SUMMARY
Polarized membrane sorting of Connexin 43 (Cx43) has not been well-characterized. Based on the presence of a putative sorting signal, Y286KLV289, within its carboxy terminal cytoplasmic domain, we hypothesized that Cx43 is selectively expressed on the basolateral surface of Madin-Darby canine kidney (MDCK) cells in a tyrosine-dependent manner. We generated stable MDCK cell lines expressing human wild-type and mutant Cx43-eYFP and analyzed the membrane localization of Cx43-eYFP within polarized monolayers using confocal microscopy and selective surface biotinylation. We found that wild-type Cx43-eYFP was selectively targeted to the basolateral membrane domain of MDCK cells. Substitution of alanine for Y286 disrupted basolateral targeting of Cx43-eYFP. Additionally, transplantation of a sequence containing the transferrin receptor internalization signal, LSYTRF, for P284GYKLV289 also disrupted basolateral targeting. Taken together, these results indicate that Y286 in its native amino acid sequence is necessary for targeting Cx43-eYFP to the basolateral membrane domain of MDCK cells. To determine whether the F52dup or L90V oculodentodigital dysplasia (ODDD)-associated mutations could affect polarized sorting of Cx43-eYFP, we analyzed the expression of these Cx43-eYFP mutant constructs and found that the L90V mutation disrupted basolateral expression. These findings raise the possibility that alteration of polarized targeting of Cx43 by some ODDD- associated mutations may have a phenotypic contribution.
Keywords: Connexin43, tyrosine, basolateral, oculodentodigital dysplasia
INTRODUCTION
Connexin 43 (Cx43) is a ubiquitously expressed gap junctional subunit that mediates intercellular communication via the formation of gap junctions and hemichannels [1]. In addition, Cx43 may promote normal cellular migration and development by enabling intercellular adhesion [2]. Cx43 is expressed in many polarized cell types such as brain endothelial cells, thyroid epithelial cells, and cholangiocytes [3–6]. Cx43 is also highly expressed in astrocytes, a cell type that exhibits a polarized phenotype and participates in polarized functions [7–10]. Thus far, there have been limited studies examining the expression of Cx43 in polarized cells, and there is little information regarding characterization of the involved sorting signals. In general, previous studies demonstrate basolateral expression of Cx43 and other connexins in various polarized cell types [5, 11–13]. The trafficking of a Cx43-GFP chimera expressed in MDCK cells has previously been examined, albeit not in the context of polarized monolayers [14].
Madin-Darby canine kidney (MDCK) cells are a well-characterized model-system for the study of polarized trafficking to distinct apical and basolateral domains that are separated by tight junctions [15–17]. In MDCK cells, basolaterally-expressed membrane proteins often depend on a tyrosine or dileucine - based sorting signal located in the cytoplasmic tail. A common tyrosine-based consensus sorting motif consists of YXXØ (Y = tyrosine, X = any amino acid, and Ø = amino acid with a bulky hydrophobic side chain) [18]. These polarized sorting signals are recognized in other cell types as well [19–21]. For example, the VSV-G protein, which is targeted to the basolateral membrane domain in MDCK cells, has a tyrosine-based signal directing it to the somatodendrite versus axon in neurons and the myelin sheath versus soma in oligodendrocytes [22, 23]. The existence of such a signal in the carboxy terminus of Cx43 led us to hypothesize that Y286 is involved in basolateral targeting of Cx43.
At least 60 mutations in Cx43 have been discovered that cause oculodentodigital dysplasia (ODDD), a rare human developmental disorder characterized by defects in the craniofacial bones, loss of tooth enamel, and abnormal soft tissue separation of two or three digits [24, 25]. Depending on the particular Cx43 mutation, a wide range of other abnormalities including neurologic and cardiac are variably observed [26–37]. As of yet, there is no clear understanding of the relationship between genotype and phenotype, although functional evaluation of most mutations that have been studied demonstrates reduced gap junction activity [38–43]. Several ODDD mutations have been found to cause altered trafficking. For example, a C260fsX306 mutation (leading to truncation of most of the Cx43 cytoplasmic tail) and a G60S mutation have been found to cause impaired cell membrane expression of Cx43 responsible for impaired intercellular communication [44, 45]. Interestingly, the G138R and G143S ODDD mutations have been found to cause enhanced hemichannel function with absent gap junctional signaling in HeLa cells. Additionally, these mutations were associated with decreased Cx43 degradation [46]. These studies provide evidence that altered membrane protein trafficking may be responsible for abnormal function of Cx43 associated with disease.
In this study, we determined that Cx43-eYFP is targeted to the basolateral membrane domain of MDCK cells. We also found that Y286 in the sequence P284GYKLV289 is necessary for basolateral targeting of Cx43-eYFP. Lastly, we found that the L90V ODDD mutation disrupts the selective delivery of Cx43-eYFP to the basolateral membrane domain. These results implicate aberrant polarized sorting of Cx43 in human disease.
RESULTS
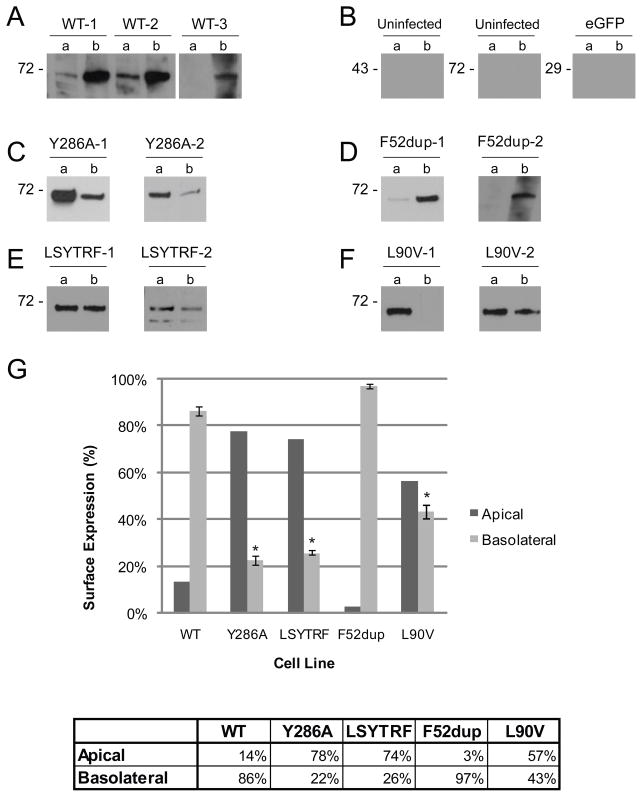
Stable expression of wild-type (WT) and mutant Cx43-eYFP constructs in MDCK cells
To examine the localization of human Cx43-eYFP constructs in MDCK cells, we stably expressed eYFP-tagged WT and mutant Cx43 cDNA fusion constructs in MDCK (strain II) cells. As indicated in Fig. 1, eYFP-tagged Cx43 constructs consist of the Cx43 sequence joined at its C-terminus to eYFP by an eight-amino-acid ‘linker’ segment (SRDPPVAT). The locations of the four mutations (Y286A, LSYTRF, L90V, and F52dup) analyzed are also indicated. We confirmed by Western blot analysis that Cx43-eYFP constructs were properly translated in MDCK cells (Fig. 2A). Using the anti-Cx43 antibody, a prominent band migrating at ~70 kDa, the predicted molecular weight of full-length Cx43-eYFP, was detected in total and surface lysates for WT and each mutant cell line, whereas this band was not detected in the uninfected control cells. Similarly, using the polyclonal anti-GFP antibody, a prominent band migrating at ~70 kDa was detected in total and surface lysates for WT and each mutant cell line, whereas this band was absent in uninfected control cells. For reference, a band at ~29 kDa was detected in the total cell lysate of cells expressing only eGFP. No GFP expression was detected after surface biotinylation of cells expressing only eGFP, demonstrating the selectivity of surface biotinylation for cell surface proteins. Since the amount of protein loaded for surface and total Cx43-eYFP Western blots were not normalized to each other, no conclusions can be made from these experiments regarding the relative level of surface expression of the constructs. Confocal images of the XY plane show that all Cx43-eYFP constructs are predominantly expressed at the cell membrane. Gap junction aggregates, or plaques, can also be seen at areas of cell-cell contacts (arrow) in all constructs except the F52dup mutant (Fig. 2B).
