Abstract
Data on human odor thresholds show disparities huge enough to marginalize olfactory psychophysics and delegitimize importation of its data into other areas. Variation of orders of magnitude from study to study, much of it systematic, threatens meaningful comparisons with animal species, comparison between in vivo with in vitro studies, the search for molecular determinants of potency, and use of olfactory information for environmental or public health policy. On the premise that good experimental results will flow from use of good tools, this report describes a vapor delivery system and its peripherals that instantiate good tools. The vapor delivery device 8 (VDD8) provides flexibility in range of delivered concentrations, offers definable stability of delivery, accommodates solvent-free delivery below a part per trillion, gives a realistic interface with subjects, has accessible and replaceable components, and adapts to a variety of psychophysical methodologies. The device serves most often for measurement of absolute sensitivity, where its design encourages collection of thousands of judgments per day from subjects tested simultaneously. The results have shown humans to be more sensitive and less variable than has previous testing. The VDD8 can also serve for measurement of differential sensitivity, discrimination of quality, and perception of mixtures and masking. The exposition seeks to transmit general lessons while it proffers some specifics of design to reproduce features of the device in a new or existing system. The principles can apply to devices for animal testing.
Keywords: absolute sensitivity, odor threshold, olfaction, olfactometer, psychophysics, volatile organic compound
Introduction
Measurement of sensitivity to odors suffers from unreliability. Compilations of thresholds show variation from study to study of about 4 to 5 orders of magnitude for almost every odorant, some of it perhaps random but much of it systematic (van Gemert 2003). A systematic portion revealed itself when compilers Devos et al. (1990) found they could assign a factor to bring the thresholds gathered by a given investigator, often over several studies, into alignment with that of other investigators. Normalization reduced the variation but still left a residual of orders of magnitude. The exercise showed that methodology contributed greatly to measured thresholds.
As long as 2 decades ago, a compilation highlighted how the unreliability lay largely in the tools used to gather thresholds (American Industrial Hygiene Association 1989). Inadequate tools equated to inadequate answers. One can hope therefore to solve the problem through use of proper tools. The work here strives toward that goal. It recounts some diagnostic background and gives examples of hardware, software, analytical measurement, and psychophysical methodology that have served to enhance testing for absolute detection. It makes no effort at standardization or regimentation. For any investigator who may find some features of the approach desirable, the text contains concrete details to facilitate development.
The factors that one would need to study to unravel the many methodological influences on threshold exceeds anyone's resources, but variables of principal interest include: 1) manner of control of the stimulus (e.g., static vs. dynamic dilution; use of a solvent), 2) measurement of level (viz., any effort to validate concentration), 3) interface between vapor and subject (e.g., flowing stream of air; puff from a bottle), and 4) psychophysical method (e.g., use of forced choice; use of “yes–no”).
The first 3 of the variables involve mass transfer. Few investigations have included any measurement of concentration made available, no less delivered, to subjects. Most studies have relied upon nominal expression of strength, such as concentration of odorant in a solvent or fraction of nominally saturated vapor. Compilers have often needed to convert results given as liquid concentration or percent “saturation” into vapor concentration. How they calculated these, they do not say. Headspace concentration over a liquid will depend not only upon mole fraction of solute but also upon the solvent (Cometto-Muñiz et al. 2003). Estimates of vapor pressure from the literature may also differ from source to source. In the absence of any attempt to confirm concentration, computation of threshold entails guesswork. To complicate matters, a sniff from a jar, or even a flowing airstream, can allow dilution of vapor with surrounding air and render even a properly estimated concentration systematically wrong.
The fourth variable, namely, psychophysical methodology, plays its most notable role with respect to delivery of realistic blanks. Subjects make false positive judgments about odors readily (Engen 1972; Cain 1988). Most modern investigators have sought to avoid the issue via use of forced choice. One could use single presentations and appropriate analysis, but measures of detection can have little credibility without use of blanks.
Although use of blanks may seem almost a trivial consideration, it has proved a stumbling block, perhaps especially for sophisticated devices. Many odorants have thresholds below parts per billion of air and, equally important, some will adsorb tenaciously to surfaces. At very low concentrations, no routine analytical procedure can guarantee delivery of contaminant-free flow as defined by the nose. Trace contamination may render a device useless for measurement of threshold. Any device with a final common path for delivery risks carry over from trial to trial. In their description of a sophisticated and useful device, Johnson and Sobel (2007) noted: “The particular olfactometer we have described here is not without drawbacks … in that it relies on a single flushable odor line from olfactometer to subject (rather than multiple lines, one for each odor used in a given experiment), it is prone to slight contamination and is therefore inappropriate for applications such as detection threshold testing.” (p. 244.) The investigators exhibited commendable honesty, for only they would know of the problem. One can, and some have, invested considerable resources in construction of a device only to find that it cannot give adequate blanks.
Although investigators may fail to state it, fear of contamination has likely caused some to study only odorants with the correlated properties of high vapor pressure, low surface adsorption, and high thresholds. The strategy excludes thousands of chemicals, including many of the more interesting such as most fragrance materials. Odor science cannot develop from thresholds for just materials with small, highly volatile molecules, such as ethyl alcohol and acetone. Furthermore, inability to decontaminate devices may account for why some investigators have used the same materials for years, even in suprathreshold investigations.
Certain designs may favor one olfactometer over another, but none can claim success without a record of performance. The device described below has survived years of use without alteration of basic design. Changes have entailed additions, such as ports for syringe sampling. Contamination has proved an issue of minor concern. Thresholds measured with the device fall among the lowest collected for a material in itself an indicator of success (Table 1).
Table 1.
