Summary
Accurate chromosome segregation during mitosis relies on the organization of microtubules into a bipolar spindle. Kinesin-5 proteins play an evolutionarily conserved role in establishing spindle bipolarity [1, 2] and clinical trials are currently evaluating inhibitors of human kinesin-5 (i.e. Eg5) for chemotherapeutic potential. However, in mammalian somatic cells Eg5 activity is dispensable for maintenance of bipolar spindles once they are formed [3, 4], suggesting distinct requirements for establishment versus maintenance of spindle bipolarity. By combining Eg5 inhibition with RNA interference of other spindle proteins, we show that mitotic cells deficient in MCAK fail to maintain spindle bipolarity in the absence of Eg5 activity. Collapse of bipolar spindles in MCAK-deficient cells is driven by pole focusing activities and is independent of MCAK function at centromeres, implicating hyperstabilized non-kinetochore microtubules in spindle collapse. Conversely, destabilizing non-kinetochore microtubules in early mitosis reduces the reliance on Eg5 for establishment of spindle bipolarity and renders cells partially resistant to Eg5 inhibitors. Thus, the temporal requirement for microtubule sliding generated by Eg5 activity during bipolar spindle assembly in mammalian cells is regulated by changes in the dynamic behavior of microtubules during mitosis.
Results and Discussion
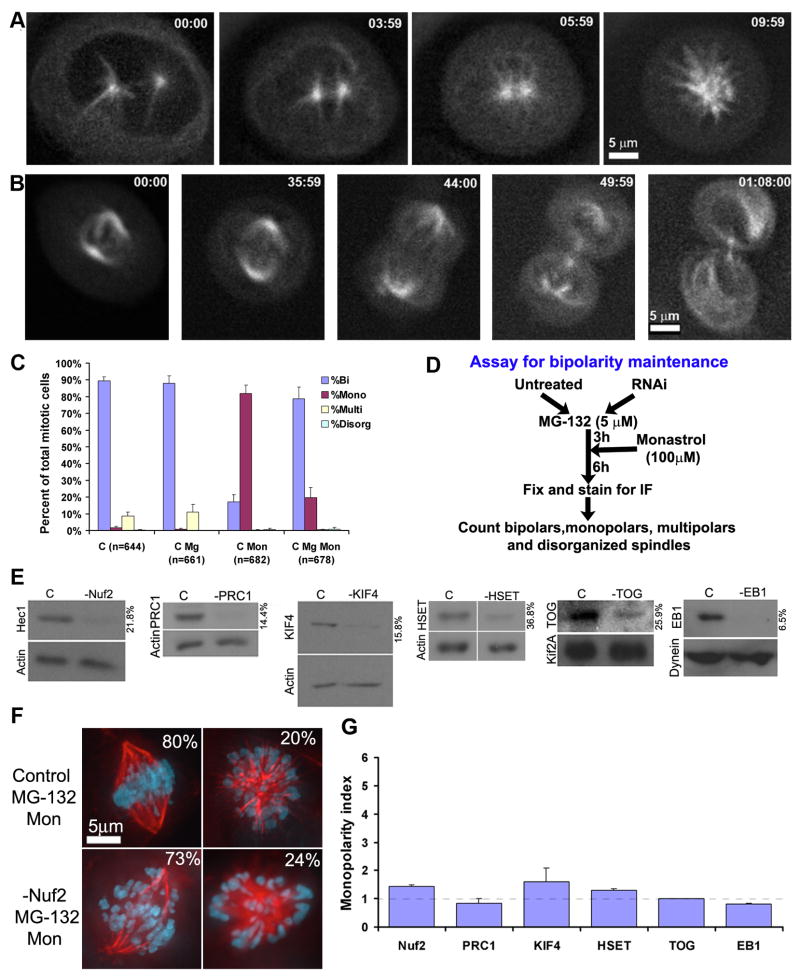
To examine mechanisms contributing to spindle bipolarity in human cultured cells we utilized monastrol to inhibit Eg5 activity [5] at different stages of mitosis. Addition of monastrol to human U2OS cells before nuclear envelope breakdown induces centrosomes to collapse resulting in a monopolar spindle, whereas monastrol addition after nuclear envelope breakdown has no deleterious effect on spindle bipolarity or mitotic progression (Fig. 1A, B; Suppl. Movies 1, 2). Likewise, monastrol induces monopolar spindles in 80% of mitotic cells in populations of unsynchronized U2OS cells but only ~20% of mitotic cells if cells are synchronized in metaphase (accumulated by treatment with MG-132; Fig. 1C). These percentages are consistent with previous values generated by inhibition of Eg5 function by antibody injection [3]. These data confirm that Eg5 activity is required for establishment but not maintenance of bipolar spindles in human somatic cells [3, 4].