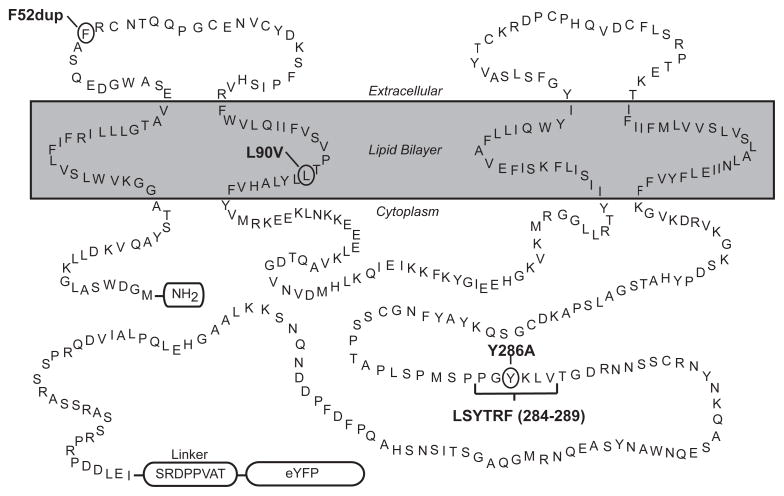
Fig. 1. Schematic diagram of Cx43-eYFP amino acid sequence.
Schematic diagram of Cx43-eYFP indicating predicted topology of Cx43 and the position of eYFP and its fusion by an eight amino acid linker (SRDPPVAT) to the C terminus. Cx43 is predicted to span the plasma membrane four times and has cytoplasmically-located N and C termini. The locations of the amino acid mutations examined in this study are indicated. F52dup is located in the extracellular domain, L90V is located in the second transmembrane domain, and Y286A and LSYTRF are located in the cytoplasmic tail. This figure is modified from a previous publication [43].
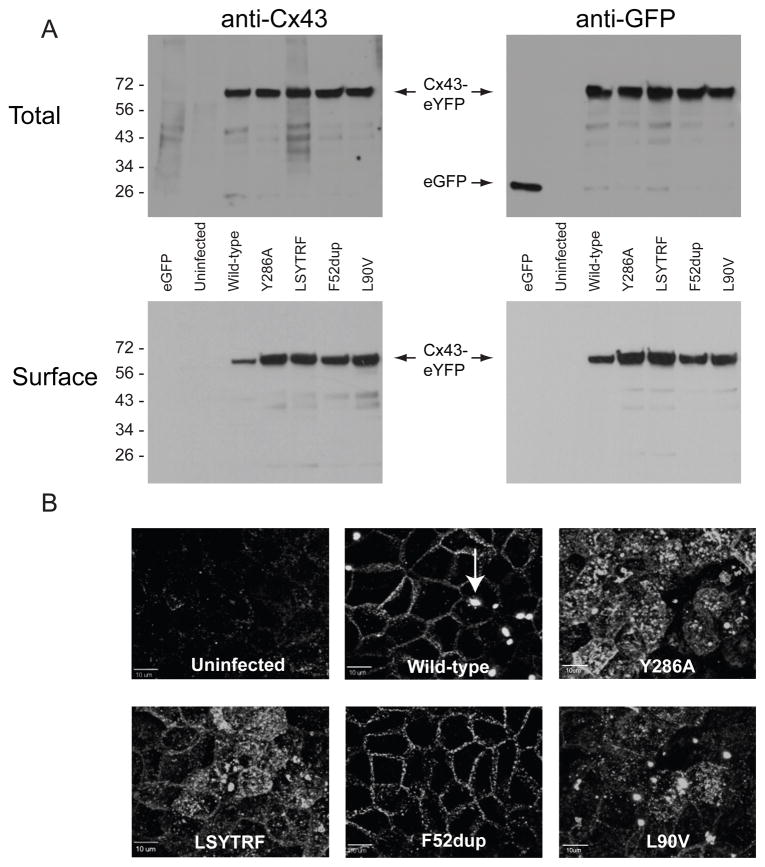
Fig. 2. Expression of Cx43-eYFP constructs in MDCK cells.
A. Surface and total protein were isolated by biotinylation and analyzed by Western blot. Using anti-Cx43 and anti-GFP antibodies to confirm proper translation of Cx43-eYFP constructs, ~70 kDa bands representing the full-length fusion proteins were produced by cells expressing WT and mutant Cx43-eYFP. B. Confocal XY images were projected to show the XY plane from above the apical surface of cell monolayers. Uninfected control MDCK cells showed minimal background fluorescence. WT and mutant Cx43-eYFP constructs were expressed on the cell membrane. Gap junction plaques (indicated by arrow in WT image) formed at points of cell-cell contact in all mutants with the same frequency and morphology as WT except for F52dup, which formed plaques much less frequently. Scale bar, 10 um.
WT Cx43-eYFP is selectively expressed on the basolateral domain of MDCK cells
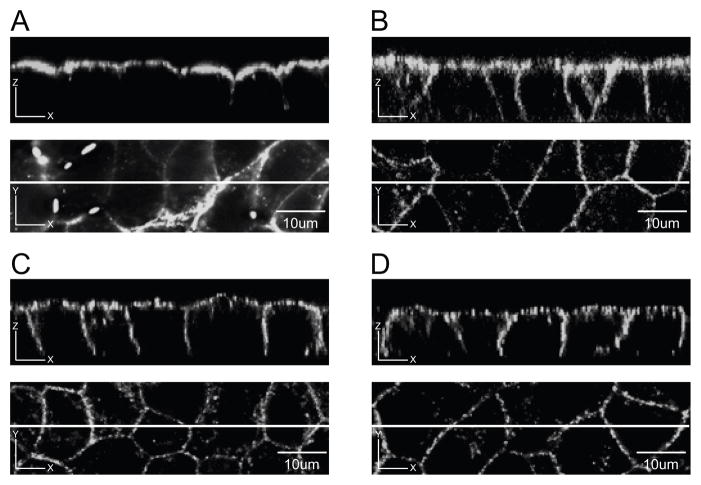
To determine the steady-state polarized membrane distribution of WT Cx43-eYFP in MDCK cells, polarized monolayers expressing WT Cx43-eYFP were cultured on filter inserts and examined using confocal microscopy. Reconstructed Z sections (XZ plane) show that WT Cx43-eYFP is selectively expressed on the basolateral surface of MDCK cells (three individual cell lines are shown) (Fig. 3A–C). For comparison, limited signal was detected in the uninfected control cells (Fig. 3D), and cells infected with only eGFP showed a diffuse cytoplasmic signal but no specific surface localization (Fig. 3E). Localization of plaques in the XZ plane is described separately (Fig. 7).