Odor thresholds in ppb obtained with VDD8 compared with compiled values from Devos et al. (1990) or others, as indicated
| Chemical | VDD8 | Devos et al. (or other) |
| Toluene | 88a | 1678 |
| Ethylbenzene | 6.6a | 93 |
| Butylbenzene | 2.5a | 4220b |
| Hexylbenzene | 5.0a | 626b |
| Octylbenzene | 96a | 369b |
| Acetone | 884c | 9832 |
| Pentanone | 91c | 5609 |
| Heptanone | 5.4c | 224 |
| Nonanone | 5.9c | 60 |
| Ethyl acetate | 269d | 8052 |
| n-Butyl acetate | 5.3d | 320 |
| n-Butyl acetate | 2.0e | 320 |
| t-Butyl acetate | 7.8e | 1291b |
| Hexyl acetate | 3.1d | 384 |
| Octyl acetate | 21d | 4.1 |
| Ethanol | 331f | 83 206 |
| 1-Butanol | 7.9f | 2377 |
| 1-Hexanol | 8.1f | 234 |
| 1-Octanol | 4.4f | 41 |
| Ethyl butyrate | 0.011 | 46 |
| Glutaraldehyde | 0.27g | 40h |
| D-Limonene | 16i | 1.8j |
| Ozone | 6.4i | 43 |
Compiled values are geometric means of unnormalized outcomes.
not actually a threshold but an extrapolation from intensity ratings but nevertheless allowed into the compilation.
Principles
Ten principles guided design of the vapor delivery device 8 (VDD8), named because it delivers vapors from 8 stations. Because the device can present levels to probe chemesthetic sensitivity (irritation), the name “olfactometer” seems too limiting.
1) “Allow for the generation of any range of concentration (100:1, 1000:1, etc.) and any series (2:1, 3:1, etc.) within that range.”
2) “Generate vapors without use of liquid solvents, except water.” Organic solvents often have some odor. With tiny proportions of odorant dissolved into a “relatively odorless solvent,” to use the euphemism, a solvent can become a masker. It can adsorb onto surfaces and impede transfer of odorant.
3) “Generate stable vapor concentrations continuously throughout a day of continuous operation.” Sensory detection varies from moment to moment, as seen in the ogival form of the psychometric function (e.g., Cain and Schmidt 2009). The function reflects all sources of variation, biological and physical, including fluctuation of the stimulus. Noise in delivered level can depress and distort the function.
4) “Permit routine measurement of both level and variability of delivery.” Some assurance of stability of delivered concentration forms the basis for any quantitative psychophysics. The delivery device needs to get around the problem that the human nose often has better sensitivity than analytical instruments (Turk et al. 2003). The olfaction laboratory should have fundamental capability to measure concentration.
5) “Provide capacity for up to 3-alternative forced-choice testing.” Forced-choice testing has become the norm. A device that permits 3-alternative testing can also permit 2-alternative testing and various types of psychophysical algorithms, for example, adaptive methods.
6) “Simulate conditions of ambient smelling.” The sniff reaches instantaneous flow rates of tens of liters per minute (Laing 1982, 1983).
7) “Allow more than one subject to interact with the device for efficiency of testing.” Testing of subjects in parallel allows an unhurried pace consistent with keeping noses fresh and undiminished by adaptation. Parallel testing affords efficiencies unavailable in serial testing and permits gathering a considerable amount of data in a day.
8) “Control the temporal sequence of testing through use of automated commands.” Programmable audible instructions can impose a regimen on testing subjects in parallel.
9) “Use interactive spreadsheets to calculate expected concentrations and other conditions.” The operator needs to decide what concentrations to deliver, whether the reservoir of material will last through testing, whether concentrations in the lines might exceed saturated vapor, whether dilution should occur in one stage or more, and so on.
10) “Prepare for the worst regarding contamination by use of parts replaceable at moderate cost.” Careful maintenance should avoid contamination but accidents may happen and should cause minimal disruption.
Antecedents
The VDD8 owes some aspects of design to Andrew Dravnieks, who created 2 devices notable for simplicity and reliability (Dravnieks 1974, 1975). The more popular device, the “Dynamic Dilution Binary Scale Olfactometer,” consists of 8 ports that emit 160 mL/min of odorized air through nozzles. Two lines feed each port (nozzle), one with vapor and the other with room air. Capillary tubes from 2-manifolds determine flow rate into the ports. Hence, a port that delivers a 2-fold dilution of the starting concentration has a line that feeds 80 mL/min of odorized air and 80 mL/min of room air to its nozzle. An aquarium pump supplies the air and a water column manostat maintains the pressure at the manifolds. The word “binary” in the name comes from 2:1 steps between dilutions. Three features make the device unique: 1) it sets up a parallel series of concentrations (fixed range of 128:1) available continuously; 2) all vapor-carrying lines hold the same concentration, only the flow rates delivered to ports varies; and 3) all parts can undergo cleaning and the lines replaced. Dravnieks's choice of delivery rate came from an intention for the device to function in any well-ventilated room. Its footprint equals just 0.17 m2 (1.9 ft2). He recommended the device for matching the perceived intensity of one odor to a reference odor (American Society for Testing and Materials 2004).
The second device, the “Dynamic Dilution Forced-Choice Triangle Olfactometer,” follows the same principles as the binary device but gives 3 choices via a triangle of nozzles held in a plastic tumbler at each of 6 stations. Dravnieks intended the device for measurement of threshold (Dravnieks and Prokop 1975). Dilution from one station to another equals 3:1, with flow rate per nozzle at 1 or 3 L/min. Whereas the features from Dravnieks's devices guided design of the VDD8, in part, choice of flow rates and the interface between subject and device did not. As noted, when subjects sniff, they achieve high rates of flow for seconds or less. If a device fails to meet the demand, surrounding air will enter and dilute concentration in the nose. Delivery of the necessary flow rates from the nozzles of Dravnieks's devices would give an aversive sensation, much as would that from a compressed air hose. As it turns out, however, use of a conical delivery port allows high flow without discomfort (Gunnarsen et al. 1994). The device described below incorporates numerous other features to meet the goals listed above.
Roadmap
The device and procedures outlined below can serve to answer certain questions that may have made some persons reluctant to venture into olfactometry. Persons who do must essentially answer such questions as:
Can I present quantitative doses of vapor without use of an organic solvent?
If I work without an organic solvent, must I foreclose on the study of materials with very low thresholds?
