Abstract
Background
Extract of globe artichoke (Cynara scolymus) is promoted as a possible preventive or cure for alcohol-induced hangover symptoms. However, few rigorous clinical trials have assessed the effects of artichoke extract, and none has examined the effects in relation to hangovers. We undertook this study to test whether artichoke extract is effective in preventing the signs and symptoms of alcohol-induced hangover.
Methods
We recruited healthy adult volunteers between 18 and 65 years of age to participate in a randomized double-blind crossover trial. Participants received either 3 capsules of commercially available standardized artichoke extract or indistinguishable, inert placebo capsules immediately before and after alcohol exposure. After a 1-week washout period the volunteers received the opposite treatment. Participants predefined the type and amount of alcoholic beverage that would give them a hangover and ate the same meal before commencing alcohol consumption on the 2 study days. The primary outcome measure was the difference in hangover severity scores between the artichoke extract and placebo interventions. Secondary outcome measures were differences between the interventions in scores using a mood profile questionnaire and cognitive performance tests administered 1 hour before and 10 hours after alcohol exposure.
Results
Fifteen volunteers participated in the study. The mean number (and standard deviation) of alcohol units (each unit being 7.9 g, or 10 mL, of ethanol) consumed during treatment with artichoke extract and placebo was 10.7 (3.1) and 10.5 (2.4) respectively, equivalent to 1.2 (0.3) and 1.2 (0.2) g of alcohol per kilogram body weight. The volume of nonalcoholic drink consumed and the duration of sleep were similar during the artichoke extract and placebo interventions. None of the outcome measures differed significantly between interventions. Adverse events were rare and were mild and transient.
Interpretation
Our results suggest that artichoke extract is not effective in preventing the signs and symptoms of alcohol-induced hangover. Larger studies are required to confirm these findings.
“So faint, so spiritless, so dull, so dead in look.” 1 Thus did Shakespeare sum up the appearance of Morton the morning after losing his battle with King Harry. The terms are equally apt to describe the appearance of any imbiber the morning after losing a personal battle with moderation. Alcohol-induced hangover consists of a varying combination of symptoms2 and may be due to a combination of ethanol, congeners (e.g., methanol), dehydration, sleep disturbance and psychosocial factors.3 It has symptomatic as well as objective effects. In the words of the Bard, alcohol “provokes the desire, but it takes away the performance.” 4
Considering the economic consequences, alcohol-induced hangover is not a trivial matter. In Britain, for instance, it is estimated that the associated symptoms account for about £2 billion (US$3.3 billion) in lost wages, mostly owing to work missed each year. The estimated costs in Australia are US$3.8 billion and in the United States US$148 billion.2
Extract of globe artichoke (Cynara scolymus) is promoted in commercial product information and on the Internet as having possible beneficial effects for symptoms of alcohol-induced hangover.5,6 The therapeutic profile and mechanism of action suggest that it has antioxidant and choleretic properties.7,8 This supports historical observations that artichoke extract may be beneficial for jaundice and liver insufficiency.9 Preclinical investigations imply choleretic activity and biliary elimination of lipids and bile acids,7,10 which have been confirmed by uncontrolled11,12 and controlled clinical studies13,14,15,16 and systematic reviews.17,18 However, no rigorous clinical trials have been conducted of the effects of artichoke extract on preventing or treating hangover symptoms. We therefore conducted a study to test whether artichoke extract is effective in preventing the signs and symptoms of alcohol-induced hangover.
