Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor involved in the regulation of multiple cellular pathways, such as xenobiotic metabolism and Th17 cell differentiation. Identification of key physiologically relevant ligand(s) that regulate AHR function remains to be accomplished. Screening of indole metabolites has identified indoxyl-3-sulfate (I3S) as a potent endogenous ligand that selectively activates the human AHR at nanomolar concentrations in primary human hepatocytes, regulating transcription of multiple genes, including: CYP1A1, CYP1A2, CYP1B1, UGT1A1, UGT1A6, IL6, and SAA1. Furthermore, I3S exhibits an ~ 500-fold greater potency in terms of transcriptional activation of the human AHR relative to the mouse AHR in cell lines. Structure-function studies reveal that the sulfate group is important determinant for efficient AHR activation. This is the first phase II enzymatic product identified that can significantly activate the AHR and ligand competition binding assays indicate that I3S is a direct AHR ligand. I3S failed to activate either CAR or PXR. The physiological importance of I3S lies in the fact that it is a key uremic toxin that accumulates to high micromolar concentrations in kidney dialysis patients, but its mechanism of action is unknown. I3S represents the first identified relatively high potency endogenous AHR ligand that plays a key role in human disease progression. These studies provide evidence that the production of I3S can lead to AHR activation and altered drug metabolism. Our results also suggest that prolonged activation of the AHR by I3S may contribute to toxicity observed in kidney dialysis patients and thus represent a possible therapeutic target.
The aryl hydrocarbon receptor (AHR)1 is a ligand-activated transcription factor that belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors, a class of proteins that are considered environmental sensors. Prior to activation, the AHR is located in the cytoplasm of the cell, complexed with two heat-shock protein 90 molecules, X-associated protein 2, and p23. Binding of ligand leads to nuclear translocation and subsequent release of the chaperone complex and formation of a heterodimer with the AHR nuclear translocator (ARNT), which then binds to dioxin responsive element (DRE; or xenobiotic responsive element) sequences in the promoter regions of target genes (1). It is of interest to note that a number of genes have recently been determined to be regulated by the AHR without direct binding of the AHR to a DRE consensus sequence (2).
Activation of the AHR is primarily known to mediate the expression of phase I and phase II drug metabolism genes, including: CYP1A1/2, CYP1B1, UGT1A1/6, and sulfotransferase (SULT) 1A1. However, a variety of studies have indicated that the AHR can regulate a diverse set of genes (e.g. slug, epiregulin and prostaglandin endoperoxidase H synthesis 2) involved in immune function, proliferation as well as inflammatory signaling and these responses are often cell-type specific (3–5). The AHR also regulates IL6 expression in tumor cells (2, 6). Additionally, the AHR can mediate repression of acute phase genes (e.g. SAA1) independent of DRE binding, further expanding the diversity of AHR-mediated gene regulation (2). The AHR has also been shown to play a crucial role in a number of physiological processes, including liver vascular development (7) and T-cell differentiation into Th17 cells (8). This latter observation has spurred considerable interest in the AHR due to the emerging role Th17 cells play in proinflammatory processes such as psoriasis and inflammatory bowel disease (9, 10). Ahr null mice have reduced reproductive success that is mediated in part by impaired ovulation and folliculogenesis (11). Thus, the AHR plays an important role in normal cellular physiology and homeostasis.
A number of exogenous AHR ligands have been identified and extensively investigated. Among these are polycyclic aromatic hydrocarbons, halogenated aromatic hydrocarbons (e.g. 2,3,7,8-tetrachlorodibenzo-p-dioxin or dioxin), and many naturally occurring flavonoids (e.g. resveratrol, quercetin). The extreme toxicity of dioxin in rodents is due to its long half-life leading to continual AHR activation. Endogenous compounds have been identified as relatively weak AHR ligands and their physiological relevance remains uncertain (12). In light of the role of the AHR in multiple pathways involved in disease processes, it becomes even more imperative to identify key endogenous ligand(s) for this receptor. The search for an endogenous ligand of the AHR has yielded a few compounds, but most have shown either low binding affinity for the AHR (e.g. bilirubin) or their role in normal physiology is uncertain (e.g. indirubin). However, bilirubin can reach concentration that can activate the AHR in certain disease states such as jaundice (13). Based on numerous reports identifying indole derivatives as AHR agonists (14), coupled with the discovery that the human AHR, appears to preferentially bind indirubin (15) relative to the mouse AHR, we examined a panel of simple indole derivatives, including: tryptophan, indole, indoxyl-3-sulfate (I3S), indoxyl-3-acetate (I3A), and indole-3-methanol. Surprisingly, I3S is a human AHR ligand and potent activator of transcriptional activity. This observation has considerable physiological relevance due to the presence of high concentrations of I3S, a uremic toxin, in humans with kidney disease and its association with vascular disease (16). This is the first report of I3S binding to a specific target protein and may represent a key molecular mechanism of I3S-mediated toxicity.
MATERIALS AND METHODS
Chemicals and reagents
Indoxyl-3-sulfate, indoxyl-3-acetate, and indoxyl-3-methanol were purchased from Alfa Aesar (Ward Hill, MA). 6, 2′, 4′-trimethoxyflavone was obtained from Indofine Chemical Co. (Hillsborough, NJ). All other chemicals used were obtained from Sigma (St. Louis, MO) at the highest grade available.
Cell culture
Huh7, HepG2 40/6, and H1L1.1c2 cells were maintained in α-MEM (Sigma), supplemented with 8% fetal bovine serum (FBS; Hyclone Laboratory, Logan, UT), 1,000 units/mL penicillin, and 0.1 mg/mL streptomycin (Sigma). MCF-7 breast tumor cells were maintained as previously described (6). The human hepatoma line HepG2 40/6 and mouse hepatoma line H1L1.1c2 are clonal cell lines stably transfected with the DRE-driven luciferase reporter construct pGudLuc 6.1 or pGudLuc 1.1, respectively (17, 18).
Primary human hepatocytes
Primary human hepatocytes from two different donors were obtained from the University of Pittsburgh, through the Liver Tissue Cell Distribution System, NIH Contract #N01-DK-7-0004 / HHSN267200700004C. Culture details have been reported previously (19). Forty-eight hours following Matrigel additions, cells were exposed to I3S or TCDD at the noted concentrations for the stated treatment times.
