Abstract
It is easier to detect mixtures of gustatory and olfactory flavorants than to detect either component alone. But does the detection of mixtures exceed the level predicted by probability summation, assuming independent detection of each component? To answer this question, we measured simple response times (RTs) to detect brief pulses of one of 3 flavorants (sucrose [gustatory], citral [olfactory], sucrose–citral mixture) or water, presented into the mouth by a computer-operated, automated flow system. Subjects were instructed to press a button as soon as they detected any of the 3 nonwater stimuli. Responses to the mixtures were faster (RTs smaller) than predicted by a model of probability summation of independently detected signals, suggesting positive coactivation (integration) of gustation and retronasal olfaction in flavor perception. Evidence for integration appeared mainly in the fastest 60% of the responses, indicating that integration arises relatively early in flavor processing. Results were similar when the 3 possible flavorants, and water, were interleaved within the same session (experimental condition), and when each flavorant was interleaved with water only (control conditions). This outcome suggests that subjects did not attend selectively to one flavor component or the other in the experimental condition and further supports the conclusion that (late) decisional or attentional strategies do not exert a large influence on the gustatory–olfactory flavor integration.
Keywords: flavor, gustation, multisensory integration, olfaction, response time
Introduction
Although the flavor of foods and beverages is typically perceived as a unitary perceptual experience, flavor perception reflects inputs from multiple sensory systems. Inputs to flavor come from gustation (through stimulation of receptors on the tongue and in the mouth, which produces sweet, sour, salty, bitter, and savory sensations), olfaction (through stimulation of receptors in the olfactory mucosa, when air-borne particles work their way from the mouth through the nasopharynx), and oral somatosensation (through stimulation of diverse receptors in the oral cavity, providing information about texture, temperature, pungency, and spiciness). Even though they derive from signals transmitted over several cranial nerves, flavors often appear remarkably coherent in phenomenal perception. The present study is part of a larger program concerned with the ways that gustatory and olfactory components of flavors combine in detecting weak flavorants (at threshold levels) and in perceiving intensity and responding rapidly to stronger flavorants (at suprathreshold levels).
Perhaps the first question to ask is whether, or when, gustatory and olfactory flavor signals combine at all. Evidence that the threshold for detecting a mixture of weak flavorants, such as the gustatory stimulus sucrose and the olfactory stimulus vanillin, is lower than the threshold for detecting either sucrose or vanillin presented alone does not necessarily mean that the gustatory and olfactory signals interact or even combine. The mixture could have a lower threshold than either single component because of probability summation. Given a mixture containing 2 components, a perceiver essentially has 2 chances to detect a flavorant, even if detection of each component is independent of detection of the other.
Marks et al. (2007) and Delwiche and Heffelfinger (2005) reported summation in the detection of gustatory–olfactory mixtures (sucrose and vanillin by Marks et al. and aspartame–acesulfame potassium mixture and pineapple extract by Delwiche and Heffelfinger). Both sets of investigators concluded that summation exceeded the amount predicted by probability summation. In a related study, Dalton et al. (2000) found that the threshold to the olfactory stimulus benzaldehyde decreased significantly when the subject tasted at the same time the congruent gustatory stimulus saccharin. In that study, however, the saccharin was presented as a flavorant and the olfactory stimulus was presented as an odorant to the nose. An important question, although one ancillary to the present study, is whether gustatory and olfactory signals combine and interact differently when the olfactory stimulus is presented retronasally, as part of a single flavor, and when it is presented orthonasally, as an odor. Pfeiffer et al. (2005) replicated the results of Dalton et al. but found a nonsignificant decrease in threshold when the olfactory and gustatory stimuli were both delivered as flavorants to the mouth.
