Summary
The objective of mitosis is to provide a copy of the genome to each progeny of a cell division. This requires the separation of duplicate chromatids by the spindle apparatus, and the delivery of one set of chromosomes to each of the daughter cells. In budding yeast, the fidelity of chromosome delivery depends on the spindle position checkpoint, which prolongs mitosis until one end of the anaphase spindle arrives in the bud. Here, we tested the hypothesis that the activity of the spindle position checkpoint depends on persistent interactions between cytoplasmic microtubules and the mother-bud neck, the future site of cytokinesis. We used laser ablation to disrupt microtubule interactions with the bud neck, and we found that loss of microtubules from the neck leads to mitotic exit in a majority of checkpoint-activated cells. Our findings suggest that cytoplasmic microtubules are used to monitor the location of the spindle in the dividing cell, and in the event of positioning errors, relay a signal to inhibit mitotic exit until the spindle is appropriately positioned.
Results and Discussion
In the budding yeast Saccharomyces cerevisiae every cell division is intrinsically asymmetric. A genome is delivered to the daughter cell by drawing one end of the mitotic spindle through the bud neck, which marks the eventual plane of cytokinesis. The position of the spindle is controlled by cytoplasmic microtubules (cMTs), which project outward from the spindle pole bodies (SPBs), and interact with the cell cortex to orient the spindle along the mother-bud axis and then move one SPB through the neck and into the bud.
When mutations disrupt the interaction of cMTs with the cortex, spindle movement is often delayed, and mitosis ensues within the mother. However, the cell arrests in late anaphase due to a cell cycle checkpoint mechanism known as the spindle position checkpoint (SPC) [1–3]. The SPC prolongs mitosis by inhibiting the mitotic exit network (MEN), thereby preventing cyclin dependent kinase inhibition, spindle disassembly, and cytokinesis. Although the molecular mechanisms by which the SPC may inhibit the MEN have been examined, how aberrant spindle position activates and maintains the SPC is poorly understood [4–9].
In principle, the checkpoint must detect the position of the spindle relative to a landmark. The bud neck may serve as this landmark, with passage of the daughter-bound SPB through the neck being a critical event [10]. A priori, one can envision sensor mechanisms that either inhibit cell cycle progression when the spindle is not in the neck or promote cell cycle progression when the spindle does enter the neck.
Based on previous studies, the extension of cMTs from the SPBs through the bud neck appears to correlate with SPC activity [3, 10, 11]. We observed that cells with mis-positioned anaphase spindles exhibit at least one cMT in the bud neck, whereas cells containing properly positioned anaphase spindles (i.e. ones that have moved into the neck) do not exhibit cMTs in the neck (Supplemental Table 1). Movies of live cells reveal that cMTs persist in the neck for 97.5% ± 0.6 (mean ± SEM; n=41 cells) of the time when the anaphase spindle is within the mother (Supplemental Movie 1). These cMTs depolymerize out of the neck at a frequency of 0.5 ± 0.1 events•cell−1•hour−1 (mean ± SEM; n=41 cells). Subsequently, the same microtubule or a new microtubule grows back into the neck after an average time of 2.5 ± 0.3 min (mean ± SEM; n=28 events). Within this data set we found that the loss of cMTs from the neck increased the likelihood of checkpoint failure. When cMTs did not leave the neck for the duration of the movie, or were absent from the neck for less than 2.5 minutes, cells remained arrested in anaphase (20/21 cells and 7/8 cells, respectively). In contrast, loss of cMTs from the neck for more than 2.5 minutes was often followed by spindle disassembly, indicative of checkpoint failure (7/10 cells). This observation is similar to the previous finding that, in some cases, inappropriate mitotic exit within the mother is preceded by the loss of cMTs from the neck [3]. These data are also consistent with the finding that mutations that disrupt cMTs (i.e. cnm67Δ and tub2-401) lead to loss of microtubules from the neck and impaired SPC fidelity [3].