If a material has some solubility in water, can I use this property to advantage?
How can I assure myself that the hardware used to dose odor has the range of capacity needed to work with materials of greatly different potencies?
Can I calculate outcomes in different scenarios, such as different starting levels and different concentrations in solvent?
If I cannot physically measure final delivery for an odorant with really high potency (e.g., threshold of 1 ppt), can I still study it; can I measure it somewhere other than at the point of delivery?
How does one calibrate an analytical instrument?
What degree of variability is common in analytical measurement?
Should I use mass flow controllers (MFCs) for all flow measurement?
Is there an absolute standard for flow measurement?
Answers to these questions and others appear below, either in the main text or the appendix (Supplementary Material). Although the text deals with a particular odor delivery device, the lessons deal with olfactometry rather than with the device. It serves as an example, just as the computation of a sample problem can illustrate mathematical principles. The logical sequence of the text below goes as follows: 1) introduction to the device, which when first seen in schematic may appear complex but should seem simpler as the reader follows the path. 2) the text instructs the reader to look at first only at the part where the stimulus material enters and it then addresses the question of what will come out at the subject's end. 3) To know outcome, an operator must anticipate the flows and dilutions involved and spreadsheets show how to accomplish an intended outcome. 4) The spreadsheets prepare the reader to understand the components of the device because these components execute what the reader has seen in the sheets. 5) To know that the device has accomplished its goal, a calibrated physical–chemical instrument must measure the vapor. 6) If the measurement confirms expectations, then the operator can collect psychophysical data, but needs to understand that the interface between device and subject should provide a guarantee that the vapor goes where intended into the nose.
Design and operation
Stimulus generation
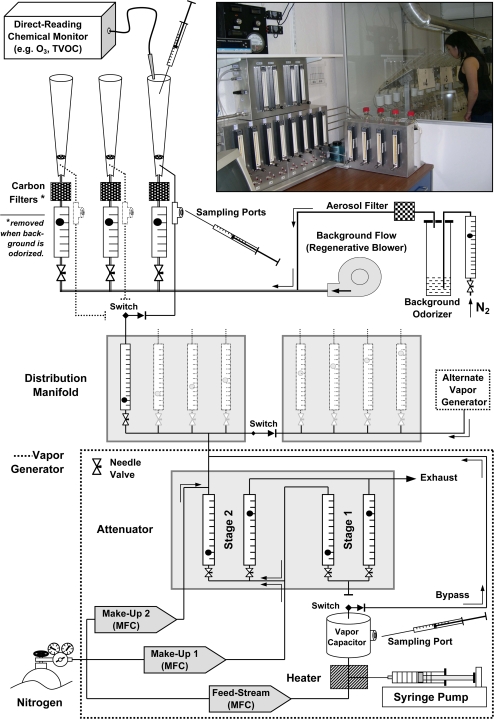
The VDD8 operates at room temperature. In most cases, the operator will feed liquid volatile organic compound (VOC) via a programmable syringe pump into a line that goes to a heating block held at a temperature just below boiling point (see Figure 1). A feed stream of nitrogen monitored through a MFC passes through the block to transport material to the vapor capacitor. The elevated temperature of the heating block raises rate of conversion of liquid into vapor. The vapor capacitor allows the newly created vapor phase combination of nitrogen and VOC to dwell for about 30 s as it enters the diluting portion of the device. The operator can sample vapor through a septum-lined cap in the wall of the vapor capacitor.
Figure 1.
A schematic shows essential parts of the VDD8. Generation of vapor begins with flow of inert nitrogen (feed stream) through a MFC to a heater that receives a cross-flow of liquid from a syringe. The vapor then goes into the 1.9-L vapor capacitor (larger cylinder). The vapor may then go through an Attenuator to dilute it one or 2 stages (up to 800 000:1) or may bypass the Attenuator. When the vapor enters the distribution manifold, it splits into 8 (or 4) lines, each to 1 cone of the 3 in a station. (The 8-path distribution manifold can become 2 four-path manifolds operated independently. The alternate vapor generator refers to a setup that duplicates the components outlined by the dashed line.) Just below where flow enters a cone, a fitting allows vapor sampling. The flow of vapor enters the bottom of a cone where it mixes with a background flow of air provided by a regenerative blower (oil-less ring compressor). All cones receive the same flow of air, typically 40 L/min, cleaned by activated carbon just before it enters a cone. A perforated disk in each cone creates turbulence to promote mixing. The mouth of the cone affords a third place to sample vapor concentration. The photo inset gives a sense of scale, with the 8-rotameter unit distribution manifold, the 4-rotameter unit Attenuator above it, and the 4-rotameter unit background odorizer. This figure appears in color in the online version of Chemical Senses.
The appendix (Supplementary Material) provides a description of certain key components. The dual exposition should allow readers who wish to capture the principles to do so and those who wish to put the principles into practice to do so, as well. The next section deals with predicting the output of the VDD8.
Interactive spreadsheets
“Interactive spreadsheets” play an essential role for understanding the VDD8. The operator should create a relevant spreadsheet before testing a new material. It can let the operator anticipate the various ways to achieve test concentrations of interest.
Odorants vary in potency over about 10 orders of magnitude. The operator needs also to consider the operating range of the syringe pump and the amount of material that hours of operation will consume.