Figure 1.
Eg5 is dispensible for maintenance of spindle bipolarity in human U2OS cells. Time lapse imaging of monastrol-treated U2OS cells expressing GFP-tubulin (A) prior to or (B) after nuclear envelope breakdown. Time is presented in minutes:seconds. Scale bar as indicated. (C) Percentages of mitotic U2OS cells with bipolar, monopolar, multipolar or disorganized spindles in populations that were either untreated (C), treated for nine hours with 5 μM MG-132 alone (C MG), nine hours of 100 μM monastrol alone (C Mon), or with MG-132 for three hours followed by monastrol for six hours (C Mg Mon). N is total numbers of mitotic cells counted for each condition. Error bars represent standard deviations. (D) Design of the assay used for the analysis of maintenance of spindle bipolarity. (E) Immunoblots demonstrating the efficiency of protein depletion using RNA interference for control cells (C) or cells depleted of PRC1, TOG, Kif4, Nuf2, HSET, or EB1 as indicated. Loading controls are identified as either actin, Kif2a, or dynein, and the numbers to the right of each blot indicate quantity of each protein remaining after RNAi compared to control. (F) Immunofluorescent images of fixed U2OS cells that were either untreated (Control MG-132 Mon) or depleted of Nuf2 (-Nuf2 MG-132 Mon) under our assay conditions. Percentages indicate the fraction of cells with bipolar or monopolar spindles in each population. (G) The monopolarity index refers to the percent of monopolar cells in the RNAi- treated population divided by the percent of monopolar cells in the control sample. Error bars represent standard errors.
Since a mechanism for maintaining spindle bipolarity has not been described in somatic cells, we reasoned that a mechanism may be revealed under “sensitized” conditions where Eg5 is inhibited with monastrol. We considered various forces that could contribute to maintaining bipolar spindles in the absence of Eg5 activity including force generated by kinetochores, chromokinesins, anti-parallel microtubule crosslinkers, and microtubule-associated proteins. To identify which of these mechanisms is responsible for maintenance of spindle bipolarity in the absence of Eg5 activity, we designed an assay that scores only bipolar spindle maintenance and not establishment. Candidate proteins were depleted in U2OS cells using RNA interference followed by MG-132 treatment to accumulate bipolar spindles, which were then subjected to monastrol treatment (Fig. 1D). Immunoblots show the efficiency of depletion of each candidate protein by RNA interference (Fig. 1E). Populations of untransfected control cells displayed 80% bipolar spindles and 20% monopolar spindles under these conditions (Fig. 1F). For ease of comparison, we converted the population percentages into a “monopolarity index” which is the percentage of monopolar spindles in the RNAi treated samples divided by the percentage of monopolar spindles in the control sample (Fig. 1G; raw population data is presented in Suppl. Fig. 1).
Kinetochores have been proposed to increase the rate of bipolar spindle formation [6] suggesting a possible role in bipolar spindle maintenance. To examine this, we applied our assay to the protein Nuf2, a component of the outer kinetochore Ndc80 complex essential for stable kinetochore-microtubule interactions [7, 8]. Populations of cells depleted of Nuf2 displayed 73% bipolar and 24% monopolar spindles under our assay conditions (Fig. 1F), resulting in a monopolarity index not significantly different from control cells (Fig. 1G; p=0.056, Fisher’s exact test). Therefore, inhibition of stable kinetochore-microtubule interactions does not affect bipolarity maintenance in our assay. Similarly, when we tested the role of anti-parallel microtubule crosslinking in bipolarity maintenance by depleting PRC1 [9], the percentage of mitotic cells with monopolar spindles did not increase significantly; indicating that anti-parallel microtubule crosslinks provided by PRC1 do not account for the stability of bipolar spindles in the absence of Eg5 function (Fig. 1G). Likewise, depletions of either a chromokinesin (Kif4) [10], a pole focusing motor (HSET) [11], or microtubule-associated proteins TOG [12] and EB1 [13] did not significantly change the monopolarity index, ruling out those proteins in maintaining spindle bipolarity in the absence of Eg5 activity as well (Fig. 1G).