Stable reporter luciferase assay
Assays were performed using the HepG2 40/6 and H1L1.1c2 stable reporter cell lines, described previously (17, 18). Following 4 h treatments with specified compounds at the given concentrations, cells were lysed and luciferase activity was determined using Luciferase Assay System Substrate (Promega, Madison, WI) according to manufacturer’s directions.
Ligand binding assay and transgenic mice
AHR ligand binding assays were performed as described previously, as was the development of the AHRTtrCreAlbAhrfx/fx mouse model from which liver cytosol was used as a source for human AHR (15). In this mouse line the mouse AHR has been deleted from hepatocytes and the human AHR is expressed from the transgene driven by a hepatocyte specific promoter.
RNA isolation and real-time PCR
RNA was isolated from cells using TRI® Reagent (Sigma) according to manufacturer’s instructions. cDNA was prepared from the RNA samples by reverse transcription with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and analyzed by quantitative real-time PCR on a BioRad iQ system using PerfeCTa™ SYBR® Green SuperMix for iQ (Quanta Biosciences, Gaithersburg, MD). The ribosomal gene product, L13a, was used to normalize the results for each gene examined. Primers used for real-time RT-PCR analysis are listed in Supplementary Table S1.
Gene silencing
AHR mRNA levels were decreased using the Dharmacon On-Target plus small interfering RNA (siRNA) oligonucleotide (Dharmacon, J004990-07).
Electroporation/nucleofection was performed using the Amaxa nucleofection system, essentially as described in manufacturer protocols. Briefly, cells were washed and suspended at a concentration of 3 × 106 per 100 µL of nucleofection solution. Control or targeted siRNA was added to the sample for a final concentration of 2 µM. Samples were electroporated using manufacturer’s high efficiency program and plated into six-well dishes in complete media.
Western blot analysis
Whole-cell extracts were prepared by lysing cells in RIPA buffer [10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 140 mM NaCl, 1% Triton X-100, 0.1% Na-deoxycholate, 0.1% SDS] supplemented with 1% NP40, 300 mM NaCl, and protease inhibitor cocktail (Sigma). Homogenates were centrifuged at 21,000 × g for 30 min at 4°C, and the soluble fraction was collected as cellular extract. Protein samples were resolved by Tricine SDS-PAGE and transferred to membrane. Immunoblotting was performed using antibodies directed against AHR (mouse monoclonal antibody RPT1, Affinity Bioreagents, Golden, CO) and p23 (provided by Dr. David Toft, Mayo Clinic, Rochester, MN). Proteins were visualized using biotin-conjugated secondary antibodies in conjunction with 125I streptavidin.
P450 Glo activity assay
The P450-Glo™ CYP1A1 Assay was purchased from Promega Corporation (Madison, WI). Cells were grown in 12-well (primary human hepatocytes) or 24-well (Huh7) plates. I3S (100 nM-50 µM), 10 nM TCDD, or DMSO was added directly to the plates (0.1% DMSO total/well). Following 18 h of treatment, cells were washed once with warm PBS and fresh media containing luciferin-CEE reagent (1:100 dilution) was added to the wells, 200 µl/well for a 24-well plate and 500 µl/well for a 12-well plate. Cells were incubated for an additional 24 h before luciferase readings were performed as described by manufacturer. Samples were normalized for total protein concentration.
Transient transfection assays for CAR and PXR activation
All transfections were performed in a 48-well format. On the morning of day 1, COS1 cells were seeded to approximately 60–70% confluency in each well. DNA transfection mixtures were assembled using Fugene6 transfection reagent (Roche Applied Science, Indianapolis, IN). Each well was transfected with 25 ng of CMV2-CAR or CMV2-PXR expression plasmid, 25 ng 3.1-RXRα expression plasmid, 100 ng of luciferase reporter, and 10 ng of pRL-CMV (for transfection normalization; Promega, Madison, WI). For all transfections, the reagent was prepared at a ratio of 1:3 (micrograms of DNA to microliters of transfection reagent) as recommended in the manufacturer's protocol. Within a given experiment, all transfections contained the same total amount of DNA. At the time of transfection (~ 1 h after seeding), cells were approximately 70% confluent. The following day (~18 hr post-transfection), cells were treated with chemical agents, which were all prepared in DMSO. Each treatment was performed in quadruplicate. Because CAR1 is constitutively active, androstanol (10 µM), an inverse-agonist of CAR1 (20), was included in the treatment mix to decrease the receptor’s constitutive activity, which can then be reversed in the presence of an agonist. The concentrations of other treatments were as follows: I3S, 5 µM; 6-(4-Chlorophenyl)imidazo[2,1-b] [1,3]thiazole-5-carbaldehyde O-3,4-dichlorobenzyl) oxime (CITCO), a positive control for the CAR receptors, 5 µM, and Rifampicin (RFPM), a positive control for PXR, 10 µM. On day 3 (24 h after chemical treatment), cells were washed with PBS and luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) and a Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, CA). Luciferase assay and stop and glow reagents were diluted with 1x Tris-buffered saline, pH 8.0, to a 0.5x final concentration. All other aspects of the assay were performed in accordance with the manufacturer's protocol.
Statistical analysis
Treatments were compared to the carrier solvent control values. One-way ANOVA was followed by Tukey’s Multiple Comparison Test to compare samples for all analyses including luciferase reporter assays and qRT-PCR using GraphPad Prism version 5.0.