In the study by Marks et al. (2007), flavor detection was measured in 2 different conditions: In one condition, each flavorant (gustatory, olfactory, and gustatory–olfactory) was presented in a separate session; in the other condition, all 3 flavorants were interleaved within the same session. Taken together, the results were consistent with predictions of a simple model of gustatory–olfactory summation, namely a model of “additive independent channels.” This model assumes 1) that gustatory and olfactory signals for intensity are stochastically independent—that neither is affected by the presence or level of the other, 2) that the magnitudes of the gustatory and olfactory signals combine additively, and 3) following the tenets of signal detection theory, that the detectability of each flavorant depends on the magnitude of the intensity signal relative to the noise in the system (the magnitude of noise that controls detection of unmixed components is smaller when each component is tested separately rather than interleaved with other flavorants within the session, see Marks et al. 2007).
The results of the threshold studies of Delwiche and Heffelfinger (2005) and, especially, of Marks et al. (2007) are consistent with the hypothesis that intensity signals from gustatory and olfactory components of flavors add linearly. Also consistent with linear addition are results of studies on intensity perception of suprathreshold gustatory–olfactory flavorants (e.g., Murphy et al. 1977; Murphy and Cain 1980; McBride 1993).
If intensity signals in the gustatory and olfactory systems are stochastically independent, then one would expect the perceived intensity of either component of a gustatory–olfactory flavorant to be unaffected by the presence or magnitude of the other component. Interactions between components—enhancement or suppression of one component by the other—provide evidence against stochastic independence but does not specify where in flavor processing independence fails. Murphy et al. (1977) found enhancement in mixtures of sucrose and citral, but they also found linear additivity of perceived intensity. Analogously, gustatory flavorants and olfactory stimuli presented as odors not only show summation (Frank et al. 1991; Schifferstein and Verlegh 1996; van der Klaauw and Frank 1996) but also show interactions between the components; these interactions, however, may be reduced or eliminated by changes in selective attention (Frank et al. 1993; Clark and Lawless 1994; van der Klaauw and Frank 1996).
With ratings of suprathreshold stimuli, it can be difficult to determine whether and when interactions arise at a relatively early, sensory level or at a later, decisional level. Although it is clear that information from different sensory systems, taste and olfaction, is integrated in flavor perception, nevertheless, ratings of intensity provide only limited information about the integration process and are especially susceptible to cognitive and decisional processes. Furthermore, evidence that interactions can accompany linear intensity summation and can be influenced by attention suggests that the interactions may reflect high-level decisional or cognitive processes rather than lower level sensory ones. Finally, when neural responses are inferred from functional magnetic resonance imaging, the responses show supra-additive summation in mixtures of congruent olfactory and gustatory flavorants, compared with the responses measured to the components presented separately (De Araujo et al. 2003; Small et al. 2004). These neural responses have been observed in secondary multisensory, functionally diverse areas. The poor temporal resolution of the neural signal as measured by functional magnetic resonance imaging, however, precludes making strong inferences about the processing stage at which taste and olfaction may interact.
We propose that multisensory interactions between gustatory and olfactory signals take place at early stages of processing of flavors, that is, prior to the decisional or cognitive processes that affect overt ratings. To reveal this process of early integration, the present study capitalizes on measures of simple response time (RT). By asking subjects to respond as quickly as possible to gustatory, olfactory, and combined gustatory–olfactory flavorants, the resulting RTs make it possible to determine whether, for example, the magnitude of the integration of signals exceeds the prediction made by a model that assumes stochastic independence without summation.
In the present study, subjects were instructed to press a button when they detected any flavor and to withhold responding if no flavor was perceived. Four different stimuli were presented: a gustatory stimulus (sucrose), an olfactory stimulus (citral), a gustatory–olfactory stimulus (sucrose–citral mixture), and water. The design was adapted for present purposes from a paradigm developed by Miller (1982, 1991) to test critically for evidence of coactivation (as opposed to independent activation) of signals in 2 processing channels. Originally developed to investigate coactivation of auditory and visual signals, to the best of our knowledge, this powerful paradigm has never been applied to the chemical senses. Basically, the model of stochastically independent activation, with no summation, assumes that when a stimulus containing 2 components is presented, the subject responds as soon as the neural signal produced by either component (in either processing channel) reaches the level needed to trigger the response. Assuming that the time needed to reach the triggering level varies randomly from trial-to-trial in each channel, with the variability being uncorrelated in the 2 channels (stochastic independence), RTs to the mixture will be smaller than RTs to either component presented separately. That is, RTs will show probability summation.