We sought to test directly whether the interaction between cMTs and the bud neck is important for SPC activity. To disrupt this interaction, we performed laser microsurgery of GFP-labeled microtubules in cells that were arrested by the SPC secondary to the loss of dynein. Dynein mutants are defective for pulling the spindle through the neck, leading to the accumulation of SPC-arrested cells. Irradiation with pulses of 539-nm light was sufficient to sever individual cMTs between the bud neck and SPB. Severing was evident by the displacement of a distal fragment from the remaining microtubule. After severing, both the fragment and remaining microtubule depolymerized (Supplemental Movie 2). Fragments appeared to shorten primarily from the newly formed minus end, although shortening from the plus end was observed occasionally. The remaining microtubule shortened back to the SPB by depolymerization from its plus end, consistent with previous evidence that minus ends at the SPB are anchored and inert [12]. Within several minutes following depolymerization, cMTs grew back from the SPB. Often, a new microtubule grew into the bud neck. Thus, microirradiation led to a transient loss of cMTs from the bud neck of SPC-arrested cells.
After cMT disruption, we asked whether cells remained arrested in anaphase by monitoring the spindle for up to 90 minutes. We observed several outcomes. First, a portion of cells corrected the spindle position defect by moving one end of the spindle across the bud neck (9/38 cells). This translocation satisfied the SPC, prompting spindle disassembly. Unirradiated control cells exhibited a similar rate of spindle correction over 90 minutes (16/83 cells).
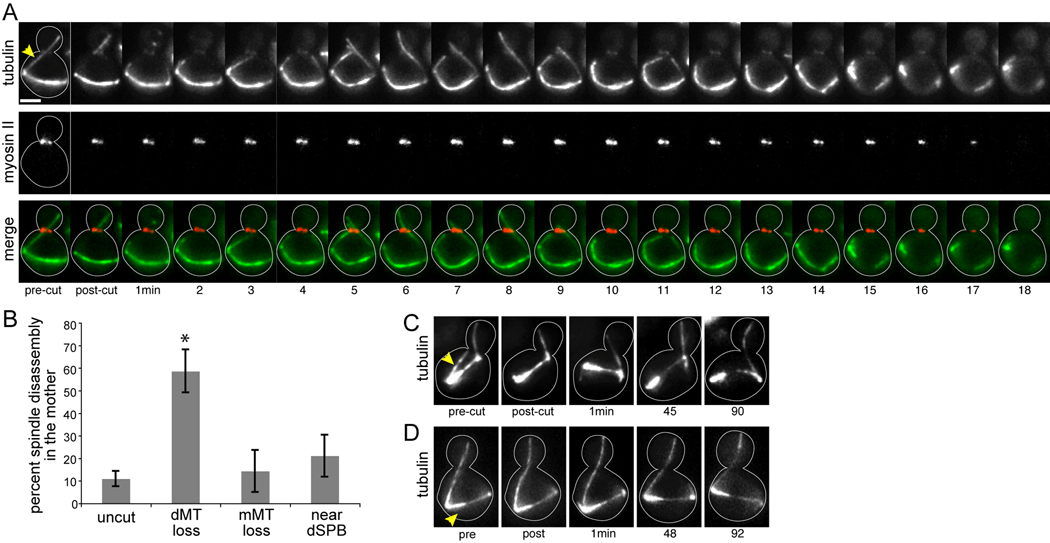
When the spindle remained in the mother after cMT disruption, 59% of cells (17/29 cells) disassembled the spindle and appeared to exit mitosis. Interpolar microtubules were totally lost, and the two SPBs moved independently. In separate experiments, we confirmed that spindle disassembly after cMT disruption was soon followed by cytokinesis, evident by the contraction and disappearance of the actomyosin ring from the bud neck (Fig 1A). Cytokinesis was similarly coupled with spindle disassembly during appropriate mitotic exit, after one SPB enters the bud (Supplemental Movie 3; [3]). Therefore, mitotic exit may be prompted by the loss of cMTs from the bud neck. In 7% of cells with the spindle in the mother (2/29 cells), the spindle became bent and elongated (Supplemental Fig 1). These events were rare, and the cell cycle status of these cells is uncertain. Finally, the morphology of the spindle was largely unchanged in 34% of cells (10/29 cells), suggesting that these cells maintained SPC-arrest despite the transient loss of cMTs from the neck.
Figure 1.
