Abstract
The aim of the present study was to investigate the morphology and function of a drug eluting metallic porous surface produced by the immobilization of poly lactide-co-glycolide microspheres bearing dexamethasone onto plasma electrolytically oxidized Ti–6Al–7Nb medical alloy. Spheres of 20 μm diameter were produced by an oil-in-water emulsion/solvent evaporation method and thermally immobilized onto titanium discs. The scanning electron microscopy investigations revealed that the size distribution and morphology of the attached spheres had not changed significantly. The drug release profiles following degradation in phosphate buffered saline for 1000 h showed that, upon immobilisation, the spheres maintained a sustained release, with a triphasic profile similar to the non-attached system. The only significant change was an increased release rate during the first 100 h. This difference was attributed to the effect of thermal attachment of the spheres to the surface.
Introduction
During 2007, there were more than 150,000 patients requiring total or partial joint arthroplasty in the United Kingdom alone [1]. At the same time, 13,000 patients required revision surgery, many of these due to septic or aseptic implant loosening. One way to reduce this proportion is to improve implant fixation. Previous work has been undertaken to control cellular activity by the generation of micro- or nanotexture onto the surface of metallic implants [2]. There is hope that drug eluting medical combination devices may afford even greater fixation by means of triggering specific cellular interactions next to providing additional biofunctionalities (e.g. antibacterial activity).
Poly (lactide-co-glycolide) (PLGA) has been studied since the 1980s [3] as a carrier for sustained drug release. It is known to degrade in water over time via hydrolytic fission [4], and the end products of this reaction are lactic and glycolic acid. Both of these chemicals are readily soluble in water and are excreted by mammals. These properties make PLGA a very attractive implant material, as no surgery is needed to remove the implant after its function is no longer required.
PLGA is known to show good chemical biocompatibility in animal studies due to a limited inflammatory response [5, 6]. As a result, PLGA has been used for many years as an implantable material, capable of being degraded in situ and require no further intervention for removal. A common example of this is its use as restorable sutures [7]. The degradation is controlled by local pH and polymer hydrophilicity. Some papers report an unusually large amount of inflammation from PLGA implants [8], usually attributed to the falling pH during polymer degradation. In an attempt to reduce this effect, research has been performed on modification of the PLGA matrix by the incorporation of basic salts [9, 10].
As a semicrystalline polymer, PLGA can also incorporate small hydrophobic molecules into its structure and release them over time when exposed to an aqueous solution by a combination of diffusion and degradation [11]. It can also be processed to encapsulate small aqueous droplets of hydrophilic drugs [12], or as a suspension of dry drug particles [13, 14]. The last two techniques are suitable for delivering proteins, whilst all three are suitable for delivery of lower molecular weight drugs. Whilst microspheres are usually used to deliver drugs to a single site of action, nanospheres have been used for drug delivery in cases needing considerable diffusion, such as intraocularly [15] or as an aerosol [16].
PLGA can be used to deliver drugs in the form of films or microparticles. PLGA films can be used on metallic surfaces to some effect, particularly in stents where sustained delivery of drugs is critical in preventing restenosis [17]. However, this type of delivery method results in an uncontrolled, usually short, diffusion-like release and can disguise the structure of the metallic implant by applying a uniform layer to the surface [18]. Whilst this may help to prevent cell proliferation in stents, in orthopedic applications it has been shown that a micro/nanostructure is very important for bone growth, even on the surface of a PLGA film [2]. An alternate method of sustained drug delivery would be to employ a particulate formulation of PLGA. By doing so, it would allow for a controlled, tunable drug release whilst preserving or enhancing the underlying texture of the implant surface, thus also promoting cellular interactions.
Titanium and its medical alloys have been the choice for many years for implanted devices as it displays very good biocompatibility relative to other metallic biomaterials such as surgical steel and cobalt–chrome alloys. They are used in a range of fields such as dental prostheses, total joint replacements as well as bone fixation devices [19]. Titanium surfaces can be modified by various processes (e.g. plasma spray deposition, sintering, plasma electrolytic oxidation) to increase the roughness of the titanium surface or create porous topographies allowing for bone on/in growth or enhanced cellular interaction [20].
