure.
Strategies to compensate for the gas body destruction might include increasing the dose or the duration of the treatment. When the circulating dose reached a constant plateau level, the gas body destruction rate was equal to the gas body infusion rate. For this equilibrium condition, bioeffects might be expected to build as GCH from each exposure accumulates. Further GCH results were determined for 120 s infusions giving total doses of 2, 4, 10 and 20 μl/kg, and for 30, 60 and 300 s infusions at 5 μl/kg/min, all including a 5 min washout (Fig. 5). The GCH increased rapidly for low doses or durations but became nearly constant at less than 100 % for higher doses or durations, a phenomenon noted previously [3]. The initial rate of rise was 112 %/(μl/kg) relative to the estimated circulating dose, with a plateau of 56.5 % GCH (r2=0.81). The initial rate of rise was 1.2 %/s relative to the infusion duration, with a plateau of 66.3 % GCH (r2=0.88). That is, added gas bodies for higher doses (> 0.5 μl/kg) or longer durations of intermittent exposure (> 60 s) are much less effective than the initial gas bodies. One factor in this trend may have been the gas body distribution at increasing numbers (Poisson distribution probability). For example, for a circulating dose with an average of 1 effective gas body per glomerulus, 36.8% of glomeruli would have none and 63.2 % would have one or more (36.8 % with one, 18.4 % with two, etc., according to the Poisson distribution). The initial injury may also lead to a physiological response, which could minimize further injury, as has been observed in lithotripsy research [23]. In addition, at higher doses or infusion durations, the GCH effect could be further limited by self-shielding with high doses increasing attenuation in intervening tissue, or by deterioration of the infusion suspension as gas bodies rise during long durations.
Finally, the efficacy of gas bodies can be approximately determined. The estimated circulating doses at the GCH plateau are not optimum for calculating the efficiency of nucleation and GCH of the infused gas bodies, since the apparent efficacy decreased with increasing dose and duration. The optimum efficiency can be estimated from the low dose (linear) range, which can be judged from the fitted curve in the low-effect linear portion of the response shown in Fig. 5a. The dose response curve for 120 scan exposures indicates 1 GCH (about 0.37 % in a histology slide) per scan for a circulating dose extrapolated to be 0.39 μl/kg. This linear-range value can be converted into an efficiency estimate for the number of gas bodies required for one GCH. For this calculation, the blood volume of rats was 64 ml/kg [11], and the capillary blood volume of a glomerulus was 0.58 10-6 ml [12]. The number of gas bodies per ml of contrast infusion was taken to be 6.9 109 ml-1, which takes into account the dilution and passage of the agent through the canula [24]. However, this value neglects losses of gas bodies from the tail vein to the kidney, which are assumed to be a small percentage. These parameters combine to indicate about 6.7 glomeruli in a histological section (2.5 % of an average 273 glomeruli per section) would have one gas body for a 0.39 μl/kg circulating dose. Thus, the circulating dose for 1 GCH per scan from the low dose data indicate a nucleation-bioeffect efficiency of 6.7 gas bodies (per GCH). That is, only about 1 in 7 gas bodies were optimum for cavitation nucleation and capillary rupture under the these conditions (with minimal gas body loss). Although this result would change for higher or lower PRPAs and with variation in some other parameters, this estimate may approximate the highest efficiency for this mode of diagnostic ultrasound. Overall, the gas body destruction, reduced response for higher doses or durations and modest efficiency for bioeffects complicate the consideration of gas body dosimetry in diagnostic and therapeutic applications.
Acknowledgments
This work was supported by PHS grant EB00338 awarded by the National Institutes of Health, DHHS.