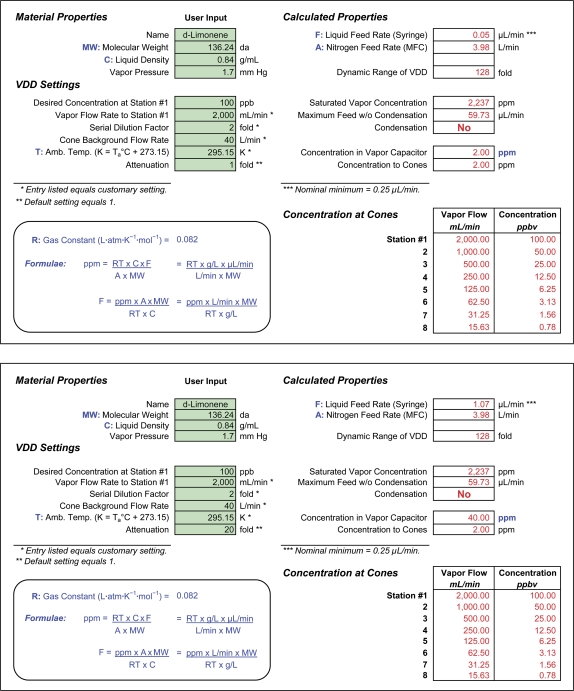
The spreadsheet shown in Figure 2 (http://chemosensory.ucsd.edu/ and Supplementary Material) would allow an operator to anticipate the parameters for delivery in an investigation of D-limonene. The operator fills in the shaded cells under “material properties” (molecular weight, density, and vapor pressure) and under “VDD settings.” Consider the cell “Desired Concentration at Station #1.” That station will present the highest concentration, so the operator should expect subjects to detect it readily. The literature may guide the choice of the number. The geometric average of the listings in van Gemert (2003) suggests a threshold of 141 ppb but contains one value far above the others. With that outlier removed, the listings imply a threshold of 70 ppb. In an effort to keep delivery in the general range, the operator may choose to enter 100 ppb. The remaining entries in the column consist of customary settings for testing. A flow rate of 2000 mL/min at Station 1 sets up a series of quite measurable flow rates down to Station 8 with progressive halving (2-fold dilution). A “cone background flow rate” of 40 L/min equals the design rate for the VDD8, though other values can also serve. The temperature of 295.15 K equals room temperature (22 °C). “Attenuation” of 1-fold means that the mixture of D-limonene and nitrogen achieved should not require any dilution (attenuation) before it enters the distribution manifold. The flow from the vapor capacitor can therefore bypass the Attenuator. The setting of 1-fold for the attenuator, however, starts as a guess. Calculations will reveal the quality of the guess.
Figure 2.
Upper part: spreadsheet to set up the VDD for a given outcome, in this case for D-limonene at a maximum concentration of 100 ppb and 2-fold dilutions over 8 stations. The user enters the information in the left columns and the spreadsheet returns the information in the right columns. Under VDD settings, the information with asterisks represents that customarily used to generate data for a psychometric function. The entry of 1 for Attenuation provides a starting point that may need adjustment. Under calculated properties, the asterisked information (top cell) lies below the nominal minimum for the liquid feed rate from the syringe. In such a case, the operator can increase the entry for Attenuation. Ignoring that for the moment, the calculated properties pose no other problems. The “dynamic range” of 128:1 merely represents 7 successive halvings from the highest concentration. The calculation for Maximum Feed w/o Condensation shows that the feed rate of 0.05 μL/min lies very far from a rate that would cause “condensation.” Hence, the spreadsheet has returned the answer “No.” With the entry of 1 for Attenuation, the “concentration in vapor capacitor” and concentration to cones both equal the same value, in this case 2 ppm. Assuming accurate calibration of the 8 rotameters of the distribution manifold, the values in the table of concentration at cones, that is, 100 ppbv, 50 ppbv, etc., should hold as well. Lower part: the lower spreadsheet differs from the upper in small but essential ways. Because the upper indicated a liquid feed rate below the critical value for uniform delivery, the operator entered 20 into Attenuation. With the calculated liquid feed rate of 1.07 μL/min, approximately 4 times the critical value, the only other change in the lower sheet appears in concentration in vapor capacitor, where concentration has increased by 20-fold. The Attenuator, designed to dilute concentration, then comes into service. This figure appears in color in the online version of Chemical Senses.
The “calculated properties” give 2 important answers in the top cells: liquid feed rate from the syringe pump and the total flow rate of nitrogen to provide appropriate flow to the stations. The liquid feed rate will have certain limitations at high and low ends. At the high end, a rate could cause oversaturation of the stream of nitrogen. The estimate shown in the cell “Saturated Vapor Concentration” indicates how much of a VOC with a vapor pressure of 1.7 mmHg a stream at 3.98 L/min can hold at room temperature. As Figure 1 indicated, conversion of the VOC into a vapor takes place at a temperature well above ambient, which can allow the stream to hold a higher concentration. Cooling in the vapor capacitor could cause the stream to give up its extra concentration via condensation. The calculation assures that the stream takes up no more than it can hold at 22 °C. The rate calculated comes nowhere near saturated vapor. This could change if the operator delivered higher concentrations, as in a study of irritation. To assure that the operator notices calculated oversaturation, a cell cautions “yes” or “no,” as appropriate.
As the footnote to the cell “Liquid Feed Rate” shows, feed rate should exceed 0.25 μL/min. At lower rates, the pump may show nontrivial hysteresis. (As the name capacitor implies, the vapor capacitor exists in part to dampen hysteresis.) In this case, the calculated rate lies almost 5-fold below the minimum, a matter to ignore for now.
The 2 lowest cells under calculated properties show concentration in the vapor capacitor and to the cones, respectively. Both equal 2 ppm, a quantity measurable by injection of syringe samples into a gas chromatograph with a flame ionization detector (GC-FID), for example. The table under “concentration at cones” shows the nominal concentration series and flows of vapor into the cones.
To deal with the problem that calculated liquid feed rate lies below the nominal minimum by almost 5-fold, the operator can increase Attenuation. An entry of 20 will increase feed rate commensurately, to an acceptable 1.07 μL/min, and will change concentration in the vapor capacitor from 2 to 40 ppm. With attenuation above 1-fold, the operator will need to switch flow from the vapor capacitor into the Attenuator (Figure 1). Some flow can then exit the device via filtered exhaust, with some retained to pass into the distribution manifold. Even with the need to exhaust some vapor, the amount of liquid used for 8 h of testing would equal just 0.51 mL.