Methods
A randomized, placebo-controlled, double-blind crossover trial was conducted. We recruited healthy participants from the pool of volunteers from the Complementary Medicine unit at Peninsula Medical School, Exeter, UK, by circulated letter. Volunteers were asked to complete a screening questionnaire of their medical history and were considered eligible for inclusion if they were healthy and were between 18 and 65 years of age. Exclusion criteria were pregnancy, breast-feeding, intestinal disorders, diabetes mellitus, gallstones, any neurological or psychological conditions, known or suspected hypersensitivity to alcohol or artichoke, and use of concomitant medications except oral contraceptives. Volunteers were also excluded if they had a history of drug or alcohol abuse or scored 2 or more points on the validated Short Michigan Alcoholism Screening Test.19 Women of child-bearing potential were advised to use adequate contraception throughout the study period and for at least 6 weeks thereafter. All volunteers were instructed to refrain from consuming any alcoholic beverages at least 24 hours before the intervention. To induce hangover symptoms but prevent excessive drinking, all volunteers were required to predefine their alcohol consumption. Specifically, we asked the volunteers to “estimate the amount and the kind of alcoholic beverages that will reliably result in having hangover symptoms the next day.” We used the UK standard value for a unit of alcohol — 7.9 g or 10 mL of ethanol (C2H5OH) — to assess alcohol consumption.
Randomization was performed in blocks of 4 by a person unconnected with the study using computer-generated random number tables. The investigators were unaware of the block size and therefore were unable to predict the treatment allocation. The codes were kept in opaque envelopes under lock at a secure location to ensure the concealment of treatment allocation throughout the trial. The study was designed as a double-blind crossover trial, and volunteers were randomly assigned to receive first either 3 capsules, each containing 320 mg of commercially available standardized artichoke extract (LI120) or indistinguishable, inert placebo immediately before and after alcohol exposure. The daily dose of 1.92 g of artichoke extract — equivalent to about 48 g of fresh artichoke5 — has been shown to be safe and potentially effective14,15 and was considered adequate for this study. All participants attended a restaurant where each received the same pasta dish on each of the 2 study days before commencing alcohol consumption. Before alcohol intake the participants were reminded to limit their alcohol consumption to the predefined quantity. Participants recorded the type, volume, volume percent alcohol and number of drinks of all alcoholic and nonalcoholic drinks as well as the time of going to bed and rising in the morning. According to the crossover design, participants received the opposite treatment after a 1-week washout period following the same regimen. Participants were advised to consume the same type and amount of alcoholic and nonalcoholic drinks during the second study day. Local accommodation and taxi transfer were provided if required.
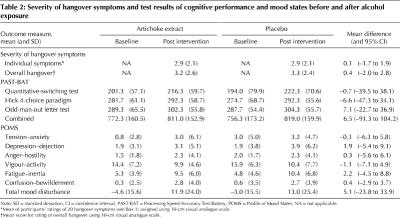
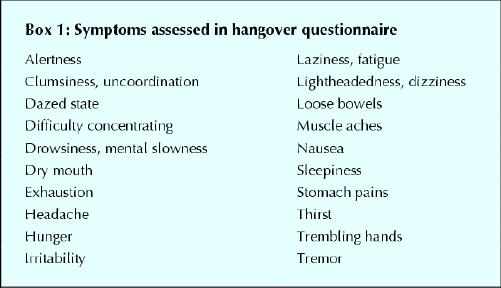
The primary outcome measure was predefined as the difference in hangover scores between artichoke and placebo. In the absence of an accepted standard instrument for hangover assessment,2 this score was derived from a set of common hangover symptoms.2,20 A list of 29 items was initially pilot tested among the group of volunteers. We asked each volunteer to indicate the severity of each symptom as it is usually experienced during a typical alcohol-induced hangover on a 5-point scale. The 20 items that were rated as the most severe by the volunteers (Box 1) were used in the final questionnaire. This questionnaire assessed the severity of each symptom, and the overall hangover, on 10-cm visual analogue scales ranging from “not at all” to “as bad as can be imagined.” Secondary outcome measures were mood and affect (as measured by the Profile of Mood States (POMS) questionnaire21) and cognitive performance (as measured by tests from the Processing Speed-Accuracy Test Battery22). The cognitive performance tests used were the quantitative-switching test, the Hick 4-choice paradigm and the odd-man-out letter test. These tests were chosen after detailed consultation with an expert in cognitive performance testing.
Box 1.