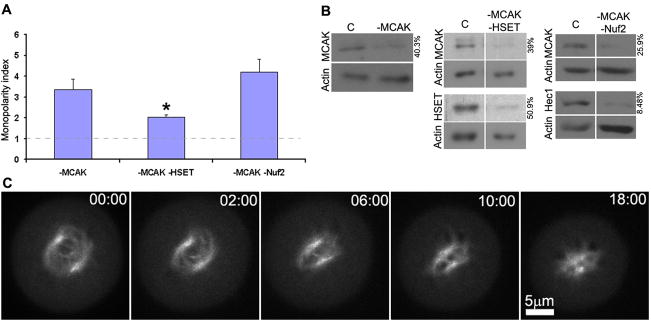
In stark contrast, cells with even modest reductions of the microtubule depolymerizing enzyme MCAK (Fig. 2B) displayed ~60% mitotic cells with monopolar spindles under our assay conditions, yielding a monopolarity index that is significantly elevated relative to control cells (Fig. 2A; raw population data is presented in Suppl. Fig. 2; p<0.0001, Fisher’s exact test). Moreover, unlike control prometaphase cells that are impervious to Eg5-inhibition after bipolar spindle formation (Fig. 1B), time lapse imaging of MCAK-deficient prometaphase cells shows nascent bipolar spindles treated with monastrol to collapse rapidly (Fig. 2C, Suppl. Movie 3). These data demonstrate that altering microtubule dynamics by depletion of MCAK renders human somatic cells reliant on Eg5 activity to maintain spindle bipolarity.
Figure 2.
MCAK contributes to maintaining spindle bipolarity in human U2OS cells. (A) Monopolarity indices of mitotic cells depleted of MCAK alone, MCAK and HSET, and MCAK and Nuf2 as indicated. Asterisk denotes significant reduction in monopolarity index compared to cells deficient in MCAK alone (p<0.0001, two tailed Fisher’s exact test). Error bars show standard errors. (B) Immunoblots demonstrating the efficiency of protein depletion using RNA interference for control cells (C) or cells depleted of MCAK, MCAK and HSET, and MCAK and Nuf2 as indicated. Actin serves as a loading control and the numbers to the right of each blot indicate quantity of each protein remaining after RNAi compared to control. (C) Time lapse imaging of monastrol-treated, MCAK-deficient U2OS cells expressing GFP-tubulin after nuclear envelope breakdown. Monastrol was added after nuclear envelope breakdown. Time is presented in minutes:seconds. Scale bar as indicated.
MCAK regulates the dynamics of both kinetochore and non-kinetochore spindle microtubules [14–18], and therefore, suppressing dynamics of either population may be responsible for the failure to maintain spindle bipolarity in MCAK-deficient cells lacking Eg5 activity. To determine if MCAK’s activity on kinetochore microtubule dynamics contributes to its role in maintaining spindle bipolarity, we subjected cells simultaneously depleted of Nuf2 and MCAK to our bipolarity maintenance assay (Fig. 2A and B). It has been shown that Nuf2-deficient cells lack stable kinetochore microtubule attachments [7]. Similar to cells lacking MCAK under our bipolarity maintenance assay conditions, mitotic cells simultaneously deficient in Nuf2 and MCAK fail to maintain spindle bipolarity and display a high monopolarity index (Fig. 2A, raw population data is presented in Suppl. Fig. 2). Thus, MCAK activity is essential to maintain spindle bipolarity in the absence of Eg5 activity, but these data suggest that it fulfills this role independent of stable kinetochore microtubules.