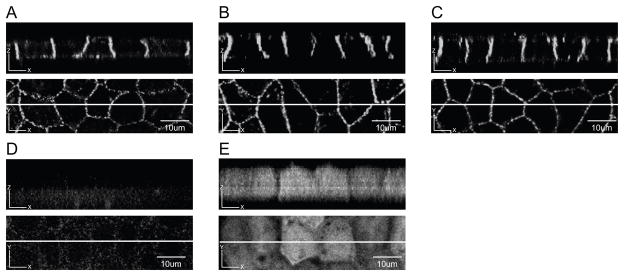
Fig. 3. WT Cx43-eYFP is selectively targeted to the basolateral domain of MDCK cells.
A,B,C. Three independent MDCK clones expressing WT Cx43-eYFP were cultured on filter inserts. Z sections of cell monolayers are shown in the top panels. Lines drawn through the XY plane in the bottom panels indicate the location of the Z sections. WT Cx43-eYFP was expressed on the basolateral membrane domain of MDCK cells. D. Z section of monolayer of uninfected MDCK cells showed background fluorescence. E. Z section of monolayer of MDCK cells expressing only eGFP showed a diffuse cytoplasmic pattern. Scale bar, 10 um.
Fig. 7. Gap junction plaques are located predominantly on the lateral surface of cell monolayers expressing WT and mutant constructs.
Z sections generated from areas containing the various types of plaques expressed by the WT construct are shown and are representative of plaques seen in all mutants. Plaques were found to span the entire membrane (A) or part of the membrane (B, C). Less frequently, plaques were found to be apparently suspended near the basal membrane (D) or the apical membrane (E), not at cell-cell junctions. F. Z section of a representative plaque formed by the Y286A mutant construct. Arrows point to plaques. Scale bar, 10 um.
To confirm these results, we performed selective surface biotinylation on the apical and basolateral surfaces in order to visualize the apical/basolateral distribution of Cx43-eYFP by Western blot analysis using the anti-Cx43 antibody. This analysis confirmed that the majority of the signal is found on the basolateral surface of cell lines expressing WT Cx43-eYFP, although there appeared to be a small amount of apically-expressed WT Cx43-eYFP (Fig. 6A). Five independent WT clones were tested, all yielding similar results. Three are shown. No signal could be detected on the apical or basolateral surfaces of the uninfected control cells at either 43 kDa or ~70 kDa, and similarly, no signal could be detected at ~29 kDa on the apical or basolateral surface of cells expressing only eGFP (Fig. 6B). Additionally, quantitative analysis of the distribution between the two domains using a plate reader revealed that 86% of the surface WT Cx43-eYFP was located on the basolateral surface (Fig. 6G).
Fig. 6. Apical/basolateral cell surface distribution of Cx43-eYFP.
Selective surface biotinylation followed by Western blot analysis using the anti-Cx43 antibody showed that WT and the F52dup mutant construct were expressed predominantly on the basolateral surface (A, D), while the Y286A, LSYTRF, and L90V mutant constructs were not exclusively distributed on the basolateral surface (C, E, F). B. Uninfected MDCK cells showed no bands at ~70 kDa or 43 kDa. Absence of bands at ~29 kDa in control cells expressing only eGFP indicates that non-specific biotinylation of cytoplasmic protein did not occur. Two or three individual cell lines for each construct are shown. G. Surface expression as quantified by a fluorescence plate reader following selective surface biotinylation. The percent of signal found on the basolateral surface of Y286A, LSYTRF, and L90V was significantly different from that of WT (*). Error bars indicate s.e.m, n = 3–6 (varies for cell lines).
As described in Methods, all experiments were performed in the presence of sodium butyrate pre-incubation to increase expression of the Cx43-eYFP constructs. We confirmed that sodium butyrate treatment did not affect results by performing confocal microscopy in the absence of sodium butyrate, dye transfer experiments with and without butyrate, and transepithelial resistance measurements with and without butyrate on a selected cell line (Fig. S1).
Y286 in the context of its native sequence P284GYKLV289 is necessary for selective basolateral expression of Cx43-eYFP in MDCK cells
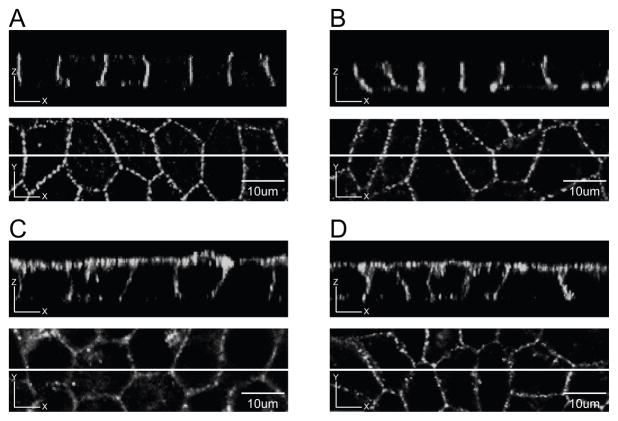
Y286 is contained within a putative tyrosine-based sorting signal (Y286KLV289) and a PPXY motif (P283PGY286), a ubiquitin ligase (NEDD4) binding site involved in internalization and degradation of Cx43 [47]. To determine whether Y286 is involved in the basolateral targeting of WT Cx43-eYFP, we substituted an alanine for Y286 of the Cx43-eYFP sequence and examined the surface distribution of the resulting Y286A mutant construct in polarized MDCK cell monolayers. Confocal analysis of XZ sections revealed that the Y286A mutation causes Cx43-eYFP to be expressed predominantly on the apical surface, indicating that Y286 is necessary for the proper basolateral distribution of Cx43-eYFP in MDCK cells (Fig. 4A, B). Western blot analysis of selective surface biotinylation fractions confirmed this predominantly apical distribution (Fig. 6C), and quantitative analysis showed that 78% of the surface signal for this mutant construct was located on the apical surface (Fig. 6G). Data from two individual cell lines are shown.
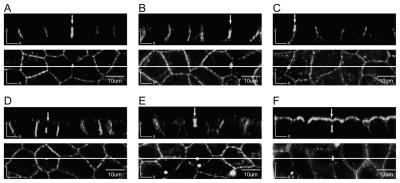
Fig. 4. Y286 in its native context is necessary for selective basolateral expression of Cx43-eYFP in MDCK cells.
A, B. Z sections of MDCK monolayers expressing the Y286A mutant construct showed predominantly apical distribution of Cx43-eYFP (2 independent clones). C, D. Z sections of MDCK monolayers expressing the LSYTRF mutant construct showed signal on both the apical and basolateral membrane domains (2 independent clones). Scale bar, 10 um.
To determine the effect of transplantation of a sequence containing the internalization signal of the transferrin receptor in place of P284GYKLV289, we expressed a mutant construct in which P284GYKLV289 was replaced by LSYTRF [48]. Confocal Z sections of MDCK cells expressing the LSYTRF mutation showed the Cx43-eYFP signal to be apparently equally distributed on the apical and basolateral surfaces (Fig. 4C, D). Western blot analysis of selective surface biotinylation fractions confirmed that cells expressing the LSYTRF mutant construct express Cx43-eYFP at similar levels on the apical and basolateral surfaces (Fig. 6E). Interestingly, the surface protein quantified using a fluorescence plate reader indicated that similar to Y286A, 74% of the LSYTRF surface signal resides on the apical surface (Fig. 6G). Since Y286 is intact in this mutant construct while its surrounding sequence is altered, these findings strongly suggest that the context in which Y286 exists is important for maintenance of basolateral targeting.