The primary outcome measure was assessed at 9 am, 10 hours after alcohol exposure, on each of the 2 study days. The secondary outcome measures, blood pressure and heart rate were assessed 1 hour before alcohol exposure and on the following morning. Body weight and height were measured at baseline only. Compliance was assessed by the primary investigator (M.H.P.), who supervised the intake of the artichoke extract and placebo capsules. The success of participant blinding was assessed, and adverse events were recorded. For safety monitoring, all participants were given a questionnaire the morning after alcohol intake and asked to record the course of their hangover symptoms during the next 24–48 hours and to indicate the exact time when they resolved. All assessments were performed at the Complementary Medicine unit. The study was approved by the University of Exeter Research Ethics Committee and conducted after written informed consent was obtained from all participants.
The data analyst was blind to the treatment allocation. Analyses were performed according to intention-to-treat principles. For each variable, participants were grouped according to the sequence of intervention (artichoke extract then placebo, or placebo then artichoke extract). The t-test was used to test for period effects and treatment-by-period interactions. Normality was tested using the Shapiro–Wilk test. Primary outcome measures were analyzed for treatment effects using independent samples t-tests to compare the means of the period differences for the 2 sequences. Analysis of covariance using baseline scores as the covariate was used for secondary outcome measures. Not normally distributed data were tested using the Mann–Whitney U test. A 2-sided p value of less than 0.05 was considered significant. Missing values were imputed using the mean values of valid observations. The sample size was estimated using a standard deviation (SD) of 2.0 and a difference of 1.5 cm on the visual analogue scale, which was considered adequate in the context of an anticipated sample of moderately hungover individuals and the claims being tested.23 A power of 80% for a 2-treatment crossover trial was suggested when including 16 participants.
Results
Twenty-five potential volunteers were assessed for eligibility. At randomization, 6 volunteers decided not to participate, and 4 were no longer available. The remaining 15 volunteers (5 men, 10 women) were randomly allocated to receive either artichoke extract or placebo first and the opposite treatment after crossover (Fig. 1). None of the participants dropped out of the study.
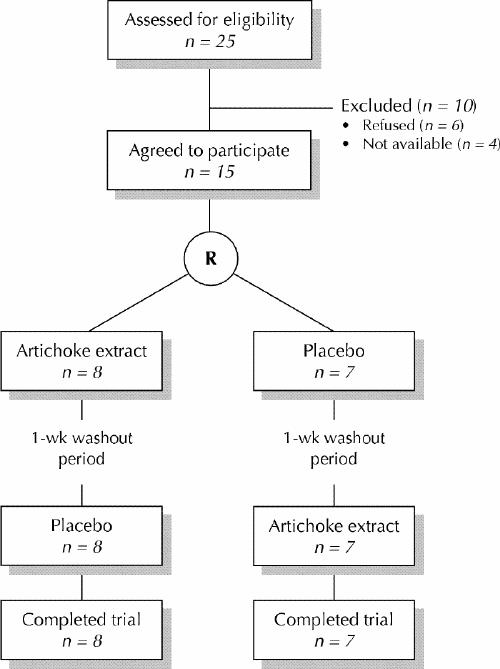
Fig. 1: Profile of trial. R = randomization.