Mitotic arrest is disrupted by the loss of cytoplasmic microtubules from the bud neck. A) Spindle disassembly following the disruption of microtubule-neck interactions. Timelapse images of microtubules labeled with GFP-tubulin and the actomyosin ring labeled with RFP-myosin-II/Myo1 in a dyn1Δ mutant. The cytoplasmic microtubule extending across the bud neck in the first panel was irradiated between the SPB and the bud neck, at the site marked by the arrowhead. After ablation, a new microtubule grew into the neck within 5 min. Spindle disassembly began approximately 14 min after ablation, evidenced by the loss of interpolar microtubules and the resolution of the two spindle poles. The actomyosin ring encircles the bud neck during mitosis, and contracts during mitotic exit, concomitant with spindle disassembly. The merge series depicts tubulin in green and myosin-II in red. Each image is a composite of 3 planes separated by 1µm. Scale bar: 2µm. Strain: yJC6877. B) Percentage of dyn1Δ cells that exhibit spindle disassembly within 90 min of the indicated manipulation. “Uncut” cells were not targeted for ablation. “dMT loss” denotes neck-interacting microtubules that were severed between the neck and SPB. “mMT loss” denotes microtubules that were present entirely within the mother compartment and did not interact with neck, and were severed near the SPB. “near dSPB” denotes cells in which the ablating beam was targeted to a region of the cytoplasm within 2µm of the SPB that harbored a neck-interacting microtubule. Error bars are the standard error of proportion. Asterisk indicates statistical significance (p<0.05; compared to uncut cells) as determined by Fisher’s exact test. C) Only microtubules that interact with the bud neck are necessary for the maintenance of the SPC. The microtubule emanating from the spindle pole that is distal to the neck was ablated at the site marked by the arrowhead, and the spindle remained intact for >90 min after ablation. Strain: yJC5603. D) Irradiation near the dSPB does not disrupt the SPC. The ablating beam was targeted to a region of the cytoplasm indicated by the arrowhead. The spindle remained in tact for >90 min afterward. Strain: yJC5603.
As one negative control, we monitored SPC-arrest in unirradiated cells within the field. The majority of these cells remained in mitosis while the spindle was positioned within the mother (84%, 56/67 cells). Spindle disassembly within the mother did occur, but at a lower frequency than that observed after cMT loss (Fig 1B; 13%, 9/67 cells; p < 0.0001). In 2/67 cells the spindle became bent and elongated within the mother, suggesting that the frequency of this outcome is not due to cMT loss (3%; p = 0.58 compared to cMT-ablated cells).
Spindle disassembly within the mother is reminiscent of checkpoint-deficient mutants that fail to inhibit the MEN [1–3]; Supplemental Movie 4). To verify that mitotic exit following the loss of neck-interacting cMTs was due to activation of the MEN, not some aberrant pathway, we ablated neck-interacting microtubules in mutants carrying the temperature sensitive cdc15-2 allele, which blocks progression through the MEN [13, 14]. These cells maintained anaphase spindles after cMT disruption (10/10 cells). Together these data indicate that the loss of cMTs from the bud neck activates the MEN, consistent with a disruption of the SPC.