RESULTS
I3S activates AHR-mediated reporter gene assay
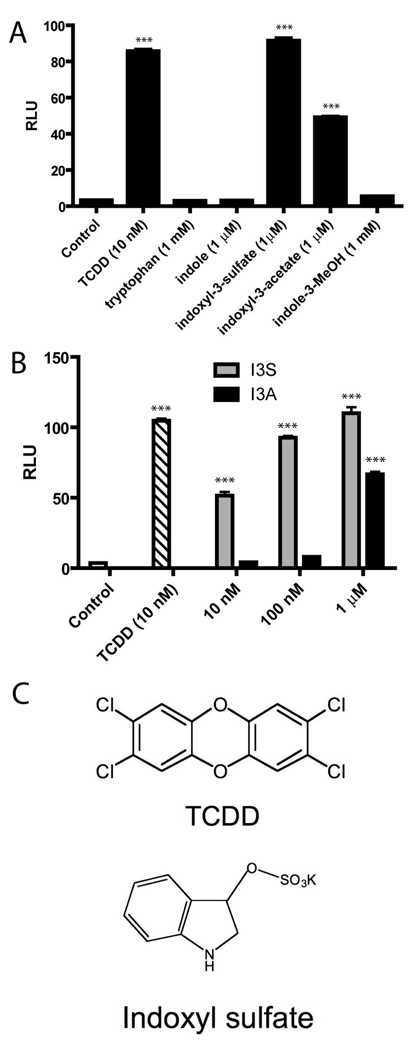
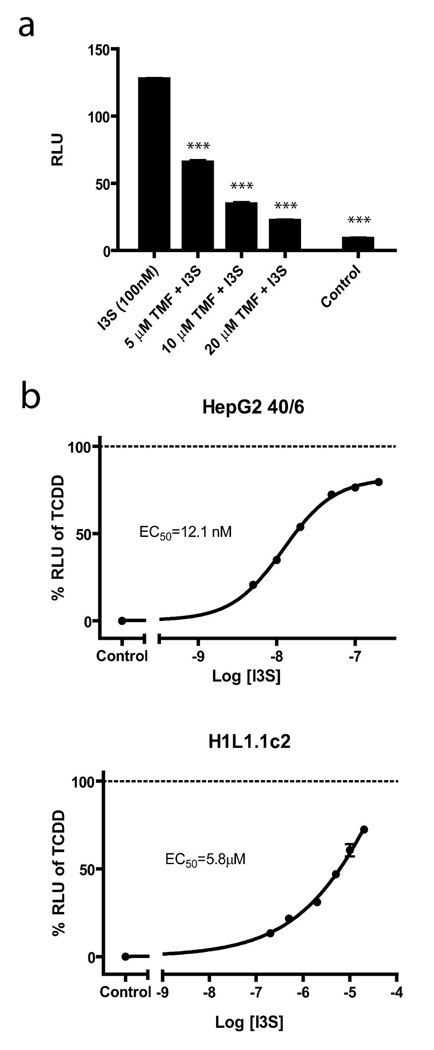
Indole and tryptophan derived products have previously been shown to be AHR ligands (12). We decided to further explore whether indole metabolites that have been shown to be present in mouse serum could activate the AHR (21). In order to screen selected indole derivatives for potential activation of the AHR, we utilized a DRE-coupled luciferase reporter stably integrated into a human hepatoma cell line (HepG2 40/6). Cells were treated with tryptophan, indole, I3S, I3A, or indole-3-methanol and assessed for AHR activation. Two compounds, I3S and I3A, were noted to exhibit significant AHR driven reporter activity (Figure 1A). Further studies with these compounds revealed I3S to be a more potent activator than I3A, with significant reporter activity observed at 10 nM (Figure 1B). To establish that the observed activity was AHR-dependent, cells were co-treated with I3S and the AHR antagonist, TMF (22). The decreased luciferase activity observed in samples treated with increasing concentrations of TMF is consistent with the concept that I3S is a direct AHR ligand (Figure 2A). The unexpected potency of I3S in activating the AHR is particularly striking, considering that it is generally believed that high affinity exogenous AHR ligands must have 3 or more cyclic or aromatic ring structures (e.g. TCDD, indolo[3,2b]carbazole). Structures for I3S and TCDD are shown for comparison (Figure 1C). To confirm that the AHR transcriptional activity observed upon exposure to I3S was not due to a trace contaminant, I3S was subjected to HPLC and NMR analysis and was determined to be greater than 99.9% pure (Supplementary Text). Therefore, these data firmly establish that the toxin I3S, associated with kidney disease (23, 24), potently activates the human AHR.
FIGURE 1.
I3S activates an AHR-dependent reporter assay. (A) Relative luciferase unit (RLU) readings for HepG2 40/6 cells treated for 4 h with the compounds listed at the indicated concentrations. (B) RLU readings for HepG2 40/6 cells treated with DMSO (control), TCDD, I3S, or I3A at noted concentrations for 4 h. (C) Structures of TCDD and I3S (potassium salt). Experiments were performed in triplicate; error bars denote standard deviation. Significance, as compared to respective control-treated samples, is noted by *** (p<0.001).
FIGURE 2.
I3S-induced DRE-mediated AHR transcriptional activity is inhibited by TMF and is species-dependent. (A) Antagonism of I3S-induced AHR-mediated luciferase activity was by co-treatment with TMF (5–20 µM) for 4 h in HepG2 40/6 cells. (B) Comparison of I3S induction of DRE-mediated transcription in HepG2 40/6 and H1L1.1c2 luciferase reporter cell lines, represented as a percentage of response to 10 nM TCDD treatment (dashed line). Experiments were performed in triplicate, with the averages displayed. Error bars represent the standard deviation. Significance, as compared to I3S, is noted by *** (p<0.001).
In addition to the human HepG2 40/6 reporter cell line, we also examined the response to I3S treatment in a mouse cell line that similarly expresses a stable DRE-driven luciferase reporter construct (H1L1.1c2). As observed previously in HepG2 40/6 cells, I3S was able to significantly increase luciferase reporter activity in H1L1.1c2 cells, although much higher concentrations (5.8 µM vs. 12.1 nM) were required to achieve 50% of the response obtained with TCDD at 10 nM (Figure 2B). The 500-fold difference in potency was atypical, as the mouse AHR is reported to exhibit a 10-fold higher affinity for ligands relative to the human homologue (25). For example, the EC50 for TCDD in Hep G2 40/6 cells is 1 µM, while in H1L1.1c2 cells it is 200 pM, thus indicating a five-fold difference in sensitivity to TCDD exposure. Based on this apparent dramatic difference in I3S-mediated AHR activity between the human and mouse forms of the AHR, we have focused on human cell lines for the remaining studies.