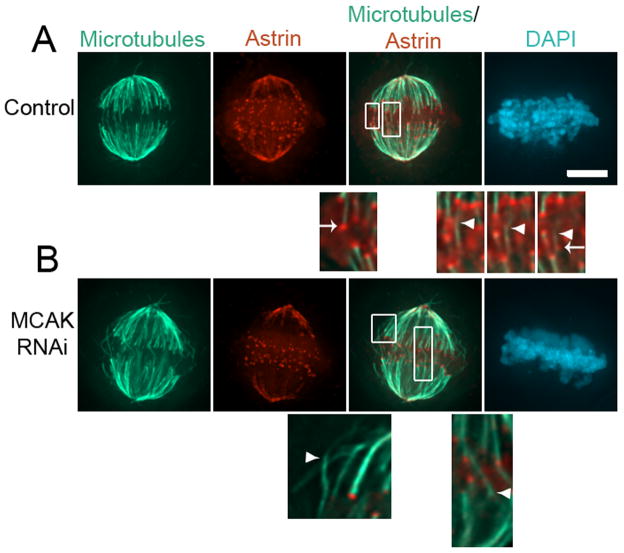
Next, we examined the role of MCAK in maintaining spindle bipolarity through regulation of non-kinetochore spindle microtubules. Most non-kinetochore spindle microtubules are depolymerized by calcium treatment prior to fixation, leaving only kinetochore microtubules (Fig. 3A) consistent with previous electron microscopy studies in PtK cells showing very few overlapping interpolar microtubules during metaphase [19]. Strikingly, MCAK-depleted metaphase spindles possess numerous calcium-stable microtubules that are not associated with kinetochores (Fig. 3B). These calcium-stable microtubules form bundles that frequently appear to extend from one half of the spindle into the other, exaggerating the population of overlapping spindle microtubules. These long overlapping microtubules may be subject to inappropriate pole focusing activities that would impair maintenance of spindle bipolarity in our assay. Pole focusing activities acting on these microtubule bundles could pull centrosomes together and collapse a bipolar spindle. To test this idea, we depleted the minus end-directed motor protein HSET which plays an established role in pole focusing [11]. Cytoplasmic dynein provides the dominant pole focusing activity, but depletion causes spindle poles to splay [11, 20], and so we were forced to specifically target HSET in these experiments. Depletion of HSET alone does not induce bipolar spindle collapse when Eg5 activity is inhibited under our assay conditions (Fig. 1E and G, Suppl Fig. 1D). Simultaneous depletion of MCAK and HSET shows a significant decrease in monopolarity index compared to cells depleted of MCAK alone (Fig. 2A and B, p<0.0001, compared to MCAK alone, Fisher’s exact test). Thus, whereas most mitotic cells lacking MCAK alone fail to maintain spindle bipolarity under our assay conditions, bipolarity maintenance is partially rescued if pole focusing is simultaneously inhibited by HSET depletion. The limited size of this effect most likely reflects the minor contribution that HSET makes to pole focusing in somatic cells [11, 20].
Figure 3.
Calcium-stable spindle microtubules. (A) Full volume projection of an untreated U2OS cell in metaphase following calcium treatment prior to fixation stained for microtubules, astrin, and DNA (DAPI) as indicated. Boxed regions are 4X magnifications and are shown as insets from single focal planes in the z-axis. Arrowheads indicate microtubule bundles and arrows indicate kinetochores. The 3 insets on the right represent sequential focal planes in the z-axis to highlight a single microtubule bundle traversing the midzone and ending at a kinetochore. (B) Full volume projection of an MCAK-deficient U2OS cell in metaphase following calcium treatment prior to fixation stained for microtubules, astrin, and DNA (DAPI) as indicated. Boxed regions are 4X magnifications and are shown as insets from single focal planes in the z-axis. Arrows indicate kinetochores. Scale bar, 5 μm.