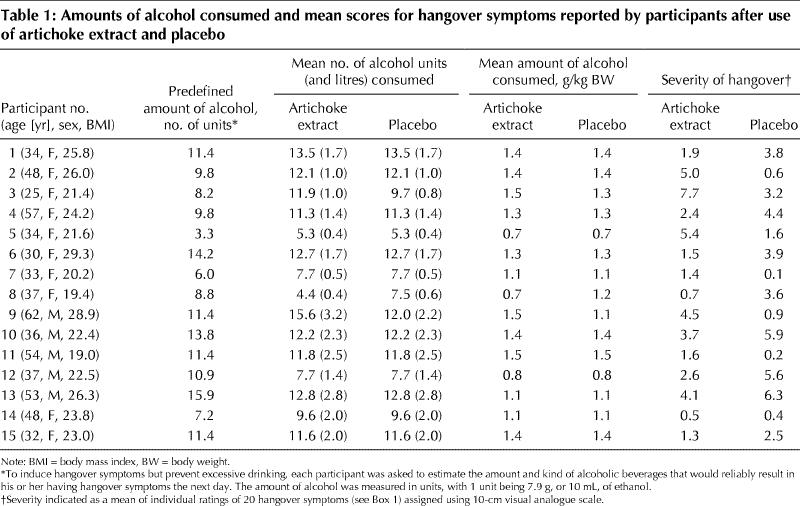
At baseline, the characteristics of the participants who received the artichoke extract first were similar to those of the participants who received placebo first: mean age (and SD) 42.0 (12.8) and 40.6 (10.2) years; weight 66.2 (8.7) and 72.0 (12.2) kg; body mass index 23.0 (3.2) and 24.3 (3.2) kg/m2 respectively. The predetermined mean number (and SD) of units of alcohol required to induce hangover symptoms was 10.2 (3.2). The actual mean number of units of alcohol consumed during treatment with artichoke extract and placebo was 10.7 (3.1) and 10.5 (2.4) respectively, equivalent to 1.2 (0.3) and 1.2 (0.2) g of alcohol per kilogram body weight (Table 1). All except 3 participants consumed identical amounts of alcohol on both study days. Of the 3 who did not, 1 drank 3.1 units of alcohol less, and 2 drank 2.2 and 3.6 units more, during the artichoke treatment period than during the placebo period. Alcohol intake took place over about 4 hours on both days. The mean volume (and SD) of nonalcoholic drinks consumed was 561.4 (305.2) and 582.9 (291.3) mL and the duration of sleep was 6.9 (0.9) and 7.1 (0.7) hours during treatment with artichoke and placebo respectively.
Table 1
There was no evidence of a treatment-by-period interaction in any of the outcome measures. The data for primary and secondary measures are presented in Table 2. Five scores for individual symptoms on the hangover questionnaire and 4 scores for the overall hangover rating were missing and were imputed. The mean scores for the individual hangover symptoms and the overall hangover rating did not differ significantly between the artichoke extract and placebo periods. The same was true for the cognitive performance test results, which showed an increase in performance from baseline to post-intervention for individual tests and for the combined score. The POMS scores indicated increased tension, depression, anger, fatigue and confusion, and decreased vigor, in the post-intervention period for both artichoke extract and placebo. This is reflected by the figures for total mood disturbance. There were no statistically significant differences for any of these outcome measures between treatments. The assessment of participant blinding indicated that, during treatment with artichoke and placebo, 7 and 6 participants respectively thought they had received the opposite intervention, 2 and 6 participants were unable to identify their group allocation, and 6 and 3 participants identified their group correctly. Compliance was 100% in both groups.
Table 2
Blood pressure and heart rate measurements remained largely unchanged throughout the study period: for artichoke extract, the mean blood pressure and heart rate (and SDs) at baseline were 124.3/75.4 (17.7/10.9) mm Hg and 69.6 (12.5) beats/min and after alcohol exposure 120.1/76.8 (15.2/10.0) mm Hg and 67.5 (8.7) beats/min. The respective values for placebo treatment were 123.2/75.0 (12.5/7.0) mm Hg and 66.2 (10.3) beats/min at baseline and 118.9/75.4 (13.4/9.0) mm Hg and 67.5 (8.6) beats/min after alcohol exposure.
One participant reported redness in the face during treatment with artichoke extract. No adverse events were reported during placebo administration. The safety monitoring indicated no serious adverse events during the course of experiencing hangover symptoms, which resolved after 23.1 (SD 15.1) hours and 25.4 (SD 9.8) hours of artichoke extract and placebo intervention respectively.
Interpretation
These data suggest that artichoke extract does not prevent the signs and symptoms of alcohol-induced hangover over and above placebo. There are also some indications that artichoke extract does not speed up recovery time. The discrepancy between the data from our randomized controlled trial and other information may be seen as a reminder of the power of expectation and make-believe.