I3S induces transcription of AHR-regulated drug metabolism genes and fails to exhibit cytotoxicity
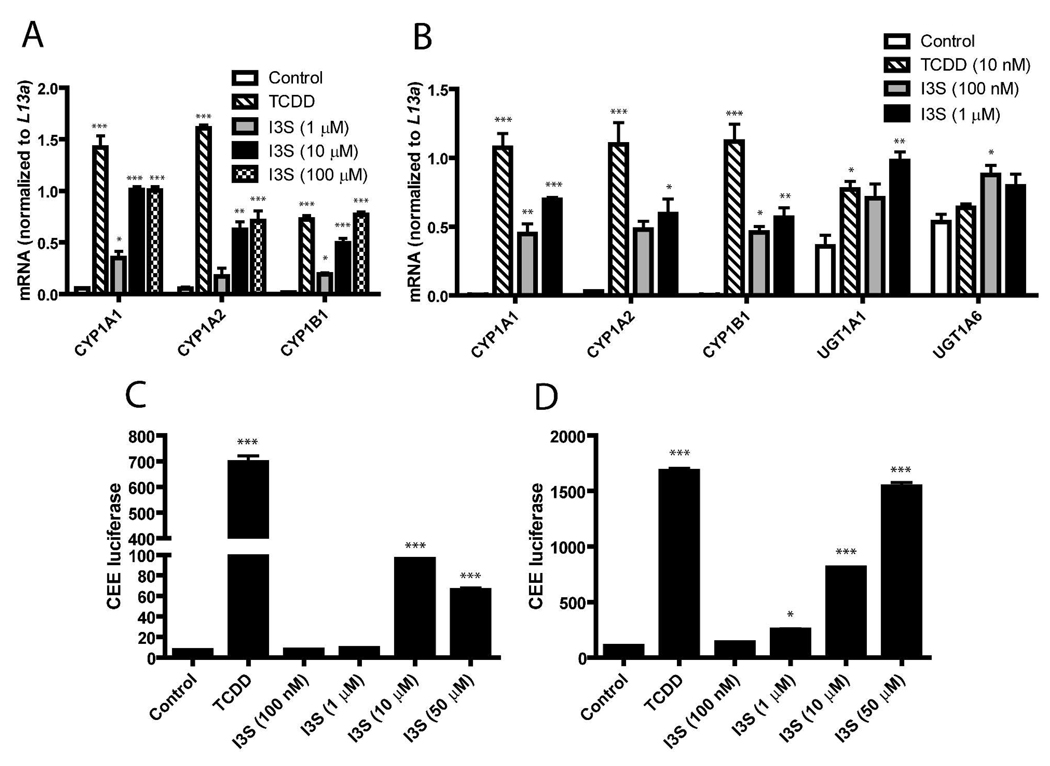
The ability of I3S to induce selected AHR-responsive genes was assessed through qRT-PCR. The expression of CYP1A1, CYP1A2, and CYP1B1 following exposure to I3S (1–100 µM) was determined in the human hepatoma cell line, Huh7. I3S treatment at 10–100 µM resulted in significant induction of CYP1A1/1A2 and CYP1B1 mRNA. (Figure 3A). Interestingly, 100 µM I3S were equally efficacious with 10 nM TCDD in stimulating CYP1B1 gene expression. Primary human hepatocytes were similarly treated with I3S (100 nM-1 µM) and significant levels of induction were also observed, compared to control, for the CYP1A1, CYP1A2, CYP1B1, UGT1A1, and UGT1A6 genes (Figure 3B). CYP1A1/2 metabolic activity was also determined for both Huh7 and primary human hepatocytes using the luciferin-CEE reporter assay. Activity was observed in Huh7 cells treated with 10 µM I3S, although only at 14% of that observed for cells treated with 10 nM TCDD (Figure 3C). However, in primary human hepatocytes, significant activity was detected with 1 µM I3S and, at higher concentrations (50 µM), activity was 92% of that for TCDD (Figure 3D). Considering that I3S is toxic in vivo we wanted to test whether I3S would cause cytotoxicity in Huh7 cells. Cells were exposed for 48 h to 10 µM I3S and no cytotoxicity was observed as assessed with a lactate dehydrogenase assay of culture supernatant (Supporting Figure S1).
FIGURE 3.
I3S induces AHR-mediated gene expression and CYP1A1/1A2 metabolic activity in Huh7 cells and primary human hepatocytes. (A) Huh7 cells were treated with DMSO (0.1% v/v, control), 10 nM TCDD, 1 µM I3S, 10 µM I3S, or 100 µM I3S for 6 h. Levels of mRNA are depicted for CYP1A1, CYP1A2, and CYP1B1 genes. (B) Primary human hepatocytes were treated with 100 nM or 1 µM I3S, 10 nM TCDD, or DMSO (control) for 6 h. Levels of mRNA are depicted for CYP1A1, CYP1A2, CYP1B1, UGT1A1, and UGT1A6 genes. CYP1A1/2 metabolic activity is measured by CEE luciferase for Huh7 cells (C) and primary human hepatocytes (D) treated with 100 nM-50 µM I3S, 10 nM TCDD, or DMSO for 18 h. Experiments were performed in triplicate; error bars denote standard deviation. Significance, as compared to respective control-treated samples, is noted by * (p<0.05), ** (p<0.01) or *** (p<0.001).