The aim of the work in this paper is to investigate the morphology and function of a drug eluting metallic porous surface, produced by the immobilization of PLGA microspheres bearing a drug onto plasma electrolytically oxidized titanium. Research has been performed on these types of surfaces before, albeit using a film or surface-covering layer to do so [21, 22]. The advantage to using a particulate drug delivery design would be to allow the incorporation of sustained drug delivery whilst preserving the controlled porous topography. The electrolyte chosen for plasma electrolytic oxidation was calcium acetate and calcium glycerophosphate, known to introduce calcium and phosphates in the oxide layers that can act as precursors for hydroxyapatite formation [23]. The PLGA microspheres were loaded with dexamethasone, a corticosteroid previously indentified with the differentiation of osteoblasts [24], as a restenosis inhibitor [25] and with the reduction of inflammatory responses [26]. Previous work performed on these spheres in an unattached, free-in-solution system [27] has shown that they can act as a sustained dexamethasone releasing system.
Materials and methods
Materials
Poly (lactide-co-glycolide) (MW: 40,000–75,000, 50:50), dexamethasone (98%), poly (vinyl alcohol) (MW: 30,000–70,000), sodium azide, disodium hydrogen phosphate, sodium dihydrogen phosphate, acetic acid, sodium acetate and all solvents were purchased from Sigma Aldrich chemicals. Distilled, deionised water (ddH2O) was provided from Millipore.
Phosphate buffered saline (PBS) was made up with an additional 0.1 g/l sodium azide.
Microspheres synthesis
Following on from our previous work [27], the synthesis of the spheres can be summarised as follows:
400 mg PLGA and 80 mg dexamethasone were co-dissolved in 40 ml dichloromethane:methanol 9:1. This solution was added in 5 ml fractions to 100 ml 0.2% w/v PVA solution in ddH2O, and stirred at 1250 rpm for 30 mins, followed by stirring at 60 rpm for 18 h. The microspheres were then filtered out of solution, washed with ddH2O, and dried fully in a lyophiliser for 24 h.
Titanium plasma electrolytic oxidation and sphere immobilisation
Ti–6Al–7Nb medical alloy (ACNIS International, France) was used as the substrate in the form of cylindrical disks with a thickness of 8 mm and a diameter of 19 mm. The disks were ground with 1,200 grit paper (Struers, Denmark) using water as lubricating liquid. The samples were ultrasonically cleaned, washed in ethanol and deionised water, then dried under compressed air.
The plasma electrolytic oxidation (PEO) process was carried out in a double-wall glass electrolytic cell with an internal volume of 800 ml. The electrolyte used was a solution of 4.2 g/l calcium glycerophosphate and 24 g/l calcium acetate in deionised water (Sigma Aldrich).
Titanium disks were suspended in the centre of the electrolytic cell by screwing them to an insulated metallic rod acting as an anode whilst a cylindrical steel jacket in the solution acted as a cathode. The temperature of the electrolyte was maintained in the range of 15–25°C during oxidation by water circulation through the cell jacket, whilst the electrolyte was stirred at a speed of 500 rpm to maintain homogeneity.
Oxidation was performed under galvanostatic conditions using a current density of 20 A/dm2 for 5 min, using an AC (50 Hz) power supply type ACS 1500 (ET Power Systems Ltd, UK). After oxidation, the samples were thoroughly cleaned with deionised water and dried in air.
Suspensions of the spheres were made up in ddH2O with 1 min of sonication at 20 W. Samples of 0.8 ml were taken from this suspension and dropped onto one face of the titanium discs as prepared above to form one continuous droplet over the entire surface. This was repeated for each oxidised and non-oxidised sample. These samples were placed into an oven (Nabertherm) to allow the water to evaporate and for the microspheres to bond with the surface oxide. The thermal treatment applied was optimised around the Tg (45°C) of the PLGA used (1 h at 50°C).