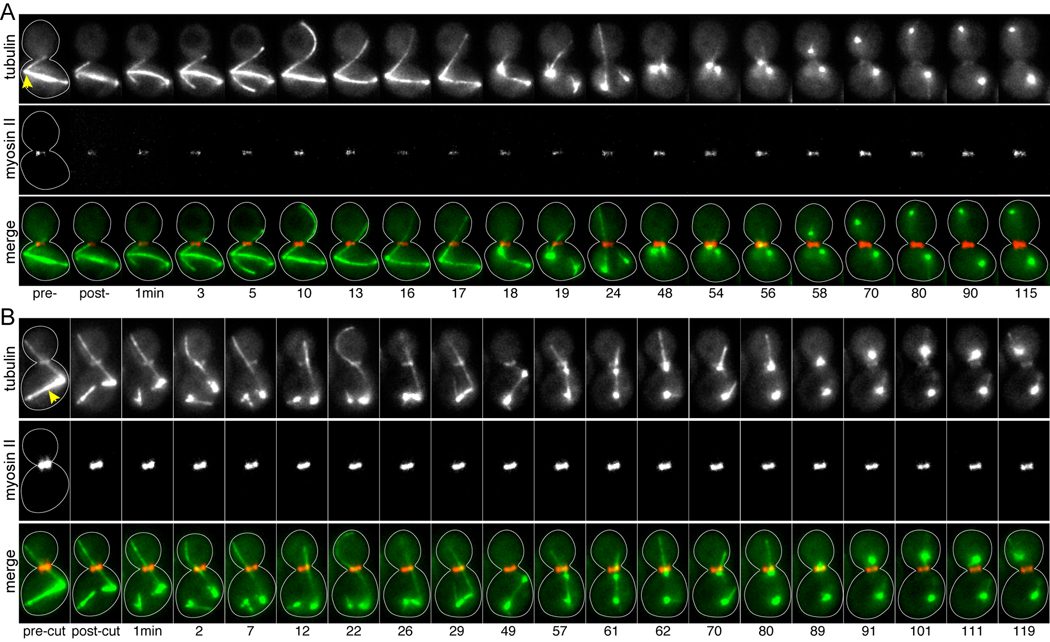
Next, we asked whether SPC failure was specifically attributable to the disruption of cMTs in the neck. To make this determination, we irradiated other sites within SPC-arrested cells. First, we tested whether loss of other cMTs (i.e. ones that did not pass through the neck) could disrupt the checkpoint. We identified SPC-activated cells with cMTs extending from one SPB through the neck, and we ablated cMTs at the alternate SPB (Fig 1C). After ablation, the majority of these cells remained in mitosis, and the frequency of spindle disassembly was similar to that observed in unirradiated cells (Fig 1B; 2/14 cells; p = 0.65). To test whether irradiation could promote spindle disassembly by damaging the SPBs, we first targeted regions of the cytoplasm near the SPB that harbored the bud-directed cMT (dSPB; Fig 1D). This did not enhance the frequency of SPC failure (Fig 1B; 4/19 cells; p = 0.47 compared to control cells). Next, we targeted irradiation directly to the SPBs, using a level of energy sufficient to sever cMTs. In nearly all of these cases, the spindle collapsed; however, the actomyosin ring did not contract, indicating that cells remained in mitosis (Fig 2A). We observed this outcome regardless of whether the dSPB (14/15 cells) or mSPB (5/6 cells) was targeted. Furthermore, this treatment did not destroy the function of the SPBs, as dynamic microtubules emanated from both SPBs after spindle collapse and occasionally formed a transient spindle-like linkage. Surprisingly, we observed that after spindle collapse, the passage of one SPB through the bud neck did not prompt mitotic exit (Fig 2A). This may be explained by the activation of alternative checkpoints in these cells, or perhaps a role for the spindle in MEN-signaling. Directly severing the microtubules of the spindle elicited similar behaviors, but did not provoke mitotic exit (7/9 cells; Fig 2B). Together, these results substantiate our experimental approach and demonstrate that SPC failure is specifically induced by the loss of cMTs from the bud neck.
Figure 2.
Damaging the SPBs or spindle microtubules does not prompt mitotic exit. Timelapse images of microtubules labeled with GFP-tubulin and actomyosin rings labeled with RFP-myosin-II/Myo1, from movies of dyn1Δ mutant cells. Merge series depicts tubulin in green and myosin-II in red. Each image is a composite of 3 planes separated by 1µm. Strain: yJC6877. A) Ablation of the dSPB. Arrowhead marks the dSPB, which harbors the neck-interacting microtubule and was targeted for ablation. The spindle collapses within 18 min of irradiation. The actomyosin ring does not contract within 115 min, despite the migration of one of the resolved spindle poles into the bud at 56 min, indicating that this cell has not exited mitosis. B) Severing the spindle. The ablation beam was targeted to spindle microtubules, denoted by the arrowhead, resulting in the destruction of the spindle and resolution of the spindle poles. The spindle reforms by 29 min; however, this association is lost by 62 min. One spindle pole enters the bud at 89 min. The actomyosin ring does not contract within 119 min, indicating that this cell has not exited mitosis.