I3S is a direct ligand of and mediates gene regulation via the AHR and fails to activate PXR or CAR
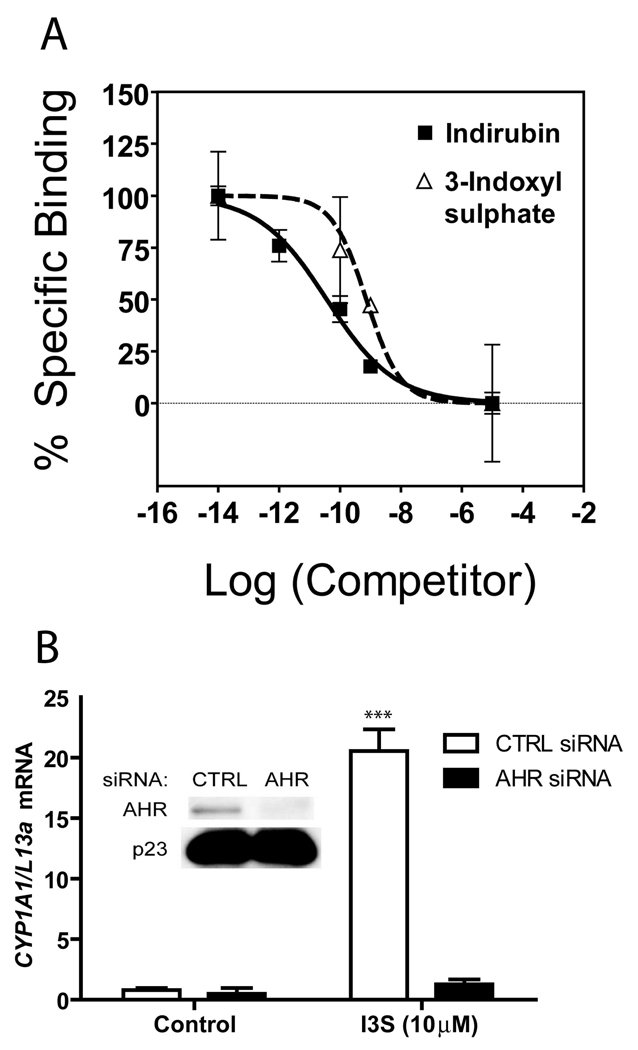
The AHR has also been shown to work through a non-classical pathway, in which binding of AHR:ARNT to a DRE sequence occurs in the absence of direct ligand binding (e.g. omeprazole (26)). Thus, to determine if I3S binds directly to the AHR, we performed ligand-binding competition assays (15, 25) using liver cytosol isolated from a transgenic mouse expressing the human homologue of AHR. This hypothesis was confirmed, as I3S was able to effectively displace the AHR photoaffinity ligand, 2-azido-3-[125I]-7,8-dibromodibenzo-p-dioxin (Figure 4A). Both I3S and indirubin exhibit the ability to potently displace the photoaffinity ligand from the human AHR, compared to a known high affinity exogenous AHR agonist (15). siRNA-mediated knockdown of AHR, performed in MCF-7 cells likewise revealed that induction of CYP1A1 by I3S is AHR dependent (Figure 4B). In this experiment, CYP1A1 mRNA levels following exposure to I3S were 94% lower in the cells treated with the AHR siRNA, as compared to control siRNA transfected cells.
FIGURE 4.
I3S mediated induction of CYP1A1 is dependent upon direct binding to and signaling through the AHR. (A) I3S competes with the binding of photoaffinity radioligand to the AHR. Indirubin is a potent human AHR agonist that served as a reference point. (B) Induced levels of CYP1A1 mRNA observed in MCF7 cells treated with 10 µM I3S following pre-treatment with control siRNA or siRNA directed against the AHR. Experiments were performed in triplicate; error bars denote standard deviation. Significance, as compared to respective control-treated samples, is noted by *** (p<0.001).
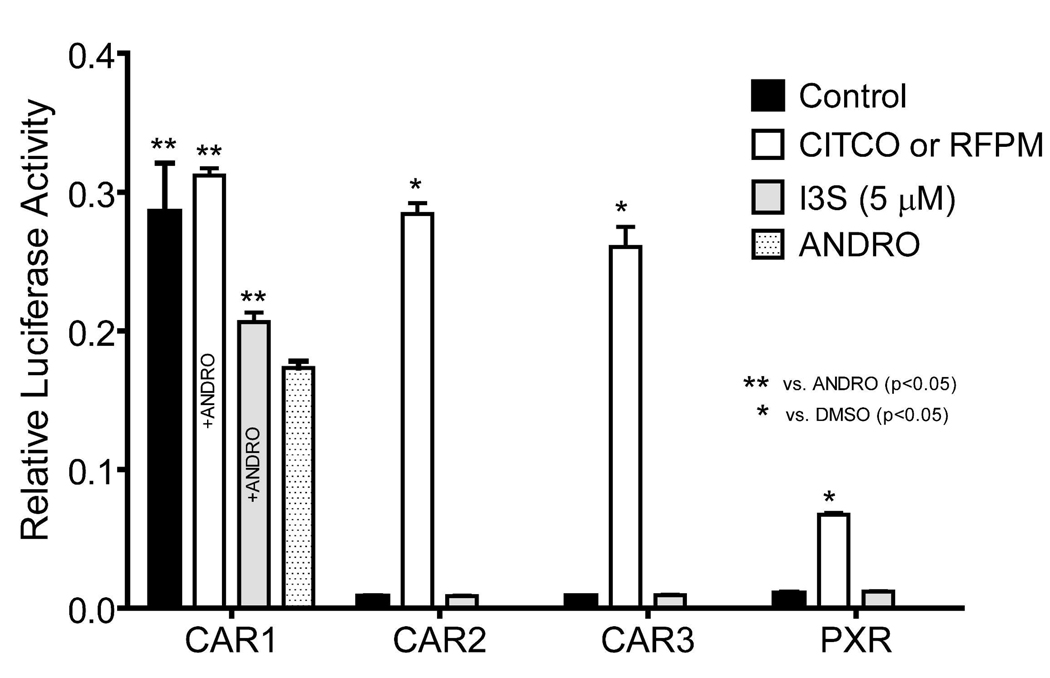
To assess the specificity of I3S we examined its potential to activate additional xeniobiotic sensors, which are co-expressed with the AHR in the liver (e.g. CAR1, CAR2, CAR3, and PXR). COS-1 cells cotransfected with CAR and PXR expression vectors along with appropriate reporter constructs and exposed to I3S. Of these receptors, only CAR1 showed a marginal activation following I3S treatment (Figure 5). However, it is still possible that high micromolar concentrations may be capable of activating these receptors, although whether that outcome would be physiologically relevant is uncertain. Nevertheless, these data suggest that the AHR is most likely the primary receptor that responds to I3S in the liver.
FIGURE 5.