Scanning electron microscopy (SEM) and size analysis
Pictures of the microspheres loaded onto the titanium samples were obtained using a JEOL 6500 SEM at 5–15 kV. Each sample was gold sputter coated before analysis, and pictures were taken at 750–1000× magnification. The images were analysed by Analysis software in order to produce a size distribution of the bonded spheres for each sample. At least 150 data points were used to get an accurate distribution of sizes.
High performance liquid chromatography (HPLC)
Samples of PBS or acetonitrile (for calibration) had their dexamethasone concentration analysed by HPLC, and thus were run on a machine fitted with a reverse phase Varian Chromosphere C18 column (250 × 4.6 mm) with a running buffer of 60% acetonitrile, 40% sodium acetate buffer at pH 4.8. The concentration of dexamethasone was resolved using a Varian Prostar spectrometer reading at 254 nm, with a dexamethasone peak at 3.75 min. The machine was calibrated every day of use with at least three fresh samples of dexamethasone in acetonitrile of known concentrations straddling the expected concentrations.
Surface loading efficiency
Surface loading efficiency (SLE) was calculated using the following formula:
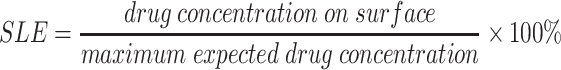 |
A microsphere loaded titanium disc was washed with approx. 9 g acetonitrile and left to soak in the solvent for 24 h to ensure complete dissolution of all bonded microspheres. A 1.8 ml sample was analysed by HPLC as noted in Sect. 2.5 for dexamethasone. Based on this result the total amount of attached spheres was estimated.
Release profile assay
Three samples of attached spheres on both oxidised and non-oxidised titanium discs were incubated in 10 ml PBS at pH 7.0, 37°C with 30 rpm of stirring for 1000 h. Samples of 1.7 ml were removed and filtered through a 220 nm polyester membrane to remove any possible detached PLGA spheres. The incubated sample was then topped up to 10 ml with fresh, filtered PBS.
Each removed sample was run on an HPLC as described in Sect. 2.5 with a dexamethasone peak at 3.75 min, and a peak corresponding to the simulated body fluid at 1.76 min.
Results
Morphology of the immobilised spheres
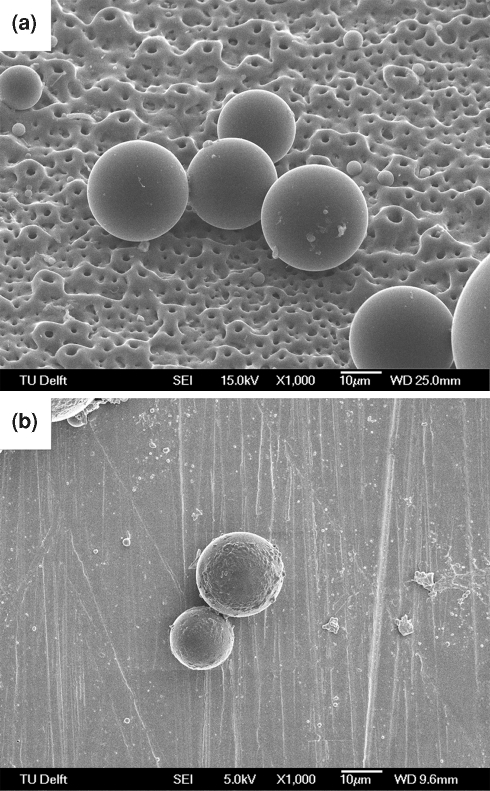
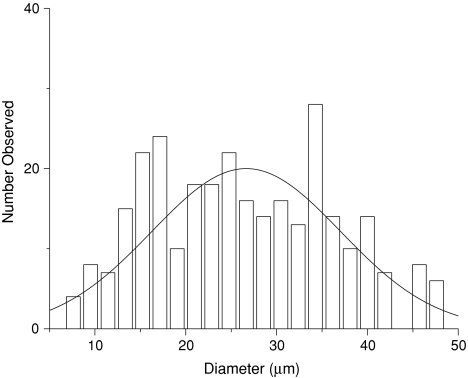
As found in our previous work [27], the applied microsphere synthesis scheme led to spheres produced of approximately 20 μm diameter. Typical SEM images of the spheres attached to oxidised and non-oxidised titanium are shown in Fig. 1. A slight union between nearby spheres or partial melting of the spheres (Fig. 1a), showed the effect of thermal treatment onto the surface. This evidence suggests that the spheres are lightly attached to the titanium oxide surface texture. Areas with agglomerated spheres were occasionally observed. The distribution of sphere sizes after thermal attachment to the titanium oxide surface can be seen in Fig. 2. Before thermal treatment, the average diameter was 20 ± 10 μm [27], whilst after thermal treatment the sphere diameters were found to be 26 ± 10 μm. These overlapping figures indicate that no significant change in diameter or shape of the spheres occurred due to thermal treatment.