Our study had limitations. We deliberately included participants from the pool of volunteers from the Complementary Medicine unit in order to optimize adherence to the study protocol and at the same time minimize the risk of excessive drinking. The recruitment strategy resulted in women being over-represented by 2:1, and thus the sample may not be representative of the average population who experience hangover symptoms. In this predominately female sample, the average ingestion of 1.2 g of alcohol per kilogram body weight resulted in moderate hangover discomfort (Tables 1 and 2). This is supported by data suggesting hangover symptoms in healthy volunteers after acute consumption of 1.0 to 1.4 g of alcohol per per kilogram body weight.20,24,25 We did not have an accepted standard instrument with data for reliability and validity with which to assess the extent of alcohol hangover. The questionnaire we designed for this purpose was based on a list of symptoms that had been used in previous studies2,20 and that had been pilot tested among the volunteers. We believe our approach provided an outcome measure with adequate face validity.26 Background symptomatology may have confounded the results of the primary outcome measure. The data for the overall hangover rating, however, suggest that the extent of background symptomatology was minimal. The increase in POMS scores for mood states indicated that the alcohol challenge had the anticipated effect. The increase in cognitive performance scores between baseline and post-intervention measurements was likely due to a learning effect, which we controlled for. The effect of hangover symptoms on the volunteers' ability to rate symptoms and complete questionnaires for the outcome measures is another inherent confounder. Another limitation relates to a possible type II error owing to the small sample, which creates a degree of uncertainty and may have obscured a possible true effect. The study was designed to detect an effect in moderately hungover individuals and was considered appropriate in the context of the claims being tested.23 The measurement variation, however, proved to be larger than expected.
Initial ethical concerns of inducing the negative effects of alcohol in healthy volunteers were considered but discarded as irrelevant given the general availability of artichoke extract, the nature of the claims of its benefits and the impact of hangover symptoms on health, family relationships and productivity. We also attempted to minimize harm by controlling alcohol consumption to levels predefined as acceptable by each participant in a sober state. This avoided standardizing alcohol intake and prevented excessive drinking. Another ethical concern was the implications of the study had it yielded a positive result. It is conceivable that such a result might have led to increased alcohol consumption in the population. However, little evidence exists to show that alleviation of hangover symptoms leads to increased alcohol consumption.2 Conversely, there is also no conclusive evidence that hangover effectively deters alcohol consumption.
In conclusion, our findings do not suggest that artichoke extract is effective in preventing alcohol-induced hangover. Larger studies are required to confirm these findings.
Acknowledgments
We thank Dr. Richard D. Roberts, Department of Psychology, University of Sydney, Australia, for his valuable advice on cognitive performance testing. We also thank Boots the Chemist for donating the artichoke capsules and manufacturing the indistinguishable placebos free of charge, and we thank the staff of the White Hart, Exeter, for their support.
Footnotes
This article has been peer reviewed.
Contributors: All of the authors contributed to the study conception and design. Max Pittler and Adrian White were responsible for the data analysis and interpretation. Max Pittler, Adrian White and Clare Stevinson drafted the article. All of the authors reviewed the manuscript critically for important intellectual content and approved the final version.
Competing interests: None declared.
Correspondence to: Dr. Max H. Pittler, Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, 25 Victoria Park Rd., Exeter UK; m.h.pittler@ex.ac.uk
References
- 1.Shakespeare W. Henry IV part 2. Act 2, scene 3.
- 2.Wiese JG, Shlipak MG, Browner WS. The alcohol hangover. Ann Intern Med 2000;132:897-902. [DOI] [PubMed]
- 3.Calder I. Hangovers. BMJ 1997;314:2-3. [DOI] [PMC free article] [PubMed]
- 4.Shakespeare W. Macbeth. Act 2, scene 3.