Effect of I3S on nuclear receptor activation. COS-1 cells were transfected with 25 ng of CMV2-CAR or CMV2-PXR expression plasmid, 25 ng 3.1-RXRα expression plasmid, 100 ng of luciferase reporter, and 10 ng of pRL-CMV. After 18 h, cells were treated as indicated and each treatment was performed in quadruplicate. At 24 h post-treatment, the cells were lysed and analyzed for luciferase activity. All values represent the mean ± standard deviation. The double asterisk indicates significant difference (p<0.05) compared with androstanol control values and the single asterisk indicates significant difference (p<0.05) compared with carrier solvent control values.
I3S modulates AHR-mediated genes involved in cellular homeostasis
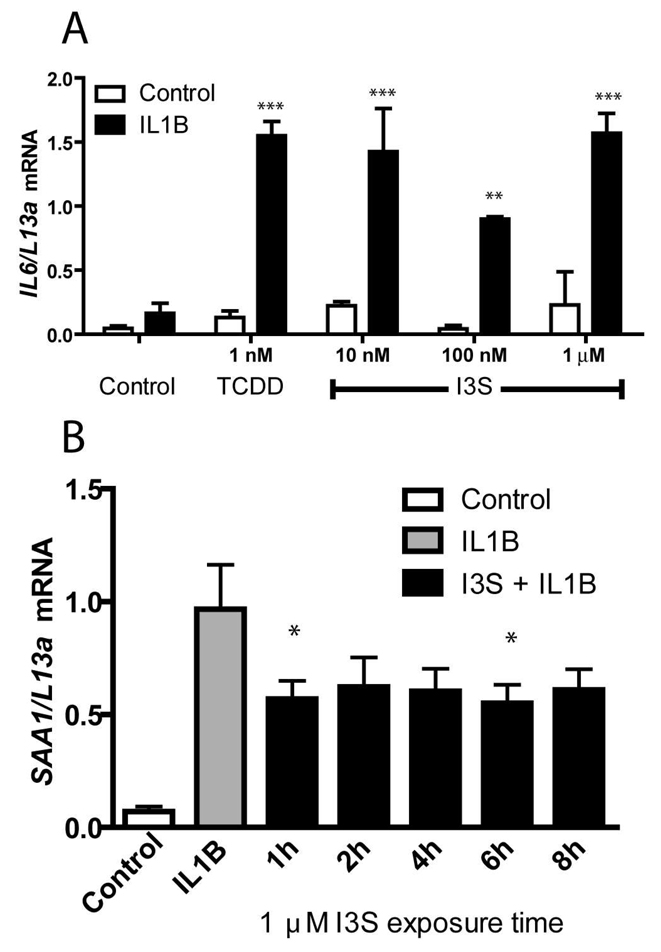
Although the data for the drug metabolizing enzymes clearly demonstrate that I3S is acting as an agonist for the AHR, we were intrigued by the possibility that I3S-activated AHR may be regulating other biological regulatory processes, such as those involved in immune function homeostasis. Co-treatment of MCF-7 human breast cancer cell line with interleukin-1β (IL1B) and an AHR agonist leads to a synergistic increase of IL6 levels (6). These findings, coupled with the results here, suggest that I3S may also synergize with cytokines to increase IL6 production in tumor tissue. In cells treated with IL1B and as little as 10 nM I3S, significant synergistic induction of IL6 was observed compared to IL1B alone (Figure 6A). Conversely, it has also been reported that activation of the AHR can repress acute phase gene expression, including SAA1, in a DRE-independent manner (2). We show here that Huh7 cells treated with IL1B exhibit an increased expression of SAA1 mRNA, which is repressed with I3S pre-treatment for 1–8 h (Figure 6B). Co-treatment of Huh 7 cells with IL1B and TCDD results in a 50 to 75% repression of SAA1 mRNA levels (2). These results indicate that I3S can mediate diverse transcriptional activities through the AHR.
FIGURE 6.
I3S modulates transcription of IL6 and SAA1. (A) Synergistic induction of IL-6 mRNA in MCF7 cells treated with 1 nM TCDD or 10 nM-1 µM I3S and IL1B (2ng/ml). (B) Induced levels of SAA1 mRNA in Huh7 cells treated with 2 ng/ml IL1B for 3 h is repressed with I3S pre-treatment for 1–8 h. Experiments were performed in triplicate and error bars represent the standard deviation. Significance, as compared to the respective IL1B-treated sample, is noted by * (p<0.05), ** (p<0.01) or *** (p<0.001).
DISCUSSION
In the intestinal tract bacteria such as Escherichia coli express tryptophanase, which leads to the production of indole from dietary tryptophan. Indole absorbed from the gut is hydroxylated in the liver by CYP2E1 to form indoxyl, followed by sulfation by sulfotransferases to form I3C, which is excreted in urine (27, 28). Whether the intracellular production of I3S in the liver in healthy individuals leads to elevated basal levels of phase II enzymes regulated by the AHR will require further investigation. The formation of significant serum levels of I3S in mice is dependent upon the presence of gut flora (21). Studies in rats have revealed that elevated serum I3S levels can mediate the progression of glomerular sclerosis (23). In healthy humans the serum level of I3S is ~2 µM, with nearly 100% bound to serum proteins (29). The proximal tubular cells within the kidney transport I3S from peritubular capillary blood to the tubular lumen, suggesting that there are normally significant levels of I3S in these cells and the AHR may have a biological role in the function of this cell type. Transport of I3S across proximal tubular cells has been shown to be mediated by organic anion transporters (OATs) (30). Interestingly, the AHR can both increase and decrease mRNA expression of a number of transporter proteins, including, Oat1a1, Oat2b1, Oat3a1, Mrp-2 and -3 in mouse liver (31, 32). Human kidney dysfunction occurring as a result of diabetic nephrophy has been shown to lead to sustained high levels of I3S in serum. In chronic hemodialysis patients the level can be between 7–343 µM, with an average concentration of 120–140 µM in serum (33, 34). In these patients ~89% of the I3S is bound to serum protein, suggesting that the effective free serum concentration of I3S would be 12 µM, a concentration that yields maximal induction of AHR transcriptional activity in cell culture experiments (29). Clearly, further studies are needed to examine the role of the AHR in proximal tubular cells. The tissue concentrations of I3S have been examined in a nephrectomized rat model system (CRF) and I3S was found at the highest concentrations in the kidney and at lower levels in lung, heart and liver (35). Within the kidney an ~ 6-fold increase in I3S concentration has been detected in CRF rats, with I3S levels of ~ 71 µM detected in kidney homogenate. If these results reflect the tissue concentrations present in patients with end stage kidney disease it would be expected that the AHR would be fully activated. Considering that I3S levels are highly elevated in rat models of CRF, it would be expected that subsequent AHR activation would enhance expression of AHR target genes. This has in fact been observed, as the AHR target gene CYP1A2 protein levels in kidney and liver are greatly enhanced in CRF rats (36), thus supporting the significance of the findings presented here. Interestingly, the kidney has relatively high levels of AHR expression, which upon activation by TCDD can mediate hydronephrosis in mice (37). The toxic effects of TCDD are solely mediated by the AHR and thus can yield clues as to the possible effects of elevated AHR activity induced by high I3S levels. Rats exposed to TCDD resulted in altered glomerular structures, an increase in renal oxidative stress and altered creatinine levels (38). Studies in primary rabbit proximal tubule cells reveal that TCDD inhibits cell proliferation, decrease glucose uptake and suppressed expression of Na+/glucose cotransporter 1 and 2 (39). These studies suggest that excessive AHR activity has a profound effect on kidney function.