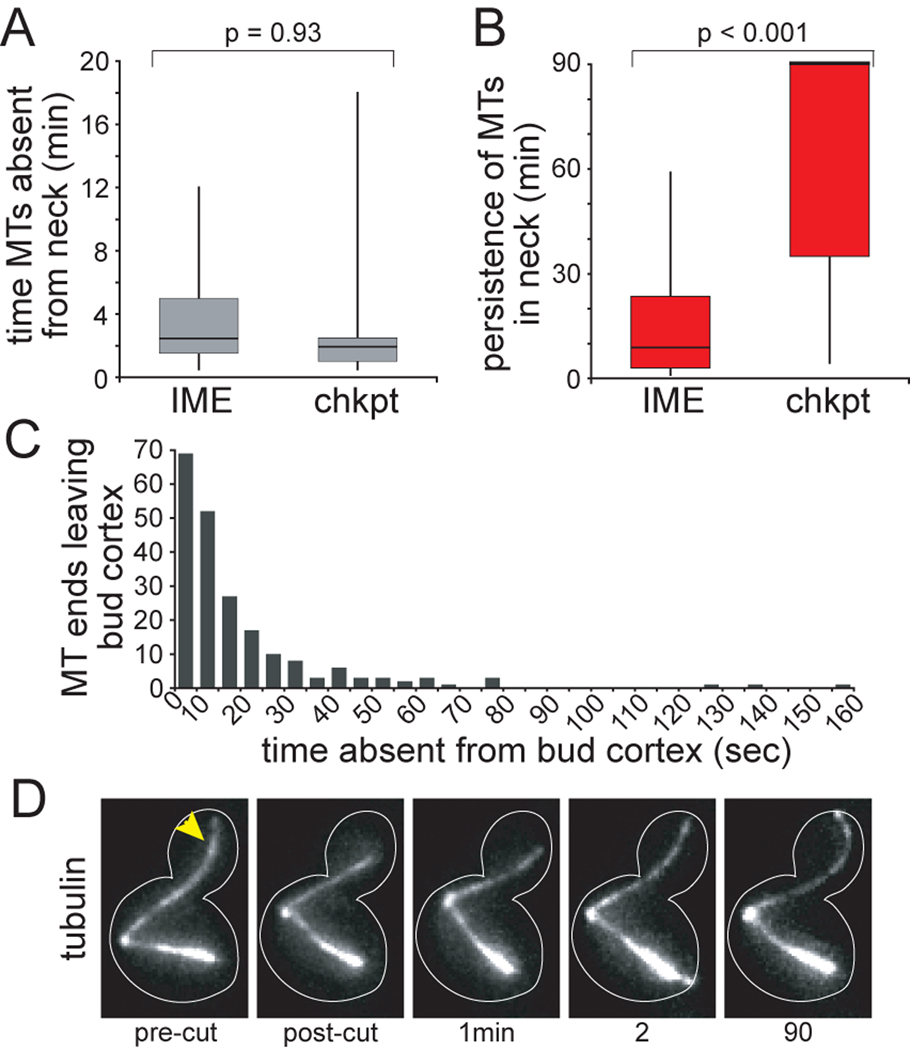
Our finding that some cells maintain the SPC after the disruption of cMTs in the bud neck suggests that the transient absence of cMTs may not be sufficient to disrupt the SPC. We reasoned that prolonged absence might diminish SPC activity; therefore, the recovery of new cMTs into the neck might determine outcome. By comparing the timing of cMT-recovery in the neck, we found that the duration of cMT absence was not significantly different between cells that exited mitosis versus those that maintained the SPC (Fig 3A). We did, however, discover a significant difference in the persistence of new cMTs in the neck. cMTs briefly visited the neck before depolymerizing away in cells that aborted the SPC (Fig 3B). In contrast, cMTs grew back into the neck and persisted - often for the remainder of the 90-minute movie - in cells that maintained the SPC. This difference was not dependent on whether the cMT that re-entered the neck grew from the SPB that harbored the original neck-interacting cMT or the alternate SPB (data not shown). These results indicate that after the disruption of cMT-neck interactions, the stability of the SPC may depend on the restoration of persistent cMTs in the neck.
Figure 3.