The L90V but not the F52dup ODDD mutation disrupts basolateral sorting of Cx43-eYFP in MDCK cells
To determine whether ODDD-associated mutations affect the basolateral targeting of Cx43-eYFP, we examined the localization of F52dup and L90V mutant Cx43-eYFP constructs in polarized MDCK cell monolayers. These mutants were selected for analysis from our earlier study of ODDD mutants in C6 rat glioma cells [43]. We chose the F52dup mutant because it failed to form gap junction plaques and we chose the L90V mutant because it appeared to have an increased amount of plaques in the processes compared to WT (unpublished observation). Confocal Z sections of cells expressing the F52dup mutant construct showed predominantly basolateral expression, indicating that this mutation does not alter polarized targeting of Cx43-eYFP (Fig. 5A, B). These results were confirmed by Western blot analysis of the selective surface biotinylation fractions with nearly all signal (97%) found on the basolateral surface (Fig. 6D, G). In contrast, confocal analysis of the distribution of the L90V mutant construct in polarized monolayers revealed that this mutation causes Cx43-eYFP to be distributed on both the apical and basolateral surfaces (Fig. 5C, D). Western blot analysis of selective surface biotinylation fractions confirmed that the surface expression of the L90V mutant construct is not restricted to the basolateral surface, with 57% of the signal being found on the apical surface. (Fig. 6F, G). Of note, the L90V-1 clone appeared to be exclusively expressed on the apical surface (Fig. 6F). Two independent clones for each mutant construct are shown.
Fig. 5. L90V but not the F52dup ODDD mutation disrupts basolateral targeting of Cx43-eYFP in MDCK cells.
A, B. Z sections of MDCK monolayers expressing the F52dup mutant construct showed that this mutation does not affect basolateral targeting of Cx43-eYFP (2 independent clones). C, D. Z sections of MDCK monolayers expressing the L90V mutant construct showed that basolateral targeting of Cx43-eYFP was disrupted (2 independent clones). Scale bar, 10 um.
Gap junction plaques reside predominantly on the lateral surface of WT and apically-distributed mutant constructs
With the exception of the F52dup mutant, all of the other mutant constructs formed plaques with the same frequency and morphology as WT (Fig. 2B). We observed that the F52Dup mutant formed the large gap junction plaques far less frequently, consistent with our previous results when the F52dup mutant was expressed in C6 rat glioma cells [43]. The various plaques expressed by the WT construct were representative of plaques seen in all mutant constructs (Fig. 7 A–E). Plaques formed most frequently on the lateral surface of the cell membrane and spanned either the entire area of cell-cell contact (Fig. 7A) or a smaller area closer to the basal (Fig. 7B) or apical (Fig. 7C) membrane. Plaques were also sometimes seen suspended near the basal surface (Fig. 7D) or near the apical membrane (Fig. 7E); however, these examples occurred less frequently. Mutants with apical expression formed plaques predominantly on the lateral surface as well; for example, a representative plaque formed by the Y286A mutant is shown (Fig. 7F). We saw no qualitative difference in the relative frequencies of these types of plaques between WT and the various mutants (data not shown).
Degradation from the cell surface is impaired for Y286A Cx43-eYFP
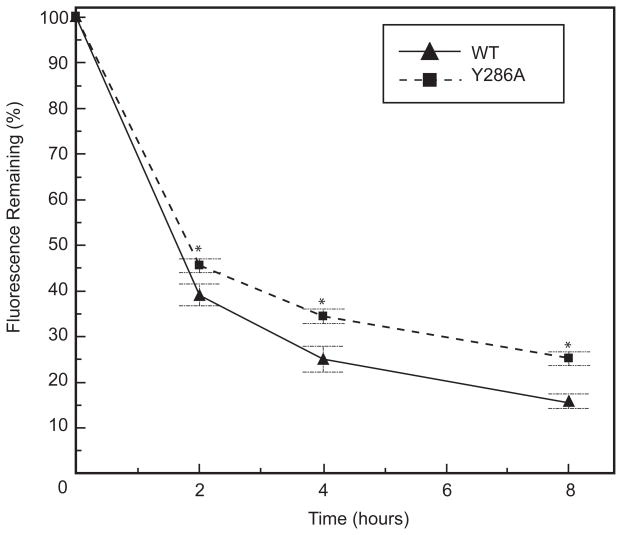
The tyrosine-based signal containing Y286 also conforms to a putative lysosomal degradation signal [47]. To determine whether any of the mutations inhibit degradation of surface Cx43-eYFP, we performed pulse-chase surface biotinylation experiments on all of the cell lines (grown as monolayers on 60mm cell culture dishes). The amount of Cx43-eYFP present after 2, 4, and 8 hour chase intervals was measured by a fluorescence plate reader and normalized to the amount present at time 0. We found that surface WT Cx43-eYFP was rapidly degraded, with a half-life of under 2 hours. We performed Western blots probed with the anti-GFP and anti-Cx43 antibodies to confirm that we were properly measuring the disappearance of intact Cx43-eYFP without tracking degradation products containing eYFP (data not shown). The Y286A mutation resulted in a slightly longer half-life of surface Cx43-eYFP compared to WT, indicating that Y286A has a role in mediating degradation from the cell surface (Fig. 8). The L90V, LSYTRF, and F52dup mutations had minimal effect on degradation of Cx43-eYFP from the surface as compared to WT (Fig. S2).
Fig. 8. The Y286A mutant construct shows impaired degradation as compared to WT.
Surface protein degradation assays on tissue culture dishes were conducted by labeling surface protein with membrane-impermeant sulfo-NHS-LC-biotin and lysing cells at 0, 2, 4, and 8 hours. eYFP fluorescence was quantified using a fluorescence plate reader. Fluorescence remaining (%) was calculated by normalizing to the reading at time 0 for each cell line. The Y286A mutation slightly impaired degradation of Cx43-eYFP from the surface as compared to WT. For each cell type, at least three independent experiments were performed on two clones. Data are graphed as mean ± s.e.m. (brackets represent s.e.m.).
DISCUSSION
We sought to determine the localization of surface expression of Cx43-eYFP in polarized MDCK cells and whether two ODDD mutations could alter this distribution. We hypothesized that Cx43-eYFP would have tyrosine-dependent basolateral expression in MDCK cells based on the presence of Y286KLV289 within the amino acid sequence of the cytoplasmic tail. Using confocal microscopy and selective surface biotinylation, we have shown that WT Cx43-eYFP is targeted to the basolateral membrane domain of MDCK cells (Fig. 3A, Fig 6A). The selective expression of Cx43-eYFP on the basolateral domain of MDCK cells is consistent with other studies showing that Cx43 and other connexins are typically distributed on the basolateral surface of polarized cells [5, 11–13]. Since all experiments were performed in the presence of sodium butyrate to increase expression of the Cx43-eYFP constructs as had previously been shown using this model system [49], we used a variety of approaches to confirm that sodium butyrate incubation did not alter the distribution of Cx43-eYFP or disrupt the monolayer integrity (Fig. S1). First, we performed confocal experiments in the absence of sodium butyrate pre-incubation and found similar distributions between the apical and basolateral surface for all of the constructs. We then conducted dye transfer assays on monolayers expressing each construct and found no consistently significant increases in dye transfer with sodium butyrate pre-incubation compared to untreated monolayers. Lastly, we conducted transepithelial resistance (TER) measurements on a representative cell line and found that the sodium butyrate did not alter the TER of the filter-grown monolayer.