The data presented here show for the first time that the indole-metabolite, I3S, specifically binds to the AHR and acts as a potent agonist. However, it is still possible that in cells that an I3S metabolite could be formed that also leads to AHR activation. Athough, considering that nM concentrations of I3S induces AHR transcriptional activity after only a few hours of treatment would argue against this possibility. Relative to TCDD, I3S appears to be a more efficacious inducer of phase II enzyme expression than cytochrome P-450 regulated by the AHR in primary human hepatocytes. This may imply that the I3S/AHR/ARNT complex regulates gene expression in a manner different from a receptor bound to TCDD. The fact that I3S is a strong AHR agonist was initially surprising. The fact that the presence of a polar sulfate moiety is necessary for AHR activation is counterintuitive to the dogma in the literature, in which hydrophobic polycyclic compounds have usually made the most potent ligands for activating the AHR. Secondly, this is the first example known by the authors of a phase II enzyme metabolite activating the AHR. Our data clearly demonstrate that I3S binds to the receptor and modulates AHR-dependent biological signaling. Another novel aspect of this study is the identification of an AHR ligand that has a ~500-fold higher human AHR potency compared to that of the mouse AHR. Exogenous AHR ligands (e.g. TCDD, benzo(a)pyrene) generally have higher affinity for the mouse AHR relative to the human AHR. The exception to this statement is the relatively higher affinity of indirubin for the human AHR (15). These observations may indicate that the human AHR has adapted to bind a different subset of ligands, or at least to bind certain ligands with higher affinity. It has been well established that I3S is a uremic toxin that contributes to kidney glomerular sclerosis, although the mechanism of toxicity has not been firmly established. In addition, I3S has been shown to induce oxidant stress and suppress erythoid colony and lymphocyte blast formation in cell culture models (40–42), however, these effects were observed only at high micromolar concentration and their physiological relevance is uncertain. In contrast, the results presented here describe for the first time a specific intracellular target of I3S activity that can be activated in the nanomolar range. Historically, the focus has been on the ability of I3S to mediate nephrotoxicity, but the fact that there are high circulating levels of I3S in dialysis patients coupled with its ability to activate the AHR would suggest that I3S could mediate altered gene expression in a variety of tissues and lead to altered xenobiotic or endogenous metabolism in the liver. Thus, it may be expected that some aspects of I3S toxicity in these patients could be a consequence of sustained AHR activation.
The sulfation of substrates generally leads to enhanced excretion of metabolites and thus is considered a detoxification process. Structure-activity studies indicate that indole or indoxyl do not exhibit the toxicity associated with I3S, thus the presence of a sulfate group leads to I3S exhibiting a unique biological activity. This is perhaps due to the ability of the sulfate group to interact with a neighboring positively charged amino acid functional group in the ligand-binding pocket of the AHR. Interestingly, there are sulfated metabolites (e.g. heterocyclic amines) that have been shown to be unstable and that can covalently bind DNA, providing one pathway through which sulfation can lead to toxicity (43). In contrast, I3S is quite stable and is excreted without further metabolism. Several reports suggest a correlation between I3S levels and an increased occurrence of cardiovascular disease (44). Studies supporting this observation have revealed an increase in oxidant stress and inflammation during chronic kidney disease, key marker of cardiovascular disease (41). However, these studies did not identify a molecular target for these effects. We propose that certain side effects associated with the need for dialysis treatment and chronic kidney disease may be a consequence of continual I3S-mediated activation of AHR, effects also seen with exposure to high affinity persistent AHR agonists (e.g. dioxin). The discovery that I3S is a potent AHR agonist should stimulate clinical studies examining whether elevated I3S in patients with kidney disease mediates toxicity through AHR activation and whether AHR antagonists could block these effects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Vivek Kapur for helpful discussions that initiated this project. We also thank Denise M. Coslo for assistance maintaining the primary human hepatocyte cultures prior to experimentation and Jacek Krzeminski for chromatography analysis of I3S.
Footnotes
This research was supported by the National Institutes of Health grants; ES04869 (G.H.P), and NIH GM066411 (C.J.O.).
Abbreviations: AHR, aryl hydrocarbon receptor; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TMF, 6, 2′, 4′-trimethoxyflavone; IL, interleukin.
SUPPORTING INFORMATION AVAILABLE
Additional supplementary information, Table S1 and Figure S1 are described in the text. This material is available free of charge via Internet at http://pubs.acs.org.