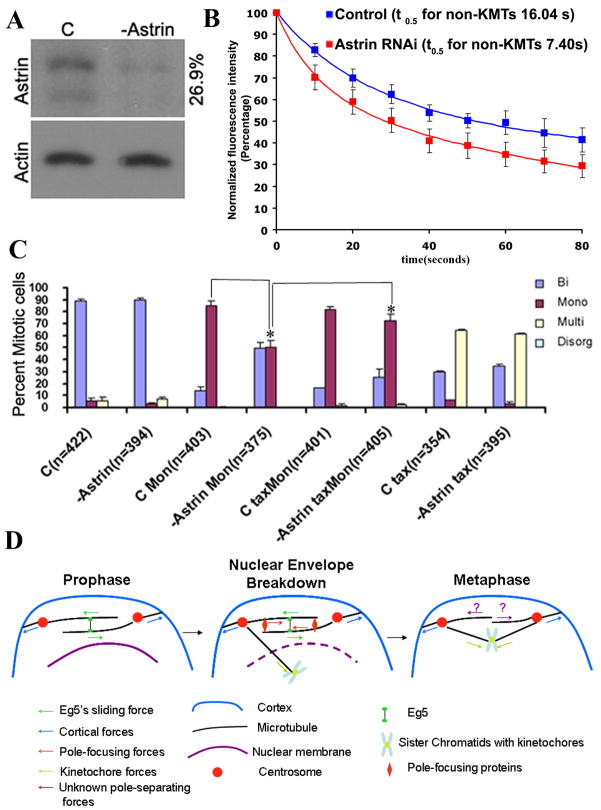
The data presented thus far indicate high microtubule dynamics ensured by MCAK renders the microtubule sliding activity of Eg5 unnecessary for maintenance of spindle bipolarity in human cells. This suggests the existence of a functional relationship between microtubule dynamics and Eg5-generated microtubule sliding. These results predict that decreasing microtubule stability early in mitosis would reduce the requirement for Eg5 activity during bipolar spindle establishment at the time of nuclear envelope breakdown. To test this prediction, we reduced non-kinetochore microtubule stability and then determined the percentage of mitotic cells with monopolar spindles formed upon monastrol treatment followed by MG-132. Due to the duration of this experiment we could not use nocodazole to destabilize microtubules, because, even at low doses (33nM –132nM), it caused spindle disorganization. Instead, we depleted cells of the spindle-associated protein astrin, a mitosis-specific microtubule-associated protein [21]. Immunoblots show the efficiency of astrin depletion by RNA interference (Fig. 4A). The half-life of non-kinetochore microtubules in astrin-deficient cells in prometaphase is 7.40 ± 2.60 seconds (n=9 cells, R2 > 0.99), a significant reduction from control cells where the half-life of non-kinetochore microtubules in prometaphase is 16.04 ± 1.14 seconds (Fig. 4B; n=9 cells, R2 > 0.98, p < 0.0005, Student t-test), indicating that astrin depletion is an efficient way to decrease microtubule stability. When astrin is depleted, fewer mitotic cells display monopolar spindles when treated with a range of monastrol concentrations (60, 80, or 100 μM) compared to control cells. For example, 85.4% of control mitotic cells form monopolar spindles in the presence of 80 μM monastrol, but only 50.7% of astrin-deficient mitotic U20S cells have monopolar spindles at this concentration of monastrol (Fig 4C). Several roles have been proposed for astrin in mitosis [22, 23]. To verify that this change in monastrol sensitivity is caused by the role of astrin in microtubule dynamics, we stabilized microtubules in astrin-deficient cells by addition of 5 nM taxol [24]. A majority of astrin-deficient mitotic cells treated with taxol and monastrol show monopolar spindles. At the concentrations used here, the established effect of taxol is to stabilize microtubules indicating that the primary effect of astrin depletion was to destabilize spindle microtubules (Fig. 4C). These data demonstrate that the role of Eg5 in establishing spindle bipolarity in U2OS cells is influenced by the dynamic state of microtubules, and that sensitivity to Eg5 inhibition with monastrol can be reduced by destabilizing microtubules in early mitosis.
Figure 4.
Decreasing MT stability alters U2OS cell sensitivity to monastrol. (A) Immunoblots demonstrating the efficiency of protein depletion using RNA interference for control cells (C) or cells depleted of astrin as indicated. Loading controls are identified as actin and the numbers to the right of the blot indicate quantity of each protein remaining after RNAi compared to control. (B) Normalized fluorescence intensity of non-KMTs over time (seconds) after photoactivation of spindles in untreated (blue squares) and astrin-depleted (red squares) prometaphase cells. Data represent mean ± s.e.m, n = 9 cells for both conditions. (C) Percent of mitotic cells with bipolar, monopolar, multipolar, or disorganized spindles in untreated cells (C) and cells depleted of astrin (-astrin) with or without treatment with 80 μM monastrol (Mon) or 5 nM taxol (tax). Asterisk (*) indicates two-tailed p-value <0.0001 using Fisher’s exact test. Error bars represent standard deviations. (D) Model for forces acting during spindle bipolarity during different stages of mitosis in mammalian somatic cells. Arrows denote the direction of microtubule movement in response to applied force. During prophase, Eg5 activity slides apart antiparallel microtubules to separate centrosomes. Centrosome separation is assisted at this time by pulling forces generated at the cell cortex. Pole focusing activities (HSET, NuMA) are released into the cytosol at nuclear envelope breakdown, and these generate inward force on overlapping microtubules between the two centrosomes which is opposed by the outward force generated by Eg5. In metaphase, microtubules are relatively dynamic and lack extensive overlap. At this time, Eg5 is not necessary for spindle bipolarity because overlapping microtubules are not present for pole focusing activities and other, as yet unknown, proteins take responsibility for maintaining spindle bipolarity (question mark).