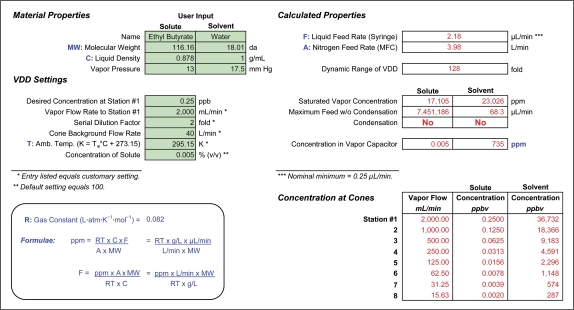
Although designed to deliver material without use of solvent, the VDD8 can operate with one material dissolved in another, preferably just water. Figure 3 shows a case for ethyl n-butyrate, using a 0.005% solution. The spreadsheet (http://chemosensory.ucsd.edu/ and Supplementary Material) now has 2 columns for input regarding material properties, one for solute and one for solvent. Regarding VDD settings, the cell “Concentration of Solute” has replaced the cell Attenuation because the solvent provides the attenuation. Under calculated properties, the cell “Maximum Feed w/o Condensation” shows a lower rate for the solvent, which makes up 99.995% of the solution. In the example for ethyl butyrate, even the concentration in the vapor capacitor lies below that measurable with a syringe sample, a matter taken up below.
Figure 3.
Showing a version of an interactive spreadsheet for use with a VOC of very low threshold and slight solubility in water (ethyl n-butyrate) and a solvent of water instead of predilution with nitrogen. As do the spreadsheets in Figure 2, this has cells for user input. Rather than a cell for Attenuation, the sheet has a cell concentration of solute. In this case, where the solution loaded into the syringe contains 99.995% water, the water vapor concentration in the stream would limit the maximum feed rate of the syringe pump. At the Liquid Feed Rate calculated to deliver a maximum VOC concentration of 0.25 ppbv, the concentration of water vapor in the stream (735 ppm) lies at only 3.2% of its saturated vapor concentration. This figure appears in color in the online version of Chemical Senses.
The concentration of water vapor lies in this instance approximately 30-fold below saturated vapor concentration. If one needed a higher injection rate and could not dissolve more VOC into water, then concentration in the water could become a concern.
Attenuator
To use the VDD8 in a mode that avoids organic solvents, the operator may need to engage the Attenuator for 1 or 2 stages of dilution (Figure 1). A stage of attenuation entails bifurcation of the flow of odorized nitrogen from the vapor capacitor. One stream goes to exhaust and the other progresses to the distribution manifold. As it does, a stream of nitrogen through a second MFC (Make-Up 1) dilutes it up to 800:1, though restores the original flow rate (3.98 L/min). With an attenuation of 20, the concentration to the cones remains as set and concentration in the vapor capacitor increases 20-fold (Figure 2).
First-stage attenuation can bring the lowest levels of a series into a range as low as tenths of parts per trillion. Because thresholds may lie in that range and one always wants to test below the threshold, a second stage can accomplish further attenuation, 1000:1. Hence, the range equals 800 000:1 and deliveries can achieve tenths of parts-per-quadrillion. With second-stage attenuation, yet another stream of nitrogen (Make-Up 2) joins and dilutes the already attenuated flow of VOC and restores the original flow rate.
Flow control in the Attenuator relies upon rotameters, which impart advantages: 1) flexibility of range because rotameters can readily be switched out from their housing. (Suitable choices could extend the range beyond 800 000:1.) 2) Although MFCs have the asset of independence of flow rate from pressure, they have the liability of possible irreversible contamination; one can wash a rotameter, not a MFC. In the VDD8, MFCs come into contact with inert gas only.
Distribution manifold
When flow leaves or bypasses the Attenuator, it splits into parallel paths, each sent to a station. Of the 3 cones of a station, only one gets flow of vapor at any given time. All 3 have background flow.
When one needs to measure sharply accelerated psychometric functions for chemesthesis, for example, 4 levels may suffice (see Cain, Schmidt, and Jalowayski 2007). Two sets of 4 levels available simultaneously can double productivity of testing. One can also run the device for 2 odorants. A valve can separate the distribution manifold into halves, and the operator can add components for generation of a second material (alternate generation unit).
Below a cone in the vapor line, a tee affords access to the stream via a septum. All tees below active cones contain the same concentration of VOC. The concentration in the vapor line will exceed that in the active cone by the flow rate of the vapor divided by that flow rate plus the make-up rate of 40 L/min. If Station 1 receives vapor at 2 L/min, then its concentration will equal [2/(2 + 40)] × 100% = 4.8% of the feeding vapor. Depending upon the VOC, one might sample directly from the cone itself.
Calibration
Calibration of the VDD8 entails measurement of flows. Rotameters (variable area flowmeters) ship with calibration charts for the gas specified and may have scales in units of interest (e.g., L/min). Individual tubes may vary and require checking against a primary standard. A flow calibrator (Gilibrator-2, Sensidyne, Inc.), based upon movement of a soap bubble in a column, provides an absolute standard. Readings will depend on temperature and pressure, so the tubes need calibration under conditions of use, a matter pertinent for the Attenuator (back pressure of 15 psi [1.03 bar]). The operator calibrates flows periodically. By the nature of the operation of the VDD, settings remain the same during testing, even across days, so the operator need not reset rotameters. This minimizes error that might arise from sighting a float differently from one adjustment to another.
Calibration extends to instruments to validate delivery. In the history of the VDD8, these have included:
For most VOCs, a gas chromatograph (GC) equipped with a broad detector, such as a flame ionization detector (GC-FID) or photoionization detector (GC-PID).
For chloropicrin, a GC with an electron-capture detector (GC-ECD).
For glutaraldehyde, a high-pressure liquid chromatograph (HPLC) with a UV detector.
For ozone, a chemiluminescent ozone analyzer (model 265A, Teledyne API).
For total VOCs, a broadband analyzer with a PID (ppbRAE, RAE Systems, Inc.).