Fig. 1.
Morphology of the immobilized PLGA spheres visualized by scanning electron microscopy: a 20 μm spheres on an oxidised titanium surface (15 kV, ×1000); b 20 μm spheres on a non-oxidised titanium surface (5 kV, ×1000)
Fig. 2.
Size distribution of the immobilised PLGA spheres. The values were calculated by visual analysis of the SEM images and include more than 150 data points
Surface loading
The titanium oxide samples prepared in this work were loaded with approx. 10 mg of the 20 spheres in order to obtain a monolayer of spheres on each surface. Two sphere-loaded titanium oxide samples were immersed in acetonitrile in order to assess the resulting dexamethasone concentration on the surface after thermal treatment, and thus by using the already known drug loading efficiency (1% [27]), the amount of attached spheres. It was found that over 80% of the spheres remained attached to the surface after thermal treatment. Therefore, each 19 mm diameter disc was loaded with approximately 20 μg of dexamethasone.
In vitro drug release profile
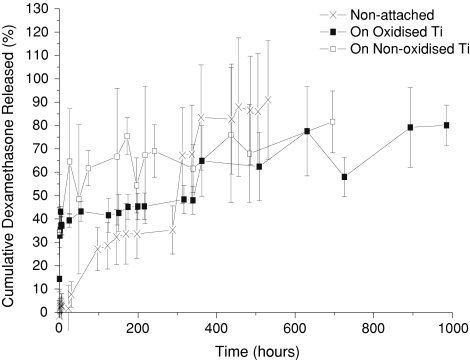
Figure 3 shows the release profile of dexamethasone from the immobilised PLGA spheres up to 1000 h. It can be seen that this trend follows the general shape of previous work on non-attached systems [11, 27]. However, a difference was observed between the free and immobilised 20 μm spheres. At the very first hour, a dexamethasone release of over 30% was reached for the attached spheres, whereas with the non-attached spheres almost no dexamethasone was released. Additionally, the release rate of both the attached sphere samples was compared to the non-attached system after 300 h (80% in 950 h vs. 90% in 550 h). No significant differences have been identified between the release profiles from the oxidised and non-oxidised loaded discs.
Fig. 3.
Dexamethasone release profiles from the immobilised 20 μm spheres on non-oxidised and oxidised titanium discs, plus non-immobilised PLGA spheres (PBS, 37°C). Average values with standard deviations are presented (n = 3)
Sphere degradation
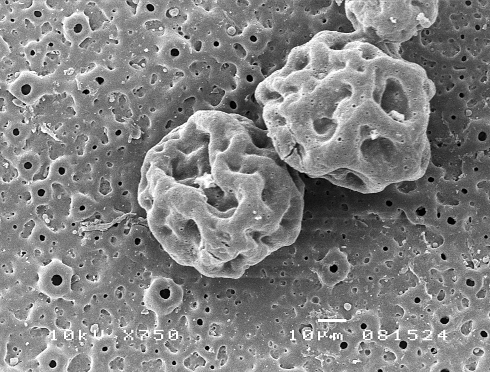
Figure 4 shows typical SEM images of the immobilised spheres on porous oxidised titanium after 800 h of in vitro release. The microspheres were deeply eroded, showing clear diffusion paths for dexamethasone release, as well as an apparent swelling in the spheres.