Checkpoint stability correlates with the recovery of persistent microtubules in the bud neck. A) Time between the ablation of neck-interacting microtubules and the arrival of new microtubules in the neck was determined for cells that subsequently underwent inappropriate mitotic exit in the mother (IME; n=17 cells) or remained arrested in mitosis (chkpt; n=10 cells). These two data sets were not significantly different, as determined by t-test (p=0.93). B) Persistence of new microtubules in the bud neck. After a new microtubule grew into the neck, the time until that microtubule left the neck was determined. For the majority of cells that remained arrested in mitosis, the new microtubule persisted in the bud neck for the remainder of the 90 min movie. These data sets were significantly different by t-test (p<0.001). C) Dynamic interactions between microtubule plus ends and the bud cortex during checkpoint-arrest. Movies of unirradiated dyn1Δ cells expressing GFP-tubulin were collected by confocal microscopy (see Experimental Procedures). When microtubule ends moved away from the cortex, the amount of time before the ends returned to the cortex was determined. 210 events were measured in 23 cells. Strain: yJC5603. D) Disruption of microtubule plus end interactions with the bud cortex does not affect the SPC. The GFP-labeled microtubule crossing the bud neck was ablated near its plus end, at the site marked by the arrowhead. After ablation of the plus end, the microtubule resumed growth within approximately 1 min and the spindle remained in tact for >90 min. Strain: yJC5603.
The cMTs that cross the bud neck often contact the bud cortex, raising the possibility that cMT-cortex interactions may contribute to the SPC. Movies of unirradiated control cells reveal that cMT-cortex interactions are dynamic, with microtubule ends leaving the cortex at a frequency of 1.0 ± 0.1 events•cell−1•min−1 (mean ± SEM; n=23 cells) for periods averaging 22.7 ± 1.4 seconds (mean ± SEM; n=210 events; Fig 3C). We tested whether increasing the duration of cMT absence from the bud cortex might promote SPC failure by severing cMTs in the bud, within several microns of the plus end. In 9 of 10 cells, the remaining microtubule did not shorten sufficiently to leave the neck (Fig 3D). Instead, the new plus end paused or briefly depolymerized before resuming growth and re-establishing contact with the cortex. In separate experiments acquired with greater time resolution, we determined that the average time between ablation near the plus end and new contact with the bud cortex was 60 ± 5 sec (mean ± SEM; n=10 cells; Supplemental Movie 5). Prolonged loss of interaction with the bud cortex did not perturb the SPC in any of the 9 cells. For the one case in which the remaining microtubule did shorten out of the neck, the cell exited mitosis. These data support the hypothesis that SPC activity relies on cMT interactions with the neck rather than the bud cortex.
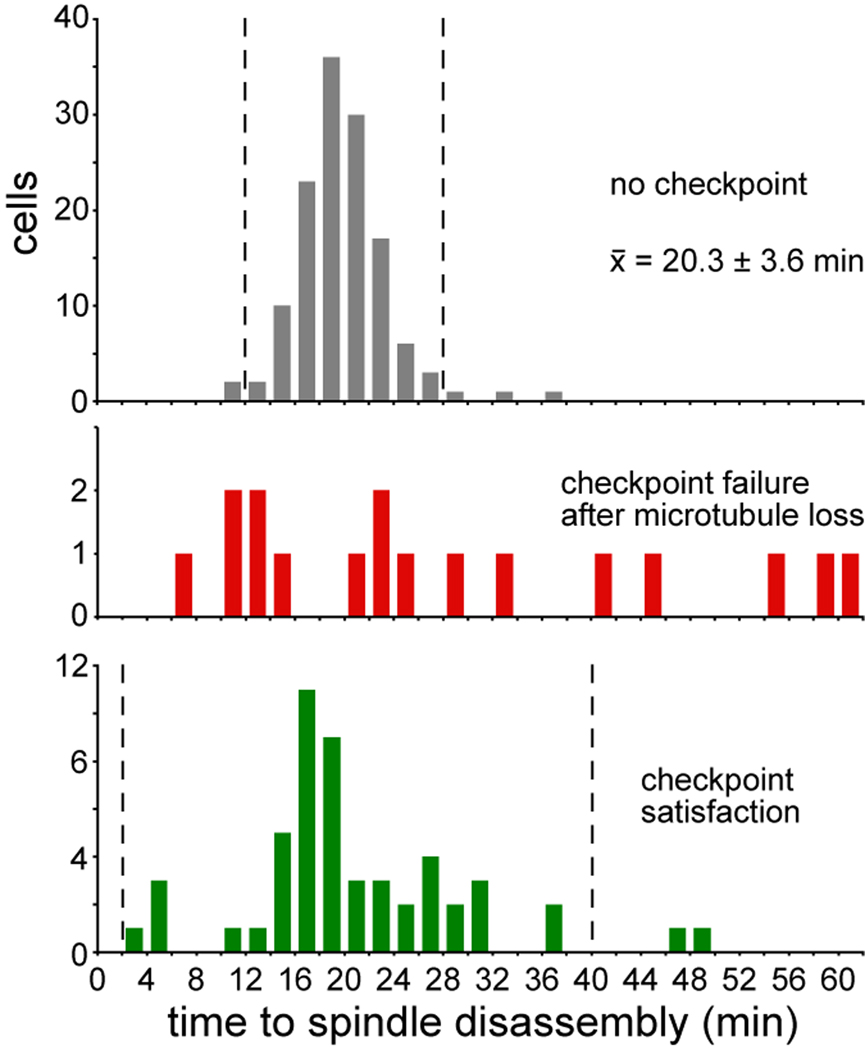