REFERENCES
- 1.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikuta T, Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp Cell Res. 2006;312:3585–3594. doi: 10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Patel RD, Kim DJ, Peters JM, Perdew GH. The aryl hydrocarbon receptor directly regulates expression of the potent mitogen epiregulin. Toxicol Sci. 2006;89:75–82. doi: 10.1093/toxsci/kfi344. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer SA, Arthur KA, Denison MS, Smith WL, DeWitt DL. Regulation of prostaglandin endoperoxide H synthase-2 expression by 2,3,7,8,-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 1996;330:319–328. doi: 10.1006/abbi.1996.0259. [DOI] [PubMed] [Google Scholar]
- 6.Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008;68:3609–3617. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harstad EB, Guite CA, Thomae TL, Bradfield CA. Liver deformation in Ahr-null mice: evidence for aberrant hepatic perfusion in early development. Mol Pharmacol. 2006;69:1534–1541. doi: 10.1124/mol.105.020107. [DOI] [PubMed] [Google Scholar]
- 8.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 9.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 10.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 11.Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 13.Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 14.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 15.Flaveny CA, Murray IA, Chiaro CR, Perdew GH. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long WP, Pray-Grant M, Tsai JC, Perdew GH. Protein kinase C activity is required for aryl hydrocarbon receptor pathway-mediated signal transduction. Mol Pharmacol. 1998;53:691–700. doi: 10.1124/mol.53.4.691. [DOI] [PubMed] [Google Scholar]
- 18.Garrison PM, Tullis K, Aarts JM, Brouwer A, Giesy JP, Denison MS. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Fundam Appl Toxicol. 1996;30:194–203. doi: 10.1006/faat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 19.Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, Omiecinski CJ. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol. 2007;222:42–56. doi: 10.1016/j.taap.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD. Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 21.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anaizi NH, Cohen JJ. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the renal tubular secretion of phenolsulfonphthalein. J Pharmacol Exp Ther. 1978;207:748–755. [PubMed] [Google Scholar]
- 23.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 24.Aoyama I, Niwa T. An oral adsorbent ameliorates renal overload of indoxyl sulfate and progression of renal failure in diabetic rats. Am J Kidney Dis. 2001;37:S7–S12. doi: 10.1053/ajkd.2001.20731. [DOI] [PubMed] [Google Scholar]
- 25.Ramadoss P, Perdew GH. Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 26.Murray IA, Perdew GH. Omeprazole stimulates the induction of human insulin-like growth factor binding protein-1 through aryl hydrocarbon receptor activation. J Pharmacol Exp Ther. 2008;324:1102–1110. doi: 10.1124/jpet.107.132241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banoglu E, Jha GG, King RS. Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur J Drug Metab Pharmacokinet. 2001;26:235–240. doi: 10.1007/BF03226377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banoglu E, King RS. Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur J Drug Metab Pharmacokinet. 2002;27:135–140. doi: 10.1007/BF03190428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa T, Takeda N, Tatematsu A, Maeda K. Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem. 1988;34:2264–2267. [PubMed] [Google Scholar]
- 30.Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H, Niwa T. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13:1711–1720. doi: 10.1097/01.asn.0000022017.96399.b2. [DOI] [PubMed] [Google Scholar]
- 31.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33:1276–1282. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 32.Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- 33.Taki K, Nakamura S, Miglinas M, Enomoto A, Niwa T. Accumulation of indoxyl sulfate in OAT1/3-positive tubular cells in kidneys of patients with chronic renal failure. J Ren Nutr. 2006;16:199–203. doi: 10.1053/j.jrn.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Komiya T, Miura K, Tsukamoto J, Okamura M, Tamada S, Asai T, Tashiro K, Kuwabara N, Iwao H, Yoshikawa J. Possible involvement of nuclear factor-kappaB inhibition in the renal protective effect of oral adsorbent AST-120 in a rat model of chronic renal failure. Int J Mol Med. 2004;13:133–138. [PubMed] [Google Scholar]
- 35.Deguchi T, Nakamura M, Tsutsumi Y, Suenaga A, Otagiri M. Pharmacokinetics and tissue distribution of uraemic indoxyl sulphate in rats. Biopharm Drug Dispos. 2003;24:345–355. doi: 10.1002/bdd.370. [DOI] [PubMed] [Google Scholar]
- 36.Sindhu RK, Vaziri ND. Upregulation of cytochrome P450 1A2 in chronic renal failure: does oxidized tryptophan play a role? Adv Exp Med Biol. 2003;527:401–407. doi: 10.1007/978-1-4615-0135-0_47. [DOI] [PubMed] [Google Scholar]
- 37.Moriguchi T, Motohashi H, Hosoya T, Nakajima O, Takahashi S, Ohsako S, Aoki Y, Nishimura N, Tohyama C, Fujii-Kuriyama Y, Yamamoto M. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc Natl Acad Sci U S A. 2003;100:5652–5657. doi: 10.1073/pnas.1037886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu CF, Wang YM, Peng SQ, Zou LB, Tan DH, Liu G, Fu Z, Wang QX, Zhao J. Combined Effects of Repeated Administration of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Polychlorinated Biphenyls on Kidneys of Male Rats. Arch Environ Contam Toxicol. 2009;57:767–776. doi: 10.1007/s00244-009-9323-x. [DOI] [PubMed] [Google Scholar]
- 39.Han HJ, Lim MJ, Lee YJ, Kim EJ, Jeon YJ, Lee JH. Effects of TCDD and estradiol-17beta on the proliferation and Na+/glucose cotransporter in renal proximal tubule cells. Toxicol In Vitro. 2005;19:21–30. doi: 10.1016/j.tiv.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Kawashima Y. Study on the uremic protein binding inhibitors as uremic toxin: toxic effect on erythroid colony formation, lymphocyte blast formation and renal function. Nippon Jinzo Gakkai Shi. 1989;31:1151–1161. [PubMed] [Google Scholar]
- 41.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 42.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5:1302–1308. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 43.Banoglu E. Current status of the cytosolic sulfotransferases in the metabolic activation of promutagens and procarcinogens. Curr Drug Metab. 2000;1:1–30. doi: 10.2174/1389200003339234. [DOI] [PubMed] [Google Scholar]
- 44.Muteliefu G, Enomoto A, Niwa T. Indoxyl sulfate promotes proliferation of human aortic smooth muscle cells by inducing oxidative stress. J Ren Nutr. 2009;19:29–32. doi: 10.1053/j.jrn.2008.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.