Fig. 4.
Morphology of the immobilized PLGA spheres visualized by scanning electron microscopy after degradation in PBS for 800 h on an oxidised titanium surface (10 kV, ×750)
Discussion
In this study a particulate drug delivery system has been incorporated into a porous oxidised titanium surface. The main aim was to assess the feasibility of such a system with respect to a sustained drug release. It has been shown before [27] that PLGA spheres of this type show a triphasic release profile, with a burst, a lag and a linear phase.
The release profiles in Fig. 3 show a similar general trend between the free system and both thermally attached systems when incubated at 37°C in PBS, however a few changes could be noticed upon closer inspection. The first one is the increase in burst release for the attached systems. The drug release before 100 h is considered the burst phase [28]. It is associated with the surface release of drugs, freed initially by the slow penetration of water into the structure of the polymer from solution. PLGA reveals a very strong increase of degradation rate as the incubation temperature increases [5]. An increase in incubation temperature from 37 to 47°C over a period of more than 17 days more than doubles the rate of polymer degradation. Consequently, one could consider that during the thermal attachment process (1 h at 50°C) the residual water from deposition could already begin hydrolysing the polymer, potentially inducing the observed increase in burst release magnitude when compared to the free system.
Between approximately 100 and 300 h, the drug release is almost zero order and can be considered to be in a lag phase. This period is associated with the continuing inclusion of water into the polymer structure, but while the average diffusion path length to solution of a drug molecule is increasing [28]. At this point, the polymer has yet to break down and result in the later drug release. All three release profiles show this phase, a clear release rate reduction after 100 h.
After 300 h the release profile changed to a first order, linear phase, associated with the breakdown of the polymer and release of bioactives from the inner core [28]. SEM investigation evidenced severe sphere breakdown after 800 h (Fig. 4). The attached microsphere systems show a lower drug release rate during this phase compared to the free system, although this is not statistically different. In these cases it is important to note that the sphere size distribution does not change, thus this effect comes most probably from the effects of the attachment process, either from the thermal treatment, resultant agglomeration, or the surface itself. Previous experiments with PLGA microspheres have shown that by immobilising spheres onto the surface of a PLLA nanofibrous scaffold [29], the release profile of encapsulated drugs is significantly slowed. This is in line with other papers where PLGA spheres are immobilised in PVA [30]. Thus, immobilising PLGA microspheres on a metallic surface should also affect the release rate in the same fashion, through a barrier effect. The surface could also act to promote agglomeration and reduce the available surface area for drug release over time.
The morphological difference between oxidised (pore size 1.1 ± 0.6 μm) and non-oxidised titanium is negligible when compared to the 20 μm spheres. In addition, the release profile from the attached system was practically the same. Thus, it is possible to say that the porosity of the oxidised titanium surface is sufficiently different from the size of the spheres to not affect the release profile significantly.
The main aim of this investigation was assessing the feasibility of sustained drug delivery directly from a oxidised titanium disc, while maintaining the topography and cellular accessibility of the metal surface. The results shown in this paper indicate that such a surface is possible to be created with significantly longer sustained release than is typically found for films [18], and other porous surfaces on titanium [21], albeit with issues in the attachment process slightly altering the release profile of the drug delivery system used.
From the results shown in this study a few applications for this system can be envisaged, although further work is needed to reduce agglomeration, enhance sphere attachment, and control the release kinetics. Previous work on dexamethasone eluting systems for immunosuppression [26] used 0.5 mg of 20 μm loaded spheres to successfully reduce inflammation around a 1 cm long cotton suture. In the present study, approximately 10 mg of microspheres were used, i.e. 20 times more dexamethasone, although on an implant significantly larger in size. Thus, it can be considered that this level of loading might be sufficient for a prolonged anti-inflammatory release system from the surface of a titanium implant. This system can also be considered for the improvement of bone cell adhesion (through porosity) as well as for cellular differentiation in an orthopedic implant as dexamethasone can act as a differentiation agent [24, 31].