During a normal mitosis, the timing of mitotic exit is tightly linked to the movement of the dSPB into the bud [3, 10]. If the disruption of cMT-neck interactions deactivates the SPC in the same fashion, then mitotic exit after cMT loss might exhibit similar kinetics. In control cells with properly aligned spindles, spindle disassembly occurred 20.2 ± 3.6 min (mean ± SD; n=132; Fig 4) after the dSPB crossed the neck. In contrast, after the disruption of cMT-neck interactions by laser irradiation, the timing of spindle disassembly was variable (Fig 4). One possible explanation is that the kinetics of mitotic exit are altered after SPC-arrest. Consistent with this notion, we found greater variability in the timing of spindle disassembly in control cells that experienced SPC-arrest before correcting spindle mis-alignment (21.1 ± 9.1 min; mean ± SD; n=52; Fig 4). Thus, the loss of cMTs from the bud neck may promote mitotic exit on a timescale similar to checkpoint satisfaction; but the delayed mitotic exit seen in some ablated cells suggests that the passage of a spindle pole into the bud prompts more efficient deactivation of the SPC.
Figure 4.
Relationship between the timing of spindle disassembly and either the passage of one SPB into the bud or the loss of microtubules from the bud neck. “No checkpoint” histogram depicts the time elapsed between the arrival of one pole of an elongating anaphase spindle in the bud and subsequent spindle disassembly in 132 cells with properly aligned spindles that do not exhibit checkpoint-dependent delay; from movies of GFP-tubulin collected on a confocal microscope (20.3 ± 3.6 min; mean ± SD). Dashed lines indicate the 95% confidence interval (mean ± 2SD). “Checkpoint failure after microtubule loss” depicts the time elapsed between the ablation of neck-interacting microtubules and spindle disassembly within the mother compartment in 17 cells. “Checkpoint satisfaction” depicts the time between the arrival of one spindle pole in the bud and spindle disassembly in cells that correct spindle alignment defects after checkpoint-dependent anaphase delay; from movies collected on a confocal microscope of 52 cells expressing GFP-tubulin (21.1 ± 9.1 min; mean ± SD). Dashed lines indicate the 95% confidence interval. Scales of the Y-axes are not equivalent. Strain: yJC5603.
In this study, we demonstrate that the persistent interaction of cMTs with the bud neck promotes the activity of the SPC. We propose that cMTs contribute to a feedback mechanism that relays SPC-activating signals from the bud neck to the SPB. The SPB is a key site of SPC signaling; the localization of checkpoint proteins to the SPB correlates with activity, and this localization is influenced by cell polarity and cMTs [7, 8, 10, 15]. The nature of the SPC-sensor that detects microtubule interactions is not clear. This mechanism could be based on forces generated by cMT plus ends contacting the bud cortex; however, we found that the disruption of cMT-cortex interactions did not affect SPC integrity. Alternatively, cMT-associated factors may stimulate SPC regulators at the bud neck. Several proteins at the neck are important for SPC function, including the septins, Bud6, and the Kin4 kinase [4–6, 11]. It will be important to determine the molecular details of this signaling mechanism.