The microtubule sliding activity of Eg5 is only required to establish bipolar spindles in mammalian somatic cells [3, 4]. Here we show that Eg5 activity becomes essential to maintain spindle bipolarity following disruption of microtubule dynamics through loss of MCAK. Multiple mechanisms influence centrosome separation and spindle bipolarity in mammalian cells (Fig. 4D) and many of those could be influenced by changes in microtubule dynamics. For example, kinetochores have been proposed to expedite centrosome separation, and stabilizing microtubule dynamics may augment that activity. However, the perseverance of spindle bipolarity observed upon disruption of stable kinetochore microtubule interactions through depletion of Nuf2 argues against kinetochore activity being the primary site of MCAK function for spindle bipolarity. Thus, it is more likely that the exaggerated non-kinetochore microtubules resulting from MCAK depletion renders Eg5 essential to maintain spindle bipolarity. Exaggerating the length and density of non-kinetochore microtubules could influence spindle bipolarity either by increasing cortical microtubule interactions or increasing the extent of microtubule overlap between the two halves of the spindle. We disfavor the former possibility because increasing microtubule contacts with the cell cortex would enhance outward forces pulling centrosomes apart rather than collapsing them, which is contrary to our observations. Instead, we favor the latter possibility because we observe calcium-stable microtubule bundles extending between the two spindle halves in MCAK-deficient cells and spindle bipolarity in MCAK-deficient cells can be preserved by simultaneously depleting the pole focusing motor HSET. Based on these observations, we propose a model for how microtubule dynamics influences the temporal requirement for the sliding activity of Eg5 (Fig. 4D). Microtubules have been shown to be relatively long and stable at the time of nuclear envelope breakdown[25], whereupon pole focusing factors (HSET, NuMA) are released from the nuclear compartment. The model suggests that Eg5 is essential at this early stage of mitosis to establish spindle bipolarity by sliding antiparallel microtubules to generate an outward force to prevent pole focusing activities from drawing spindle poles together into monopolar spindles. Microtubules then become shorter and less stable as cells transition into prometaphase after nuclear envelope breakdown[25], reducing the extent of overlapping microtubules. This renders Eg5 activity irrelevant for the maintenance of spindle bipolarity in somatic mammalian cells and other, as yet unknown, factors take over responsibility for maintaining spindle bipolarity. Thus, temporal changes in microtubule dynamics determine the temporal requirement for Eg5 activity to establish spindle bipolarity early in mitosis. This model provides a straight forward explanation for the disparities reported in the requirements of kinesin-5 motor activity in different experimental systems. For example, Eg5 activity is required for both the establishment and maintenance of spindle bipolarity in frog egg extracts [4]. Spindles in those extracts contain a network of short, crosslinked microtubules surrounded by a barrel array of astral microtubules that form antiparallel overlap [26]. Recent data shows that reducing the overlapping barrel array of microtubules in that system eliminates the requirement for Eg5 activity to maintain spindle bipolarity [27]. Moreover, spindles in C. elegans embryos do not require kinesin-5 activity for bipolarity [28], and it may be that those spindles have few overlapping non-kinetochore microtubules as judged by electron microscopy [29]. Thus, the model suggests that the difference in requirement for Eg5 activity to maintain spindle bipolarity between different model systems arises through disparities in the degree of spindle microtubule overlap governed by dynamics and not through inherently unique mechanisms. Finally, our data reveal that increasing microtubule dynamics in early mitosis reduces the sensitivity of cells to the inhibition of Eg5 activity and unveils pathways that are independent of mutation of the Eg5 gene through which tumor cells may be (or become) recalcitrant to Eg5 inhibitors.
Supplementary Material
Acknowledgments
We thank Wei Jiang for providing PRC-1-specific antibodies. We also thank Emily Hood and other members of the Compton lab for helpful discussions. This work was supported by National Institutes of Health grant GM51542.