By using another drug loaded into the PLGA spheres, it is relatively easy to extend the application range of the system investigated. Cellular proliferation in tissue scaffolds has been researched extensively [29], and such a delivery system could be applied directly onto the titanium surface, using a morphogenetic protein or a growth factor.
Conclusions
In this study a particulate drug delivery system was loaded on a oxidised titanium surface with the aim to achieve a sustained drug release whilst preserving the underlying morphology. Research into the morphology and function of this dexamethasone eluting drug delivery system showed that thermal attachment did not alter the sphere shape and size dramatically and only had a noticeable effect on drug release before 100 h. Thus, this system allows the retention of the porous metallic topography and maintains a sustained release of dexamethasone from the attached 20 μm spheres for the period of 41 days.
Acknowledgments
The support of Kees Kwakernaak from the Department of Materials Science and Engineering and Michel van der Brink from the Laboratory for Process Equipment for the assistance with scanning electron microscopy is appreciated greatly.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.British National Joint Registry. NJR StatsOnline In: British national joint registry. 2008. http://www.njrcentre.org.uk/. Accessed 15 Nov 2008.
- 2.Smith LJ, Swaim JS, Yao C, Haberstroh KM, Nauman EA, et al. Increased osteoblast cell density on nanostructured PLGA-coated nanostructured titanium for orthopedic applications. Int J Nanomedicine. 2007;2:493–499. [PMC free article] [PubMed] [Google Scholar]
- 3.Wise DL, Rosenkrantz H, Gregory JB, Esber HJ. Long-term controlled delivery of levonorgestrel in rats by means of small biodegradable cylinders. J Pharm Pharmacol. 1980;32:399–403. doi: 10.1111/j.2042-7158.1980.tb12951.x. [DOI] [PubMed] [Google Scholar]
- 4.Wu XS, Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J Biomat Sci-Polym E. 2001;12:21–34. doi: 10.1163/156856201744425. [DOI] [PubMed] [Google Scholar]
- 5.Wildemann B, Sander A, Schwabe P, Lucke M, Stockle U, et al. Short term in vivo biocompatibility testing of biodegradable poly(D, L-lactide)—growth factor coating for orthopaedic implants. Biomaterials. 2005;26:4035–4040. doi: 10.1016/j.biomaterials.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Maran A, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng M, Chen G, Burkley D, Zhou J, Jamiolkowski D, et al. A study on in vitro degradation behavior of a poly(glycolide-co-L-lactide) monofilament. Acta Biomater. 2008;4:1382–1391. doi: 10.1016/j.actbio.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliver Rev. 1997;28:5–24. doi: 10.1016/S0169-409X(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 9.Li JK, Wang N, Wu XS. A novel biodegradable system based on gelatin nanoparticles and poly(lactic-co-glycolic acid) microspheres for protein and peptide drug delivery. J Pharm Sci. 1997;86:891–895. doi: 10.1021/js970084i. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal CM, Athanasiou KA. Technique to control pH in vicinity of biodegrading PLA-PGA implants. J Biomed Mater Res. 1997;38:105–114. doi: 10.1002/(SICI)1097-4636(199722)38:2<105::AID-JBM4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Wei GB, Jin QM, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–110. doi: 10.1016/j.jconrel.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61:180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 13.Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16:153–157. doi: 10.1038/nbt0298-153. [DOI] [PubMed] [Google Scholar]