Experimental Procedures
Microscopy
Details of sample preparation and microscope systems are provided in the Supplementary Material. Time-lapse analysis of microtubule dynamics in live cells was performed using asynchronous mid-log cultures of dyn1Δ cells expressing GFP-Tub1 (strain yJC5603). Images were captured on a spinning disc confocal microscope. Preliminary ablation experiments were carried out on a custom-built laser-microsurgery system that utilizes 532-nm 8-ns pulses [16]. Subsequent experiments were performed using a Micropoint Laser Ablation Unit (Photonic Instruments, St. Charles, IL), which included a NL100 Nitrogen Laser (Stanford Research Systems, Sunnyvale, CA) tuned to 539-nm by Coumarin dye.
Data Analysis
Statistical significance of spindle disassembly events was determined by Fisher’s exact test. Statistical significance of the timing of microtubule absence or recovery was determined by t-test.
Supplementary Material
Acknowledgments
This research was supported by grants from NIH to JAC (GM47337) and AK (GM59363). JM was supported by a postdoctoral fellowship from the Molecular Oncology program of the Siteman Cancer Center at Washington University, funded by NIH T-32-CA113275. We are grateful to Drs. Scott Nelson, Boyd Butler, and Mark Longtine for advice and suggestions; and to Dr. Kyung Lee for the gift of the GFP-tubulin construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pereira G, Hofken T, Grindlay J, Manson C, Schiebel E. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10. [PubMed] [Google Scholar]
- 2.Bloecher A, Venturi GM, Tatchell K. Anaphase spindle position is monitored by the BUB2 checkpoint. Nat Cell Biol. 2000;2:556–558. doi: 10.1038/35019601. [DOI] [PubMed] [Google Scholar]
- 3.Adames NR, Oberle JR, Cooper JA. The surveillance mechanism of the spindle position checkpoint in yeast. J Cell Biol. 2001;153:159–168. doi: 10.1083/jcb.153.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira G, Schiebel E. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol Cell. 2005;19:209–221. doi: 10.1016/j.molcel.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 5.D'Aquino KE, Monje-Casas F, Paulson J, Reiser V, Charles GM, Lai L, Shokat KM, Amon A. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell. 2005;19:223–234. doi: 10.1016/j.molcel.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Nelson SA, Cooper JA. A novel pathway that coordinates mitotic exit with spindle position. Mol Biol Cell. 2007;18:3440–3450. doi: 10.1091/mbc.E07-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maekawa H, Priest C, Lechner J, Pereira G, Schiebel E. The yeast centrosome translates the positional information of the anaphase spindle into a cell cycle signal. J Cell Biol. 2007;179:423–436. doi: 10.1083/jcb.200705197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caydasi AK, Pereira G. Spindle alignment regulates the dynamic association of checkpoint proteins with yeast spindle pole bodies. Dev Cell. 2009;16:146–156. doi: 10.1016/j.devcel.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Chan LY, Amon A. The protein phosphatase 2A functions in the spindle position checkpoint by regulating the checkpoint kinase Kin4. Genes Dev. 2009;23:1639–1649. doi: 10.1101/gad.1804609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molk JN, Schuyler SC, Liu JY, Evans JG, Salmon ED, Pellman D, Bloom K. The differential roles of budding yeast Tem1p, Cdc15p, and Bub2p protein dynamics in mitotic exit. Mol Biol Cell. 2004;15:1519–1532. doi: 10.1091/mbc.E03-09-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castillon GA, Adames NR, Rosello CH, Seidel HS, Longtine MS, Cooper JA, Heil-Chapdelaine RA. Septins have a dual role in controlling mitotic exit in budding yeast. Curr Biol. 2003;13:654–658. doi: 10.1016/s0960-9822(03)00247-1. [DOI] [PubMed] [Google Scholar]
- 12.Maddox PS, Bloom KS, Salmon ED. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nat Cell Biol. 2000;2:36–41. doi: 10.1038/71357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973;74:267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mah AS, Jang J, Deshaies RJ. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc Natl Acad Sci U S A. 2001;98:7325–7330. doi: 10.1073/pnas.141098998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monje-Casas F, Amon A. Cell polarity determinants establish asymmetry in MEN signaling. Dev Cell. 2009;16:132–145. doi: 10.1016/j.devcel.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magidson V, Loncarek J, Hergert P, Rieder CL, Khodjakov A. Laser microsurgery in the GFP era: a cell biologist's perspective. Methods Cell Biol. 2007;82:239–266. doi: 10.1016/S0091-679X(06)82007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.