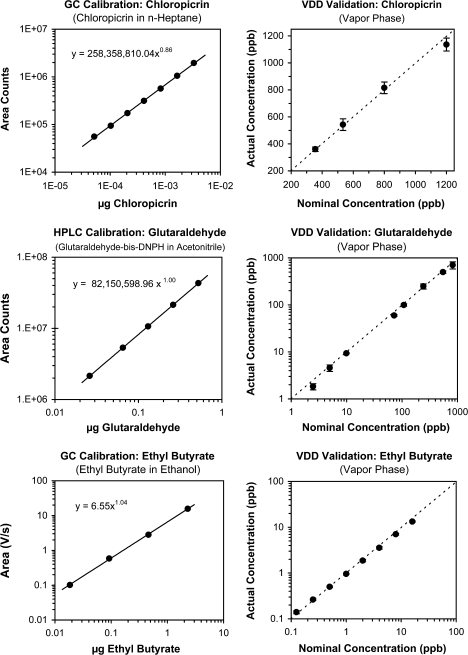
One can calibrate a GC and an HPLC with injections of liquid samples in a solvent that elutes with a different retention time from the solute (Figure 4). GC responses maintain linearity with concentration over orders of magnitude. The operator may or may not need to calibrate a material-specific analyzer. The chemiluminescent analyzer underwent calibration at the factory. One can calibrate a broadband analyzer with vapor standards.
Figure 4.
Showing 3 examples of calibration of analytical instruments and validation of delivery for the VDD8. “Top row” shows calibration of response from a GC-ECD to liquid injections (0.5 μL) of chloropicrin in n-heptane and validation of delivery for 250-μL vapor samples from the cones. The average CV of the direct vapor samples equaled 10%. “Middle row” shows calibration of response from an HPLC to liquid injections (20 μL) of glutaraldehyde-bis-DNPH in acetonitrile and validation of delivery with injected liquid samples of the same reaction product (derivative) obtained after trapping glutaraldehyde onto treated filters and reacting the trapped material with DNPH and phosphoric acid. CV equaled 10%. “Bottom row” shows calibration of response from a GC-FID to liquid injections (0.5 μL) of ethyl n-butyrate in ethanol and validation of delivery from thermally desorbed vapor samples collected from the cones onto Tenax.
Validation
Validation of output from the VDD8 comes from measurement of concentration by a calibrated instrument. The complexity of validation may have prompted some and perhaps many olfactory researchers to say, “Since I cannot measure concentration down to the threshold level, I might as well not measure it at all.” Hence, avoidance of the measurement became routine.
The examples below give some sense of how to validate concentration. The list hardly exhausts the possibilities. The operator should consider the option of “no validation” impermissible at this stage. Even measurement of just the concentration in the vapor capacitor provides a major step over no validation at all (Figure 1).
Direct sampling requires an instrument that can read mass in a grab sample. Figure 4 illustrates a case of direct syringe sampling (250 μL) of chloropicrin from cones and analysis of the halogen-containing material chloropicrin with a GC-ECD. The left side shows a calibration function for the GC, where liquid injections (0.5 μL) of chloropicrin in n-heptane gave the area counts shown on the ordinate. The operator recalibrated periodically in case the detector changed its sensitivity. In general, a calibration curve will show little random error (coefficient of variation [CV] of a few percent) because the sources of error will come just from making solutions, injection of consistent volume, and the intrinsic variability of the instrument. Insofar as measurements of validation deal with vapor, then they will commonly have higher CVs, perhaps 10–15%. Measurements have compared repeated syringe injections from the headspace over neat VOC and that from the VDD8 (Cain and Schmidt 2009). Both have exhibited CVs of about 10–15%, which implies that the VDD8 itself added no variability of consequence.
In one investigation with the VDD8, a Teledyne Instruments API Model 265A chemiluminescence ozone analyzer monitored levels of ozone created by ultraviolet irradiation of oxygen from 100 ppb down to 0.6 ppb (Cain, Schmidt, and Wolkoff 2007). The monitor indicated stability within a few percent over full days of operation of the VDD8.
Trapping entails use of a medium, such as activated carbon, Tenax, or a treated filter, to trap VOC from a large volume. This may prove necessary when a grab sample has too little mass to register a response or when a required standard method calls for trapping. The latter applied in a study of glutaraldehyde (Cain, Schmidt, and Jalowayski 2007). The United States Occupational Safety and Health Administration (OSHA) requires a method (#64) that entails sampling a volume of air through open-face monitoring cassettes that contain 2 glass fiber filters, each coated with 2,4-dinitrophenylhydrazine (DNPH) and phosphoric acid (Occupational Safety and Health Administration 1998). (Personal sampling pumps drew either 15 or 60 L through the filters, depending upon the concentration sampled.) Analysis entailed extraction with acetonitrile and analysis of injections of glutaraldehyde-bis-DNPH by HPLC. Trapping means loss of information about variability of momentary samples. The CVs of the repeated samples in Figure 4 equaled 10%. Multiplication by a factor of 2 implied a range of ±20%, that is, 2 standard deviations above and below the mean, which essentially matches the range promulgated by OSHA, ±25%. Hence, the VDD8 led to no discernible additional variability.
Amplification involves calibration with higher concentrations than one might plan to deliver to subjects. An operator may use amplification alone or with trapping. Amplification can preserve information about variability on individual trials whereas integration cannot, an inevitable loss for some materials with very low thresholds. Figure 4 shows an example of amplification and integration for ethyl n-butyrate.
As has often happened, data in the literature prompted the operator to begin at concentrations far above the threshold measured. An important handbook (Cheremisanoff 1999) listed the threshold for ethyl butyrate at 150 ppb. To accommodate that within a range of deliveries, one might use a maximum of about 2.5 ppm and minimum of 39 ppb. The spreadsheet in Figure 3 shows the range actually needed with the VDD8, a maximum of 250 ppt (0.250 ppb) and minimum of 2 ppt (0.002 ppb), that is, 4 orders of magnitude below the value suggested by the threshold in the handbook. Figure 5 shows how well individual subjects could detect these levels. The calibration curve in Figure 4 came from 0.5-μL injections of ethyl butyrate in ethanol. The validation came from 30-L vapor samples adsorbed onto Tenax-TA/Carboxen-1000/Carbosieve S-III from sampling in the cones then thermally desorbed into a GC-FID. For this, the operator amplified the delivery by 62.5-fold to a series of 125 ppt to 16 ppb. Staged measurement, as described below, generally obviates the need for amplification.