References
- 1.Miller DL, Averkiou MA, Brayman AA, Everbach EC, Holland CK, Wible JH, Jr, Wu J. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med. 2008;27:611–632. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- 2.Wible JH, Jr, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, Brandenburger GH. Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med Biol. 2002;28:1535–1546. doi: 10.1016/s0301-5629(02)00651-8. [DOI] [PubMed] [Google Scholar]
- 3.Miller DL, Dou C, Wiggins RC, Wharram BL, Goyal M, Williams AR. An in vivo rat model simulating imaging of human kidney by diagnostic ultrasound with gas-body contrast agent. Ultrasound Med Biol. 2007;33:129–135. doi: 10.1016/j.ultrasmedbio.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Ann Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Nyborg WL, editors. Emerging Therapeutic Ultrasound. World Scientific Publishing; Singapore: 2006. [Google Scholar]
- 6.Miller DL, Thomas RM. Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound Med Biol. 1995;21:1059–1065. doi: 10.1016/0301-5629(95)93252-u. [DOI] [PubMed] [Google Scholar]
- 7.Shi WT, Forsberg F, Tornes A, Ostensen J, Goldberg BB. Destruction of contrast microbubbles and the association with inertial cavitation. Ultrasound Med Biol. 2000;26:1009–1019. doi: 10.1016/s0301-5629(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg F, Merton DA, Goldberg BB. In vivo destruction of ultrasound contrast microbubbles is independent of the mechanical index. J Ultrasound Med. 2006;25:143–144. [PubMed] [Google Scholar]
- 9.Miller DL, Dou c, Wiggins RC. Doppler mode pulse sequences mitigate glomerular capillary hemorrhage in contrast-aided diagnostic ultrasound of rat kidney. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:1802–1810. doi: 10.1109/tuffc.2007.464. [DOI] [PubMed] [Google Scholar]
- 10.Dalecki D, Raeman CH, Child SZ, Penney DP, Carstensen EL. Remnants of Albunex nucleate acoustic cavitation. Ultrasound Med Biol. 1997;23:1405–1412. doi: 10.1016/s0301-5629(97)00142-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 12.Nyengaard JR. Number and dimensions of rat glomerular capillaries in normal development and after nephrectomy. Kidney Int. 1993;43:1049–1057. doi: 10.1038/ki.1993.147. [DOI] [PubMed] [Google Scholar]
- 13.Kamiyama N, Moriyasu F, Mine Y, Goto Y. Analysis of flash echo from contrast agent for designing optimal ultrasound diagnostic systems. Ultrasound Med Biol. 1999;25:411–420. doi: 10.1016/s0301-5629(98)00182-3. [DOI] [PubMed] [Google Scholar]
- 14.Schlosser T, Pohl C, Veltmann C, Lohmaier S, Goenechea J, Ehlgen A, Köster J, Bimmel D, Kuntz-Hehner S, Becher H, Tiemann K. Feasibility of the flash-replenishment concept in renal tissue: which parameters affect the assessment of the contrast replenishment? Ultrasound Med Biol. 2001;27:937–944. doi: 10.1016/s0301-5629(01)00397-0. [DOI] [PubMed] [Google Scholar]
- 15.Kaps M, Seidel G, Algermissen C, Gerriets T, Broillet A. Pharmacokinetics of echocontrast agent infusion in a dog model. J Neuroimaging. 2001;11:298–302. doi: 10.1111/j.1552-6569.2001.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 16.Averkiou M, Powers J, Skyba D, Bruce M, Jensen S. Ultrasound contrast imaging research. Ultrasound Q. 2003;19:27–37. doi: 10.1097/00013644-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Miller DL, Dou C, Wiggins RC. Frequency dependence of kidney injury induced by contrast-aided diagnostic ultrasound in rats. Ultrasound Med Biol. 2008;34:1678–1687. doi: 10.1016/j.ultrasmedbio.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehgal CM, Arger PH, Pugh CR. Sonographic enhancement of renal cortex by contrast media. J Ultrasound Med. 1995;14:741–748. doi: 10.7863/jum.1995.14.10.741. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal CM, Arger PH. Mathematical modeling of the dilution curves for ultrasonographic contrast agents. J Ultrasound Med. 1997;16:471–479. doi: 10.7863/jum.1997.16.7.471. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Schwarz KQ, Phillips D, Steinmetz SD, Schlief R. A mathematical model for the assessment of hemodynamic parameters using quantitative contrast echocardiography. IEEE Trans Biomed Eng. 1998;45:754–765. doi: 10.1109/10.678610. [DOI] [PubMed] [Google Scholar]
- 21.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–1140. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- 22.Köster J, Schlosser T, Pohl C, Lentz C, Lohmaier S, Veltmann C, Kuntz-Hehner S, Omran H, Lüderitz B, Becher H, Tiemann K. Blood flow assessment by ultrasound-induced destruction of echocontrast agents using harmonic power Doppler imaging: which parameters determine contrast replenishment curves? Echocardiography. 2001;18:1–8. doi: 10.1046/j.1540-8175.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 23.Handa RK, Bailey MR, Paun M, Gao S, Connors RA, Willis LR, Evan AP. Pretreatment with low-energy shock waves induces renal vasoconstriction during standard shock wave lithotripsy (SWL): a treatment protocol known to reduce SWL-induced renal injury. BJU Int. 2009;103:1270–1274. doi: 10.1111/j.1464-410X.2008.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Armstrong WR, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: Comparison of three different contrast agents. Ultrasound Med Biol. 2004;30:83–91. doi: 10.1016/j.ultrasmedbio.2003.09.004. [DOI] [PubMed] [Google Scholar]