- 14.Wang JJ, Chua KM, Wang CH. Stabilization and encapsulation of human immunoglobulin G into biodegradable microspheres. J Colloid Interf Sci. 2004;271:92–101. doi: 10.1016/j.jcis.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 15.Vega E, Gamisans F, Gracia ML, Chauvet A, Lacouloche F, Egea MA. PLGA nanospheres for the ocular delivery of flurbiprofen: drug release and interactions. J Pharm Sci. 2008;97:5306–5317. doi: 10.1002/jps.21383. [DOI] [PubMed] [Google Scholar]
- 16.Niwa T, Takeuchi H, Hino T, Kawashima Y. Aerosolization of lactide glycolide copolymer (PLGA) nanospheres for pulmonary delivery of peptide-drugs. Yakugaku Zasshi. 1995;115:732–741. doi: 10.1248/yakushi1947.115.9_732. [DOI] [PubMed] [Google Scholar]
- 17.Pan CJ, Tang JJ, Shao ZY, Wang J, Huang N. Improved blood compatibility of rapamycin-eluting stent by incorporating curcumin. Colloid Surf B. 2007;59:105–111. doi: 10.1016/j.colsurfb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Pan CJ, Tang JJ, Weng YJ, Wang J, Huang N. Preparation and characterization of rapamycin-loaded PLGA coating stent. J Mater Sci-Mater M. 2007;18:2193–2198. doi: 10.1007/s10856-007-3075-9. [DOI] [PubMed] [Google Scholar]
- 19.Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. Journal of the Royal Society Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyan BD, Lohmann CH, Dean DD, Sylvia VL, Cochran DL, et al. Mechanisms involved in osteoblast response to implant surface morphology. Ann Rev Mat Res. 2001;31:357–371. doi: 10.1146/annurev.matsci.31.1.357. [DOI] [Google Scholar]
- 21.Aves EP, Estevez GF, Sader MS, Sierra JCG, Yurell JCL, et al. Hydroxyapatite coating by sol-gel on Ti-6Al-4 V alloy as drug carrier. J Mater Sci-Mater M. 2009;20:543–547. doi: 10.1007/s10856-008-3609-9. [DOI] [PubMed] [Google Scholar]
- 22.Xiao JW, Zhu YC, Liu YY, Zeng Y, Xu FF. An asymmetric coating composed of gelatin and hydroxyapatite for the delivery of water insoluble drug. J Mater Sci-Mater M. 2009;20:889–896. doi: 10.1007/s10856-008-3631-y. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X, Kim K-H, Jeong Y. Anodic oxide films containing Ca and P of titanium biomaterial. Biomaterials. 2001;22:2199–2206. doi: 10.1016/S0142-9612(00)00394-X. [DOI] [PubMed] [Google Scholar]
- 24.Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/en.134.1.277. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Valero S, Santos B, Pajin F, Canton T, Lazaro E, Moreu J, Hernandez G, Padial LR. Clinical outcomes of dexamethasone-eluting stent implantation in ST-elevation acute myocardial infarction. Catheter Cardio Interv. 2007;70:492–497. doi: 10.1002/ccd.21131. [DOI] [PubMed] [Google Scholar]
- 26.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23:1649–1656. doi: 10.1016/S0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 27.Dawes GJS, Fratila-Apachitei LE, Mulia K, Apachitei I, Witkamp GJ, et al. Size effect of PLGA spheres on drug loading efficiency and release profiles. J Mater Sci-Mater M. 2009;20:1089–1094. doi: 10.1007/s10856-008-3666-0. [DOI] [PubMed] [Google Scholar]
- 28.Siepmann J, Faisant N, Benoit JP. A new mathematical model quantifying drug release from bioerodible microparticles using Monte Carlo simulations. Pharmaceut Res. 2002;19:1885–1893. doi: 10.1023/A:1021457911533. [DOI] [PubMed] [Google Scholar]
- 29.Wei GB, Jin QM, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Zhang SM, Chen PP, Cheng L, Zhou W, et al. Controlled release of insulin from PLGA nanoparticles embedded within PVA hydrogels. J Mater Sci-Mater M. 2007;18:2205–2210. doi: 10.1007/s10856-007-3010-0. [DOI] [PubMed] [Google Scholar]
- 31.Jager M, Fischer J, Dohrn W, Li XN, Ayers DC, et al. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. J Orthopaed Res. 2008;26:1440–1448. doi: 10.1002/jor.20565. [DOI] [PubMed] [Google Scholar]