We demonstrated that Y286 in the cytoplasmic domain of Cx43 is necessary for basolateral sorting of Cx43-eYFP in MDCK cells, as evidenced by the absence of selective basolateral targeting of the Y286A mutant construct (Fig. 4A, B). Therefore, Y286 of the tetrapeptide sequence Y286KLV289 represents a critical tyrosine residue found in the common basolateral sorting motif YXXØ [18]. To further characterize this signal, we then transplanted the LSYTRF sequence containing the transferrin receptor internalization signal (YTRF) in the position of P284GYKLV289 and found that selective basolateral targeting of Cx43-eYFP was not preserved (Fig. 4C, D). This finding was not unexpected considering that the transferrin receptor internalization signal does not contain basolateral targeting information [48]. Interestingly, we found that gap junction plaques were found predominantly on the lateral membrane domain even in cell lines expressing constructs that have apical expression (Fig. 7). This raises the possibility that the biotinylation assay does not capture this population completely, possibly due to poor accessibility of sulfo-NHS-LC-biotin to fully assembled gap junctions. Alternatively, this plaque population may be a component of the basolateral fraction detected for Y286A and the other apically-expressed mutant Cx43-eYFP constructs. Despite our inability to determine which assembly states are efficiently captured by the biotinylation assay, the combination of the confocal microscopy with the biotinylation data strongly suggest that trafficking of Cx43, presumably as undocked Cx43 connexons (hemichannels), is directed to the basolateral surface. From our data, it remains unclear by what mechanism gap junctional plaques are retained at the basolateral surface.
We found that the ODDD-associated L90V mutant disrupts basolateral expression of Cx43-eYFP without affecting the rate of surface degradation, whereas F52dup does not affect either basolateral expression or degradation (Fig. 5, 6). The finding of altered basolateral expression of the L90V mutant Cx43-eYFP construct may indicate the presence of another basolateral sorting determinant located in the second transmembrane domain of Cx43. Although not as common as cytoplasmic sorting signals, transmembrane sorting signals have been identified. For example, the gastric H,K-ATPase has an apical sorting signal in its fourth transmembrane domain, although the exact amino acids responsible are not identified [50]. Studies have shown that Cx43 oligomerizes into connexons in the ER or Golgi prior to delivery to the cell membrane [1]. Therefore, an alternate hypothesis is that the L90V mutation may affect oligomerization of Cx43 subunits, which impairs recognition of the Y286-based sorting signal. Overall, these findings may provide an explanation for the additional phenotypic features of neurodegeneration and hearing loss observed in ODDD patients with the L90V and not the F52dup mutation [25]. Characterization of polarized trafficking of other ODDD-associated mutants in MDCK cells will be necessary to correlate aberrant polarized trafficking with a particular phenotype.
Consistent with other studies, we found that Cx43-eYFP is rapidly degraded from the surface, with a half-life of about 2 hours (Fig. 7) [47, 51]. We detected a slight but significant decrease in surface protein degradation between WT and the Y286A mutant but not between WT and any of the other mutants. We expected to see a greater difference between WT and Y286A given that assay of the Y286A mutant in SKHep1 cells demonstrated that the mutation increased the half-life of total cellular Cx43 from 2 to 6 hours [51]. The disparity between our results may be due to the difference in cell lines used or to the fact that our assay examined degradation of surface protein as opposed to total protein. However, the lack of an appreciable effect on degradation of the construct containing the transplanted transferrin internalization signal suggests that another signal may be involved in the degradation of Cx43 outside of the P284GYKLV289 sequence.
Our findings imply that targeting of Cx43 to specific domains of polarized cells may be crucial for its functional regulation by concentrating or restricting intercellular interactions to a specific plasma membrane domain. For example, astrocytes have a polarized morphology with formation of specialized endfeet that make contacts with endothelial cells [9, 10]. Cx43 has been found to be abundantly expressed at the connection of blood vessels and astrocytic endfeet [52]. Although there are no known studies correlating targeting to the basolateral domain of MDCK cells with targeting to astrocytic endfeet, we would predict that Cx43 is selectively targeted to the astrocytic endfeet based on the finding that the VSV-G protein is targeted to the processes that form the myelin sheath in oligodendrocytes, another glial cell type [22]. We would also predict that Cx43 is targeted to the basolateral domain of endothelial cells based on findings that other basolateral sorting signals active in MDCK cells are also recognized for basolateral targeting in endothelial cells [53]. By similar mechanisms, during development, Cx43-dependent neuronal migration along glial fibers via gap junctional adhesion may require polarized targeting of Cx43 [17]. Alteration of polarized expression may explain central nervous system developmental abnormalities found in ODDD. Lastly, processes such as glioma migration along white matter or endothelial basement membrane paths may also utilize Cx43-dependent mechanisms that rely on proper targeting of Cx43 in polarized cells [54, 55].
MATERIALS AND METHODS
Cell culture
MDCK (strain II) cells expressing the RSV(A) receptor (obtained from Dr. G. Odorizzi, University of Colorado, Boulder, CO) and DF-1 cells (purchased from American Type Culture Collection, Manassas, VA) were maintained in DMEM/F12 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Lonza, Walkersville, MD) and 100 U/ml penicillin and 100 ug/ml streptomycin (Lonza, Walkersville, MD). 293T cells (obtained from Dr. P. Mischel, University of California, Los Angeles, CA) were maintained in IMDM (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 ug/ml streptomycin. All cells were grown in a 5% CO2 humidified atmosphere.
Generation of wild-type and mutant Cx43-eYFP fusion constructs
Generation of the WT, L90V, and F52dup Cx43-eYFP fusion constructs in the pEYFP-N1 vector has been previously described [43]. To introduce the Y286A and LSYTRF mutations into the Cx43 sequence, two-stage mutagenesis was performed using the WT plasmid as the template. An upstream forward primer and a mutagenic reverse primer were used to amplify a 5′ product carrying the mutation, and an overlapping 3′ product was amplified with a forward mutagenic primer (complement of the mutagenic reverse primer) and a downstream reverse primer. The 5′ and 3′ Cx43 amplification products were combined and amplified with the HindIII forward and XmaI reverse adapter primers and the resultant altered Cx43 sequences were cloned into pEYFP-N1. The following mutagenic forward primers and their complementary sequences as reverse primers were used: Y286A: 5′ – GATCATGAATTGTTTCTGTCGCCA GTAACCAGCTTGGCCCCAGGAGGAGACATAGGCG – 3′; LSYTRF: 5′ – GCAAGAAGAATTGTTTCTGTCGCCAGTGAACCGGGTATATGACAAAGGAGACATAGGCGAGAGGGGAGC – 3′.
Subcloning of Cx43-eYFP constructs into BH-RCAS and pLPCX retroviral expression vectors
Mutant and WT Cx43-eYFP fusion constructs were amplified using the following adapter primers containing the underlined ClaI sites: forward 5′ – GATCATATCGATACAGCAGCGGAGTTT – 3′ and reverse 5′ –GATCATATCGATGCCGCTTTACTTGTA – 3′. PCR products were digested with ClaI and ligated into ClaI-linearized BH-RCAS, a replication-competent retroviral vector derived from the Rous sarcoma virus [56]. Additionally, the insert encoding Y286A-eYFP was excised from the BH-RCAS vector using ClaI and inserted into the ClaI-linearized pLPCX vector. Therefore, one Y286A cell line was made using pLPCX and one was made using BH-RCAS. Cx43-coding sequences were verified at the UCLA Sequencing Core Facility.