Footnotes
Supplemental Data
Supplemental data include Experimental Procedures, two figures, and three movies and can be found with this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sawin KE, Leguellec K, Philippe M, Mitchison TJ. Mitotic Spindle Organization by A Plus-End-Directed Microtubule Motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, Mitchison TJ, Scholey JM. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. Journal of Cell Biology. 1999;144:125–138. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blangy A, Lane HA, dHerin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34(cdc2) regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. Journal of Cell Biology. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 6.Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. Journal of Cell Biology. 2009;184:365–372. doi: 10.1083/jcb.200809055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLuca JG, Moree B, Hickey JM, Kilmartin JV, Salmon ED. hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. Journal of Cell Biology. 2002;159:549–555. doi: 10.1083/jcb.200208159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 9.Mollinari C, Kleman JP, Jiang W, Schoehn G, Hunter T, Margolis RL. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. Journal of Cell Biology. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YM, Lee S, Lee E, Shin H, Hahn H, Choi W, Kim W. Human kinesin superfamily member 4 is dominantly localized in the nuclear matrix and is associated with chromosomes during mitosis. Biochemical Journal. 2001;360:549–556. doi: 10.1042/0264-6021:3600549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mountain V, Simerly C, Howard L, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. Journal of Cell Biology. 1999;147:351–365. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, Gard DL, Larroque C. The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. Journal of Cell Science. 1998;111:1371–1383. doi: 10.1242/jcs.111.10.1371. [DOI] [PubMed] [Google Scholar]
- 13.Berrueta L, Kraeft SK, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, Pellman D, Bierer BE. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10596–10601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wordeman L, Wagenbach M, von Dassow G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. Journal of Cell Biology. 2007;179:869–879. doi: 10.1083/jcb.200707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kline-Smith SL, Walczak CE. The microtubule-destabilizing kinesin XKCM1 regulates microtubule dynamic instability in cells. Molecular Biology of the Cell. 2002;13:2718–2731. doi: 10.1091/mbc.E01-12-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walczak CE, Mitchison TJ, Desai A. XKCM1: A Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 17.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nature Cell Biology. 2009;11:27–U51. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizk RS, Bohannon KP, Wetzel LA, Powers J, Shaw SL, Walczak CE. MCAK and Paclitaxel Have Differential Effects on Spindle Microtubule Organization and Dynamics. Molecular Biology of the Cell. 2009;20:1639–1651. doi: 10.1091/mbc.E08-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar Spindle Microtubules in Ptk Cells. Journal of Cell Biology. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walczak CE, Vernos I, Mitchison TJ, Karsenti E, Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Current Biology. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- 21.Mack GJ, Compton DA. Analysis of mitotic microtubule-associated proteins using mass spectrometry identifies astrin, a spindle-associated protein. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14434–14439. doi: 10.1073/pnas.261371298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du J, Jablonski S, Yen TJ, Hannon GJ. Astrin regulates Aurora-A localization. Biochemical and Biophysical Research Communications. 2008;370:213–219. doi: 10.1016/j.bbrc.2008.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thein KH, Kleylein-Sohn J, Nigg EA, Gruneberg U. Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. Journal of Cell Biology. 2007;178:345–354. doi: 10.1083/jcb.200701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell-proliferation by Taxol at low concentrations. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rusan NM, Tulu US, Fagerstrom C, Wadsworth P. Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. Journal of Cell Biology. 2002;158:997–1003. doi: 10.1083/jcb.200204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang G, Houghtaling BR, Gaetz J, Liu JZ, Danuser G, Kapoor TM. Architectural dynamics of the meiotic spindle revealed by single-fluorophore imaging. Nature Cell Biology. 2007;9:1233–U1245. doi: 10.1038/ncb1643. [DOI] [PubMed] [Google Scholar]
- 27.Houghtaling BR, Yang G, Matov A, Danuser G, Kapoor TM. Op18 reveals the contribution of nonkinetochore microtubules to the dynamic organization of the vertebrate meiotic spindle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15338–15343. doi: 10.1073/pnas.0902317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders AM, Powers J, Strome S, Saxton WM. Kinesin-5 acts as a brake in anaphase spindle elongation. Current Biology. 2007;17:R453–R454. doi: 10.1016/j.cub.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Toole ET, McDonald KL, Mantler J, McIntosh JR, Hyman AA, Muller-Reichert T. Morphologically distinct microtubule ends in the mitotic centrosome of Caenorhabditis elegans. Journal of Cell Biology. 2003;163:451–456. doi: 10.1083/jcb.200304035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.