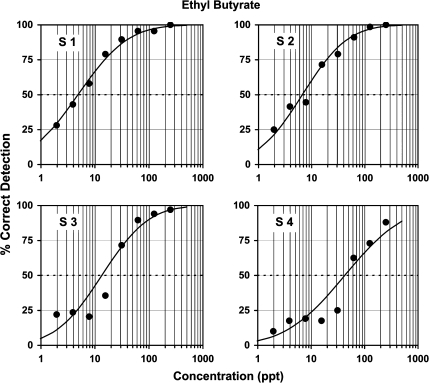
Figure 5.
Showing how well 4 young subjects (3 males and a female) detected the tutti-frutti odor of ethyl butyrate in 3-alternative forced-choice testing, with concentrations down to 2 ppt. The subjects gave informed consent to participate in a protocol approved by an Institutional Review Board of the University. Each contributed 100 judgments per point over 3 days of testing. Threshold occurred at an average of 15 ppt, 4 orders of magnitude below that listed in the Handbook of Industrial Toxicology and Hazardous Materials (Cheremisanoff 1999). For details of protocol, such as timing, see appendix (Supplementary Material).
Staged measurement refers to assessment of concentration prior to final dilution. The validation for ethyl butyrate took place before installation of sampling tees into the VDD8. With the tees available, the operator can take samples before the dilution by background air (Figures 1 and 3). In the case of D-limonene, for example, the operator could measure a quantity of 2 ppm at any line to an active cone rather than the quantities of 100 ppb down to 0.78 ppb. Samples from the stations, followed by simple calculation of dilution from the background, gave an estimate of the variation of delivered level of limonene of 12%. Samples taken from the tees have become the norm for both the level and the variability of delivery. During psychophysical testing, the operator takes a sample an hour.
If other options for validation offer insufficient concentration, the vapor capacitor can afford a check. It can at least verify concentration generated by the syringe drive.
Mixtures and masking
The cones could accommodate injection of more than one material. Two or even 3 stainless steel tubes could penetrate the stopper and mix with the background flow. The VDD8 can also deliver mixtures by the addition of VOC to the background (Figure 1). This maneuver permits the study of masking whereby subjects seek to detect presence of signal of one quality in the background of another. A unit with 4 parallel channels can provide injection of one VOC into the backgrounds of 2, of 4, of 6, or of all 8 stations or of up to 4 different VOCs distributed among the stations 2 at a time. Hence, Stations 1 and 2 can have one VOC in the background, Stations 3 and 4 another, and so on. When set up for masking, the concentration of VOC in the background would remain constant but one can trade species for level. Hence, the 4 pairs of stations could have 4 different levels of one VOC or 2 levels of 2.
To run the VDD8 in a masking mode, the operator needs to remove the carbon from the background lines. The ring compressor draws its air from the room. The air goes through a particle filter at intake. It can go through a bed of carbon as well. Fortunately, the compressor generates no perceptible contamination. It has no oil or condensate in its lines. Selective use of carbon filtration in the odor lines can actually produce a station with VOC in the background for cones 1 and 2, but not 3, and so on.
Chambers
The VDD8 can feed vapor into chambers and thereby create ambient exposures (see appendix [Supplementary Material]). Chambers can serve not only to expose subjects to vapors but also to control the environment prior to testing. Treatment of the atmosphere of a chamber with activated carbon can reduce exposure to contaminants brought into the laboratory by ventilation. If the chambers sit just a pace or 2 from the VDD8, then subjects can make the transition without exposure to unfiltered air (Cain, Schmidt, and Wolkoff 2007).
Discussion
If shown a sample of a VOC, a scientist may ask, “How does it smell?” A whiff can quickly give that answer, however subjective. The scientist may also ask, “What is its threshold?” A whiff alone cannot give that answer. Some materials with overwhelming odors when neat may have high thresholds and some with more tempered odors may have extraordinarily low thresholds. To know the answer, one must measure, a task that may seem so simple that any chemistry student could do it. A history of data collection says otherwise. Researchers have not trusted other researchers values and therefore have repeated them, generally to find their skepticism rewarded by different values. The next researcher does the same, with skepticism rewarded again. Eventually, one has a database with an error of ±1000%. When stated in this way, it might appear that studies differ because subjects differ that one group may exceed the sensitivity of another by orders of magnitude. Data obtained by the VDD8 say otherwise. They say that the sense of smell of normal people does not differ by amounts greater than the sense of hearing, about one and a half orders of magnitude. One group of subjects should not differ from another by more than a small factor. If beyond that, it should achieve statistical significance and one should ask why the difference exists. In a study of the effect of carbon filtration in the room on the threshold for D-limonene, a group of 13 subjects differed by less than 2:1 for this systematic effect. The results proved significant at P < 0.005 (Cain, Schmidt, and Wolkoff 2007). In a field where orders of magnitude have seemed like just ripples on water, a difference of just 80% might seem hopelessly inconsequential.
The VDD8 represents an actual instrument but more importantly embodies an approach to odor measurement. In a recent comparison, Cometto-Muñiz et al. (2008) found that thresholds for acetates obtained with the VDD8 lay about 200-fold below those they obtained earlier via plastic squeeze bottles (Cometto-Muñiz and Cain 1991). Table 1 shows a broader picture of how thresholds measured with the VDD8 compared with compiled values, for example, from Devos et al. (1990) or, when Devos lacks the values, others. The lessons here make no pretense of hegemony, for they cut across the field. What matters is useful archival data.