Retroviral Expression of Cx43-eYFP constructs in MDCK cells
These procedures have been previously described [43, 56, 57]. Briefly, transfection of DF-1 cells with BH-RCAS constructs encoding wild-type and mutant Cx43-eYFP was performed using Superfect (Qiagen, Valencia, CA) in 60mm dishes according to the manufacturer’s instructions. Prior to infection, the RSV(A) receptor had been expressed in the MDCK cells, rendering them susceptible to infection. MDCK cells containing the RSV(A) receptor were selected using 0.5 mg/ml G418 (Sigma, St. Louis, MO). For transfection with pLPCX vectors, 293T cells were co-transfected with 10 ug of the designated pPLCX construct, 5 ug Hit-60, and 5 ug VSV-G using HEPES-Buffered Saline (HeBS) and 150 mM CaCl2. For infection of MDCK cells, conditioned medium was collected from transfected DF-1 or 293T cells (containing recombinant virus particles), filtered using a 0.45 um filter (Whatman, Florham Park, NJ), supplemented with 5 ug/ml polybrene (Sigma, St. Louis, MO) and added to target MDCK cells. For each construct, multiple clonal cell lines were derived from the selected populations by limited dilution.
Isolation of surface protein by biotinylation on tissue culture plates
To extract protein for Western blot analysis (Fig. 2A), MDCK cells expressing wild-type and mutant Cx43-eYFP constructs were cultured on 60mm tissue culture plates. 10 mM sodium butyrate (Alfa Aesar, Ward Hill, MA) dissolved in complete cell culture medium was added to cells 24 hours prior to experiment to boost protein expression. It was confirmed by confocal microscopy, paracellular dye flux assays, and transepithelial resistance measurements that the addition of 10 mM sodium butyrate does not alter the polarized membrane properties of MDCK cells (Fig. S1). Cells were kept on ice for the duration of the experiment. Cells were rinsed three times with cold DPBS. 2 ml of membrane impermeant sulfo-NHS-LC-biotin (Pierce, Rockford, IL) dissolved in DPBS (at a concentration of 0.5 mg/ml) was applied to each plate for 30 minutes. The labeling reaction was quenched with 3 rinses of 100 mM glycine (Fisher, Fair Lawn, NJ) dissolved in DPBS, and cells were washed once more with DPBS. Cells were lysed with RIPA buffer containing 0.5% SDS (Teknova, Hollister, CA), 1% NP-40 (United States Biological, Swampscott, MA), and 0.25% sodium deoxycholate (Sigma, St. Louis, MO) supplemented with Complete Mini protease inhibitors (Roche Diagnostics, Indianapolis, IN), 1 uM sodium vanadate (Fisher, Fair Lawn, NJ), 1 uM sodium fluoride (Fisher, Fair Lawn, NJ), and 1 uM phenylmethanesulfonyl fluoride (Sigma, St. Louis, MO) for 30 minutes. Lysates were passed through a 25⅝ G syringe three times (Becton-Dickinson, Franklin Lakes, NJ). Lysates were centrifuged for 10 minutes at 13,200 RPM at 4°C, then 750 ul of the total lysate was combined with 75 ul streptavidin-agarose beads (Novagen, Gibbstown, NJ) and incubated overnight on a rotating shaker at 4°C. On the following day, beads were rinsed four times with DPBS. After the fourth rinse, the beads (which remained suspended in about 175 ul of DPBS) were transferred to a 96-well plate and fluorescence was quantified with a Wallac Victor 2 plate reader using 485 nm excitation and 535 nm emission filters. Then, these beads were prepared for Western blot as indicated below.
Selective isolation of surface protein from the apical and basolateral domains by biotinylation
For polarized protein distribution studies, cells were seeded at a high density (500,000-1×106 cells per filter, depending on cell line) and cultured on Corning PET Transwell permeable filter supports for 5 days (Corning Incorporated, Corning, NY). Experiments were performed as described above with a few adjustments. Membrane impermeant sulfo-NHS-LC-biotin was added to either the apical or basolateral side, and DPBS was added to the side not receiving sulfo-NHS-LC-biotin. Prior to lysing, filters were cut out, and placed into new 6-well plates.
Isolation of total protein by biotinylation on tissue culture plates
This procedure was performed as described above with a few changes. Membrane permeant NHS-LC-biotin (Pierce, Rockford, IL) was used instead of sulfo-NHS-LC-biotin. A 40 mM solution of NHS-LC-biotin was prepared in DMSO, and then diluted 10X in PBS. 2 ml of NHS-LC-biotin were applied to each plate for 4 hours on a shaker at 4°C. All rinses were done as described above using PBS or 100mM glycine dissolved in PBS instead of DPBS.
Paracellular Permeability of MDCK Monolayers
MDCK cells expressing WT and mutant Cx43-eYFP were plated at high density on filter inserts and cultured for 5 days. The night before the experiment, either 10 mM sodium butyrate was added to both sides of the filter, or complete growth medium for the control conditions. Experiments were conducted at room temperature. Cells were rinsed three times with DPBS. 2 ml of 10 uM sodium fluorescein (Sigma, St. Louis, MO) dissolved in DPBS were added to the apical side, and 3 ml of DPBS were added to the basolateral side. Because sodium fluorescein is membrane impermeable, it can only cross the monolayer via the paracellular pathway, thus the accumulation of dye on the basolateral side represents paracellular flux. Accumulation of the dye was determined by taking 25 ul aliquots every 30 minutes for 3 hours from the basolateral chamber, dissolving them in 1.5ml DPBS, and quantifying the fluorescence using a Wallac Victor 2 Plate Reader at 485 emission, 535 excitation. The slope of the linear regression of the fluorescence intensity plotted versus time was used as a measure of the paracellular permeability and determined as a function of the paracellular flux in calcium-free conditions, which disrupts tight junctions [58]. One experiment in triplicate was performed for each cell line.
Transepithelial Resistance Measurements
MDCK cells expressing WT and mutant Cx43-eYFP were plated at high density on filter inserts and cultured for 5 days. 24 hours before experiment, either 10 mM sodium butyrate was added to both sides of the filter, or complete growth medium for the control conditions. Transepithelial resistance was determined by injecting a 1 ms, 50 uA current (stimulator A365, WPI, Sarasota, FL) over the membrane and measuring the induced voltage drop. Resistance was calculated using Ohms Law (V=IR). The current was delivered using a silver chloride electrode in both the apical and basolateral compartments of the culture dishes. Apart from the epithelial membrane these compartments are electrically separated. The voltage drop over the membrane was measured with an instrumentation amplifier (Brownlee, San Jose, CA). The background resistance (of the culture dish/mesh, electrodes, etc.) was measured independently and subtracted so that true membrane resistance could be compared. The injected current was verified by measuring the voltage drop over a 1000 Ω resistor in series with the membrane. Data was sampled using custom software and analyzed using MS Excel (Microsoft, Seattle, OR). Measurements were made in DPBS, and as a control, in PBS, which creates calcium-free conditions that disrupt tight junctions in MDCK cells and therefore cause transepithelial resistance to be drastically decreased.