- 5.CynaraTM Artichoke. Bucks (UK): Lichtwer Pharma UK. Available: www.lichtwer.co.uk/Packets/cynarapk.html (accessed 2003 Nov 7).
- 6.BBC Essex Website. Xmas excess in Essex. Available: www.bbc.co.uk/essex/students/student_savvy/xmas_xcess.shtml (accessed 2003 Nov 7).
- 7.Kraft K. Artichoke leaf extract — recent findings reflecting effects on lipid metabolism, liver and gastrointestinal tracts. Phytomedicine 1997;4:369-78. [DOI] [PubMed]
- 8.Fintelmann V. Therapeutic profile and mechanisms of action of artichoke leaf extract: hypolipemic, antioxidant, hepatoprotective and choleretic properties. Phytomedicine 1996;(11 Suppl):50.
- 9.Ernst E. The artichoke — a welfare plant with history and future prospects. Naturamed 1995;10:30-5.
- 10.Saénz Rodriguez T, GarcÍa Giménez D, de la Puerta Vázquez R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002;9:687-93. [DOI] [PubMed]
- 11.Fintelmann V. Antidyspeptic and lipid-lowering effect of artichoke leaf extract. Z Allg Med 1996;72(Suppl 2):48-57.
- 12.Fintelmann V. Artischockenextrakt bei dyspeptischem Symptomenkomplex. Methodik und Ergebnisse einer Anwendungsbeobachtung. Z Phytother 1999; 20:93-5.
- 13.Kupke D, von Sanden H, Trinzcek-Gärtner H, Lewin J, Blümel G, Reiman HJ. Prüfung der choleretischen Aktivität eines pflanzlichen Cholagogums. Z Allg Med 1991;67:1046-58.
- 14.Kirchhoff R, Beckers CH, Kirchhoff GM, Trinczek-Gaertner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine 1994;1:107-15. [DOI] [PubMed]
- 15.Marakis G, Walker AF, Middleton RW, Booth JCL, Wright J, Pike DJ. Artichoke leaf extract reduces mild dyspepsia in an open study. Phytomedicine 2002;9:694-9. [DOI] [PubMed]
- 16.Ernst E, Pittler MH, Stevinson C, White AR. The desktop guide to complementary and alternative medicine. Edinburgh: Mosby; 2001.
- 17.Pittler MH, Ernst E. Artichoke leaf extract for serum cholesterol reduction. Perfusion 1998;11:338-40.
- 18.Pittler MH, Thompson Coon J, Ernst E. Artichoke leaf extract for treating hypercholesterolaemia [Cochrane review]. In: The Cochrane Library; Issue 2, 2003. Oxford: Update Software.
- 19.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcohol Screening Test (SMAST). J Stud Alcohol 1975;36:117-26. [DOI] [PubMed]
- 20.Streufert S, Pogash R, Braig D, Gingrich D, Kantner A, Landis R, et al. Alcohol hangover and managerial effectiveness. Alcohol Clin Exp Res 1995;19:1141-6. [DOI] [PubMed]
- 21.McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1971.
- 22.Roberts RD. The Processing Speed-Accuracy Test (PAST) Battery. Sydney: Entelligent Testing Products; 1998.
- 23.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimeters? Pain 1997;72:95-7. [DOI] [PubMed]
- 24.Verster JC, van Duin D, Volkerts ER, Schreuder AHCML, Verbaten MN. Alcohol hangover effects on memory functioning and vigilance performance after and evening of binge drinking. Neuropsychopharmacology 2003;28:740-6. [DOI] [PubMed]
- 25.Lemon J, Chesher G, Fox A, Greeley J, Nabke C. Investigation of the hangover effects of an acute dose of alcohol on psychomotor performance. Alcohol Clin Exp Res 1993;17:665-8. [DOI] [PubMed]
- 26.Smith CM, Barnes GM. Signs and symptoms of hangover: prevalence and relationship to alcohol use in a general adult population. Drug Alcohol Depend 1983;11:249-69. [DOI] [PubMed]