An instrument such as the VDD8 can afford flexibility in range of concentrations studied, avoidance of solvents, stability of delivery, realistic interface with subjects, accessibility of components, and accommodation of variations in psychophysical methodology. It could readily serve for measurement of differential sensitivity. It could serve for measurement of quality and for measurement of mixtures and masking. The VDD8 encourages simultaneous testing of subjects, collection of numerous responses within a day, but yet a leisurely pace. In a typical day, a subject spends 95% of the time sitting out or smelling blanks. (Total duration of exposure to VOC equals about 8–10 min for 6–7 h of testing.) Collection of numerous responses adds stability to the measurements. A threshold measured in one study should have archival value, not applicable to just a given device or methodology. It should give someone who needs to apply the value, for example, a person who needs to write a material safety data sheet (MSDS), assurance that it will withstand scrutiny and replication. Research with the VDD8 on glutaraldehyde odor led to downward revision of the odor threshold of its MSDS by more than 100-fold (Cain, Schmidt, and Jalowayski 2007). The odor of glutaraldehyde affords a much greater margin of safety than thought regarding when the material may irritate the eyes or nose.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by grant [R01 DC05602] from the U.S. National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Supplementary Material
References
- American Industrial Hygiene Association. Odor thresholds for chemicals with established occupational health standards. Akron (OH): AIHA; 1989. [Google Scholar]
- American Society for Testing and Materials. ASTM E544-99(2004) standard practices for referencing suprathreshold odor intensity. Philadelphia (PA): ASTM; 2004. [Google Scholar]
- Ballantyne B, Jordan SL. Toxicological, medical and industrial hygiene aspects of glutaraldehyde with particular reference to its biocidal use in cold sterilization procedures. J Appl Toxicol. 2001;21(2):131–151. doi: 10.1002/jat.741. [DOI] [PubMed] [Google Scholar]
- Cain WS. Olfaction. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, editors. Stevens’ handbook of experimental psychology, Vol. I: perception and motivation, rev. ed. New York: Wiley; l988. pp. 409–459. [Google Scholar]
- Cain WS, Schmidt R. Can we trust odor databases? Example of t- and n-butyl acetate. Atmos Environ. 2009;43:2591–2601. [Google Scholar]
- Cain WS, Schmidt R, Jalowayski AA. Odor and chemesthesis from exposures to glutaraldehyde vapor. Int Arch Occup Environ Health. 2007;80:721–731. doi: 10.1007/s00420-007-0185-0. [DOI] [PubMed] [Google Scholar]
- Cain WS, Schmidt R, Wolkoff P. Olfactory detection of ozone and d-limonene: reactants in indoor spaces. Indoor Air. 2007;17:337–347. doi: 10.1111/j.1600-0668.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Cheremisanoff NR, editor. Handbook of industrial toxicology and hazardous materials. New York: Dekker; 1999. [Google Scholar]
- Cometto-Muñiz JE, Abraham MH. Human olfactory detection of homologous n-alcohols measured via concentration-response functions. Pharmacol Biochem Behav. 2008;89(3):279–291. doi: 10.1016/j.pbb.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Abraham MH. Olfactory detectability of homologous n-alkylbenzenes as reflected by concentration-detection functions in humans. Neuroscience. 2009a;161(1):236–248. doi: 10.1016/j.neuroscience.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Abraham MH. Olfactory psychometric functions for homologous 2-ketones. Behav Brain Res. 2009b;201(1):207–215. doi: 10.1016/j.bbr.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS. Nasal pungency, odor, and eye irritation thresholds for homologous acetates. Pharmacol Biochem Behav. 1991;39(4):983–989. doi: 10.1016/0091-3057(91)90063-8. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chem Senses. 2003;28(6):467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Gil-Lostes J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol Behav. 2008;95:658–667. doi: 10.1016/j.physbeh.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos M, Patte F, Rouault J, Laffort P, van Gemert LJ. Standardized human olfactory thresholds. Oxford: IRL Press at Oxford University Press; 1990. [Google Scholar]
- Dravnieks A. A building-block model for the characterization of odorant molecules and their odors. Ann NY Acad Sci. 1974;237:144–163. doi: 10.1111/j.1749-6632.1974.tb49851.x. [DOI] [PubMed] [Google Scholar]
- Dravnieks A. Instrumental aspects of olfactometry. In: Moulton DG, Turk A, Johnston JW, editors. Methods in olfactory research. London: Academic Press; 1975. pp. 1–61. [Google Scholar]
- Dravnieks A, Prokop WH. Source emission odor measurement by a dynamic forced-choice triangle olfactometer. J Air Pollut Control Assoc. 1975;25:28–35. doi: 10.1080/00022470.1975.10470045. [DOI] [PubMed] [Google Scholar]
- Engen T. The effect of expectation on judgments of odor. Acta Psychol. 1972;36:450–458. doi: 10.1016/0001-6918(72)90025-x. [DOI] [PubMed] [Google Scholar]
- Gunnarsen L, Nielsen PA, Wolkoff P. Design and characterization of the CLIMPAQ, chamber for laboratory investigations of materials, pollution and air quality. Indoor Air. 1994;4:56–62. [Google Scholar]
- Johnson BN, Sobel N. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods. 2007;160(2):231–245. doi: 10.1016/j.jneumeth.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Laing DG. Characterization of human behaviour during odour perception. Perception. 1982;11:221–230. doi: 10.1068/p110221. [DOI] [PubMed] [Google Scholar]
- Laing DG. Natural sniffing gives optimum odour perception for humans. Perception. 1983;12:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration. Glutaraldehyde. In: Cassinelli ME, O'Connor PF, editors. Second supplement to NIOSH manual of analytical methods (NMAM) Cincinnati (OH): U.S. National Institute for Occupational Safety and Health; 1998. [Internet]. Available from: URL http://www.osha.gov/dts/sltc/methods/organic/org064/org064.html. [Google Scholar]
- Turk A, Lipták BG, Durden WP. Odor detection. In: Lipták BG, editor. Instrument engineers’ handbook, 4th ed. Vol. 1, process measurement and analysis. Boca Raton (FL): CRC Press; 2003. pp. 1480–1485. [Google Scholar]
- van Gemert LJ. Odour thresholds. Compilations of odour threshold values in air, water and other media. Utrecht (The Netherlands): Oliemans Punter & Partners BV; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.