Western blot analysis of Cx43-eYFP fusion proteins
Streptavidin-agarose beads were boiled in sample buffer containing β-mercaptoethanol and DTT for 10 minutes to strip protein off. Then the samples were centrifuged for 10 minutes at 13,200 RPM at 4°C. All of the supernatant (containing the protein) was then loaded into pre-cast Tris-HEPES-SDS gels (Pierce, Rockford, IL). Protein was transferred to nitrocellulose paper using a Transblot SD Semi-Dry Transfer Cell apparatus (Biorad, Hercules, CA). Immunoblotting was performed by incubation in primary antibodies diluted in 1% milk in TTBS using a 1:500 dilution of polyclonal anti-GFP HRP antibody (Santa Cruz Biotechnology, Santa Cruz, CA, sc-8334) or a 1:400 dilution of a rabbit polyclonal anti-Cx43 antibody (Invitrogen, Carlsbad, CA 71-0700) followed by incubation with 1:1200 dilution of secondary antibody conjugated to HRP (Cayman Chemical Company, Ann Arbor, MI 0004301). Protein bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and exposed to film.
Confocal microscopy
MDCK cells expressing the Cx43-eYFP mutant constructs were seeded at a high density and cultured on filter inserts for 5 days. 10mM sodium butyrate was added to cells 24 hours before experiment. (Microscopy was also performed in absence of pre-incubation with sodium butyrate to confirm that polarized membrane properties of the cell lines were not altered, See Fig. S1). Cells were fixed using 4% paraformaldehyde (Alfa Aesar, Ward Hill, MA). Filters were mounted onto glass cover slips with glycerol. A spin-disc confocal microscope (Olympus BX61) equipped with a camera was used. Images were acquired at an exposure of 742 ms using a 40x oil-immersion objective. Z stacks were reconstructed and analyzed using Slidebook software (Intelligent Imaging Innovations, Inc., Denver, CO). Background was corrected for all images using the “Constrained Iterative Deconvolution” function. For XY images, projection images were created using the “Max” function.
Surface protein degradation assay
Cells were grown on 60mm tissue culture plates and lysed at 0, 2, 4 and 8 hours. Surface protein was marked with sulfo-NHS-LC-biotin as described above. T0 plates were lysed (as described above) immediately, while the plates corresponding to the various time points were treated as follows. After the last DPBS rinse was completed, DPBS was aspirated, pre-warmed (37°C) complete cell culture medium was added to each plate and cells were immediately places into the tissue culture incubator (37°C, 5% CO2, humidified). Plates corresponding to the appropriate time point were taken out of the incubator and immediately placed on ice. They were rinsed twice with ice-cold DBPS and the lysing procedure was performed as described above. At least three independent experiments were performed in duplicate on two independent clones for each Cx43-eYFP variant (n varies for cell lines). Results are normalized to time 0 for each cell line. Data are graphed as mean ± s.e.m. Significance was determined using the Student t-test. P values ≤ 0.05 were considered to be significant.
Supplementary Material
Fig. S1. Sodium butyrate does not affect the polarized distribution of Cx43-eYFP constructs or the integrity of MDCK cell monolayers.
Fig. S2. LSYTRF, F52dup, and L90V mutant constructs show the same rate of degradation as WT.
Acknowledgments
This work was made possible through funding from the American Brain Tumor Association/Michael Reiss Fellowship (A.L.), Art of the Brain (A.L.), unrestricted funds from Cancer Center of Santa Barbara (A.L.), and NIH R01 DE13849 (E.W.J.). We thank Seema Tiwari-Woodruff for the use of her microscope, Guido Faas for conducting all of the TER measurements, and Olga Vagin for her guidance with dye transfer experiments.
Abbreviations
- MDCK
Madin-Darby canine kidney
- ODDD
Oculodentodigital dysplasia
- Cx43
Connexin43
References
- 1.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 2.Prochnow N, Dermietzel R. Connexons and cell adhesion: a romantic phase. Histochem Cell Biol. 2008;130:71–77. doi: 10.1007/s00418-008-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729–739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- 4.Virgintino D, Robertson D, Errede M, Benagiano V, Bertossi M, Ambrosi G, Roncali L. Expression of the gap junction protein connexin43 in human telencephalon microvessels. Microvasc Res. 2001;62:435–439. doi: 10.1006/mvre.2001.2345. [DOI] [PubMed] [Google Scholar]
- 5.Guerrier A, Fonlupt P, Morand I, Rabilloud R, Audebet C, Krutovskikh V, Gros D, Rousset B, Munari-Silem Y. Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J Cell Sci. 1995;108 (Pt 7):2609–2617. doi: 10.1242/jcs.108.7.2609. [DOI] [PubMed] [Google Scholar]
- 6.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, Okazaki K, Sears ML, Meda P, Nathanson MH, et al. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36:631–640. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 7.Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 8.Barcia C, Sanderson NS, Barrett RJ, Wawrowsky K, Kroeger KM, Puntel M, Liu C, Castro MG, Lowenstein PR. T cells’ immunological synapses induce polarization of brain astrocytes in vivo and in vitro: a novel astrocyte response mechanism to cellular injury. PLoS ONE. 2008;3:e2977. doi: 10.1371/journal.pone.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etienne-Manneville S. Polarity proteins in glial cell functions. Curr Opin Neurobiol. 2008;18:488–494. doi: 10.1016/j.conb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- 11.Clair C, Combettes L, Pierre F, Sansonetti P, Tran Van Nhieu G. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res. 2008;314:1250–1265. doi: 10.1016/j.yexcr.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Breidert S, Jacob R, Ngezahayo A, Kolb HA, Naim HY. Trafficking pathways of Cx49-GFP in living mammalian cells. Biol Chem. 2005;386:155–160. doi: 10.1515/BC.2005.019. [DOI] [PubMed] [Google Scholar]
- 13.Wiszniewski L, Sanz J, Scerri I, Gasparotto E, Dudez T, Lacroix JS, Suter S, Gallati S, Chanson M. Functional expression of connexin30 and connexin31 in the polarized human airway epithelium. Differentiation. 2007;75:382–392. doi: 10.1111/j.1432-0436.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 14.Jordan K, Solan JL, Dominguez M, Sia M, Hand A, Lampe PD, Laird DW. Trafficking, assembly, and function of a connexin43-green fluorescent protein chimera in live mammalian cells. Mol Biol Cell. 1999;10:2033–2050. doi: 10.1091/mbc.10.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 17.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 18.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 19.Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- 20.Favoreel HW. The why’s of Y-based motifs in alphaherpesvirus envelope proteins. Virus Res. 2006;117:202–208. doi: 10.1016/j.virusres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Arnold DB. Neuronal polarity: controlling the sorting and diffusion of membrane components. Pflugers Arch. 2007;453:763–769. [Google Scholar]
- 22.Klunder B, Baron W, Schrage C, de Jonge J, de Vries H, Hoekstra D. Sorting signals and regulation of cognate basolateral trafficking in myelin biogenesis. J Neurosci Res. 2008;86:1007–1016. doi: 10.1002/jnr.21556. [DOI] [PubMed] [Google Scholar]
- 23.Winckler B, Mellman I. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–640. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- 24.Laird DW. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J Biol Chem. 2008;283:2997–3001. doi: 10.1074/jbc.R700041200. [DOI] [PubMed] [Google Scholar]
- 25.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 26.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vreeburg M, de Zwart-Storm EA, Schouten MI, Nellen RG, Marcus-Soekarman D, Devies M, van Geel M, van Steensel MA. Skin changes in oculo-dento-digital dysplasia are correlated with C-terminal truncations of connexin 43. Am J Med Genet A. 2007;143:360–363. doi: 10.1002/ajmg.a.31558. [DOI] [PubMed] [Google Scholar]
- 28.Frasson M, Calixto N, Cronemberger S, de Aguiar RA, Leao LL, de Aguiar MJ. Oculodentodigital dysplasia: study of ophthalmological and clinical manifestations in three boys with probably autosomal recessive inheritance. Ophthalmic Genet. 2004;25:227–236. doi: 10.1080/13816810490513424. [DOI] [PubMed] [Google Scholar]
- 29.de la Parra DR, Zenteno JC. A new GJA1 (connexin 43) mutation causing oculodentodigital dysplasia associated to uncommon features. Ophthalmic Genet. 2007;28:198–202. doi: 10.1080/13816810701538620. [DOI] [PubMed] [Google Scholar]
- 30.van Es RJ, Wittebol-Post D, Beemer FA. Oculodentodigital dysplasia with mandibular retrognathism and absence of syndactyly: a case report with a novel mutation in the connexin 43 gene. Int J Oral Maxillofac Surg. 2007;36:858–860. doi: 10.1016/j.ijom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Loddenkemper T, Grote K, Evers S, Oelerich M, Stogbauer F. Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol. 2002;249:584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- 32.Joss SK, Ghazawy S, Tomkins S, Ahmed M, Bradbury J, Sheridan E. Variable expression of neurological phenotype in autosomal recessive oculodentodigital dysplasia of two sibs and review of the literature. Eur J Pediatr. 2008;167:341–345. doi: 10.1007/s00431-007-0468-1. [DOI] [PubMed] [Google Scholar]
- 33.Amador C, Mathews AM, Del Carmen Montoya M, Laughridge ME, Everman DB, Holden KR. Expanding the neurologic phenotype of oculodentodigital dysplasia in a 4-generation Hispanic family. J Child Neurol. 2008;23:901–905. doi: 10.1177/0883073808317730. [DOI] [PubMed] [Google Scholar]
- 34.Musa FU, Ratajczak P, Sahu J, Pentlicky S, Fryer A, Richard G, Willoughby CE. Ocular manifestations in oculodentodigital dysplasia resulting from a heterozygous missense mutation (L113P) in GJA1 (connexin 43) Eye. 2008 doi: 10.1038/eye.2008.77. [DOI] [PubMed] [Google Scholar]
- 35.Feller L, Wood NH, Sluiter MD, Noffke C, Raubenheimer EJ, Lemmer J, van Rensburg EJ. Report of a black South African child with oculodentodigital dysplasia and a novel GJA1 gene mutation. Am J Med Genet A. 2008;146A:1350–1353. doi: 10.1002/ajmg.a.32272. [DOI] [PubMed] [Google Scholar]
- 36.Honkaniemi J, Kalkkila JP, Koivisto P, Kahara V, Latvala T, Simola K. Letter to the editor: Novel GJA1 mutation in oculodentodigital dysplasia. Am J Med Genet A. 2005;139:48–49. doi: 10.1002/ajmg.a.30925. [DOI] [PubMed] [Google Scholar]
- 37.Wiest T, Herrmann O, Stogbauer F, Grasshoff U, Enders H, Koch MJ, Grond-Ginsbach C, Schwaninger M. Clinical and genetic variability of oculodentodigital dysplasia. Clin Genet. 2006;70:71–72. doi: 10.1111/j.1399-0004.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 38.Roscoe W, Veitch GI, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder GM, Laird DW. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem. 2005;280:11458–11466. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- 39.Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLachlan E, Manias JL, Gong XQ, Lounsbury CS, Shao Q, Bernier SM, Bai D, Laird DW. Functional characterization of oculodentodigital dysplasia-associated Cx43 mutants. Cell Commun Adhes. 2005;12:279–292. doi: 10.1080/15419060500514143. [DOI] [PubMed] [Google Scholar]
- 41.Seki A, Coombs W, Taffet SM, Delmar M. Loss of electrical communication, but not plaque formation, after mutations in the cytoplasmic loop of connexin43. Heart Rhythm. 2004;1:227–233. doi: 10.1016/j.hrthm.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 42.Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res. 2005;96:e83–91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- 43.Lai A, Le DN, Paznekas WA, Gifford WD, Jabs EW, Charles AC. Oculodentodigital dysplasia connexin43 mutations result in non-functional connexin hemichannels and gap junctions in C6 glioma cells. J Cell Sci. 2006;119:532–541. doi: 10.1242/jcs.02770. [DOI] [PubMed] [Google Scholar]
- 44.Manias JL, Plante I, Gong XQ, Shao Q, Churko J, Bai D, Laird DW. Fate of connexin43 in cardiac tissue harbouring a disease-linked connexin43 mutant. Cardiovasc Res. 2008;80:385–395. doi: 10.1093/cvr/cvn203. [DOI] [PubMed] [Google Scholar]
- 45.Gong XQ, Shao Q, Lounsbury CS, Bai D, Laird DW. Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma. J Biol Chem. 2006;281:31801–31811. doi: 10.1074/jbc.M605961200. [DOI] [PubMed] [Google Scholar]
- 46.Dobrowolski R, Sommershof A, Willecke K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J Membr Biol. 2007;219:9–17. doi: 10.1007/s00232-007-9055-7. [DOI] [PubMed] [Google Scholar]
- 47.Leithe E, Rivedal E. Ubiquitination of gap junction proteins. J Membr Biol. 2007;217:43–51. doi: 10.1007/s00232-007-9050-z. [DOI] [PubMed] [Google Scholar]
- 48.Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odorizzi G, Pearse A, Domingo D, Trowbridge IS, Hopkins CR. Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism. J Cell Biol. 1996;135:139–152. doi: 10.1083/jcb.135.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunbar LA, Aronson P, Caplan MJ. A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J Cell Biol. 2000;148:769–778. doi: 10.1083/jcb.148.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas MA, Zosso N, Scerri I, Demaurex N, Chanson M, Staub O. A tyrosine-based sorting signal is involved in connexin43 stability and gap junction turnover. J Cell Sci. 2003;116:2213–2222. doi: 10.1242/jcs.00440. [DOI] [PubMed] [Google Scholar]
- 52.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haller C, Kiessling F, Kubler W. Polarized expression of heterologous membrane proteins transfected in a human endothelial-derived cell line. Eur J Cell Biol. 1998;75:353–361. doi: 10.1016/S0171-9335(98)80068-X. [DOI] [PubMed] [Google Scholar]
- 54.Oliveira R, Christov C, Guillamo JS, de Bouard S, Palfi S, Venance L, Tardy M, Peschanski M. Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol. 2005;6:7. doi: 10.1186/1471-2121-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorsen F, Tysnes BB. Brain tumor cell invasion, anatomical and biological considerations. Anticancer Res. 1997;17:4121–4126. [PubMed] [Google Scholar]
- 56.Hughes SH. The RCAS vector system. Folia Biol (Praha) 2004;50:107–119. [PubMed] [Google Scholar]
- 57.Remington M, Chtchetinin J, Ancheta K, Nghiemphu PL, Cloughesy T, Lai A. The L84F polymorphic variant of human O6-methylguanine-DNA methyltransferase alters stability in U87MG glioma cells but not temozolomide sensitivity. Neuro Oncol. 2009;11:22–32. doi: 10.1215/15228517-2008-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na, K-ATPase beta1 subunit. J Biol Chem. 2008;283:2192–2202. doi: 10.1074/jbc.M704713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Sodium butyrate does not affect the polarized distribution of Cx43-eYFP constructs or the integrity of MDCK cell monolayers.
Fig. S2. LSYTRF, F52dup, and L90V mutant constructs show the same rate of degradation as WT.