The model of probability summation in RT provides a yardstick against which we measure performance in the present task. We hypothesize that the flavor system integrates signals from gustation and olfaction, and, as a result, the time needed to respond to a gustatory–olfactory flavorant should not only be smaller than the time needed to respond to either component alone but also should be smaller than the time predicted by a statistical model of (stochastically) independent activations. We shall follow the convention and use the term coactivation to refer to this process of integration, here, gustatory–olfactory integration. Further, if the coactivation arises before attentional processes or decisional strategies, then RTs measured to gustatory and olfactory flavorants presented separately should be the same under different conditions of selective attention. To test this second prediction, we also measured RTs in blocks of control trials containing either water or just 1 of the 3 possible target flavorants (gustatory alone, olfactory alone, mixture), a condition that allows the subjects to attend to a single flavorant within each block of trials.
Materials and methods
Subjects
Fourteen subjects, aged 18–33 years (mean 26.14 ± standard deviation [SD] 4.16) years, were paid to participate. In all, 7 of the subjects participated in condition A (ages 18–33 years, mean = 27.42 ± 5.25) and the other 7 in condition B (ages 23–30 years, mean = 24.85 ± 2.47). As described below, conditions A and B differed only in the test stimuli used in the initial baseline measurements that preceded the main experiment. Most of the subjects were students at Yale University. All gave informed consent per protocols approved by Yale University's Human Subjects Committee. All subjects were nonsmokers who reported no taste impairments. Each subject was instructed not to eat or drink anything except water for 1 h prior to the experiment.
Stimulus selection procedure
The gustatory stimulus was sucrose (J.T. Baker, CAS#57-50-1 C12H22O11) dissolved in deionized water. The olfactory stimulus was citral (International Flavors and Fragrances, CAS# 5392-40-5, chemical characterization: 3,7-dimethyl-2,6 octadienal, a mixture of cis- and trans-isomers). For the citral solutions, we first created a stock solution of 3% citral dissolved in ethyl alcohol (ethanol, 200 Proof Ethyl Alcohol, CAS# 64-17-5). This stock solution was then diluted to 0.015%, 0.02%, and 0.03% citral (0.485%, 0.647%, and 0.97% ethanol) in deionized water. These concentrations are similar to those used by Cerf-Ducastel and Murphy (2001, 2004). Both the ethanol and citral concentrations used here are below trigeminal and taste thresholds (Wilson et al. 1973; Cometto-Muniz and Cain 1990; Cerf-Ducastel and Murphy 2001).
Sucrose and citral were chosen because they are generally perceived to be congruent and/or harmonious in combination. Congruence is generally defined as “the extent to which 2 stimuli are appropriate for combination in a food product” (Schifferstein and Verlegh 1996). Presumably, congruence arises because most people have experienced citrus flavorants together with a sweet taste, for example, in fruits, soda, and lemonade. The congruence of citral and sucrose percepts is consistent with the observation that their mixture is perceived as pleasant (Cerf-Ducastel and Murphy 2004).
Pilot testing (results not reported) showed that, for most subjects, RTs to citral were longer than RTs to sucrose. Because the present paradigm requires the RT distributions of the individual components to overlap at least partly (the greater the overlap, the greater the power of the computational analysis), we tailored concentrations of sucrose and citral to each subject individually, based on preliminary, baseline measurements, as described below. All stimuli were made fresh every day or 2 and refrigerated until being warmed to room temperature (23.5 °C) before testing.
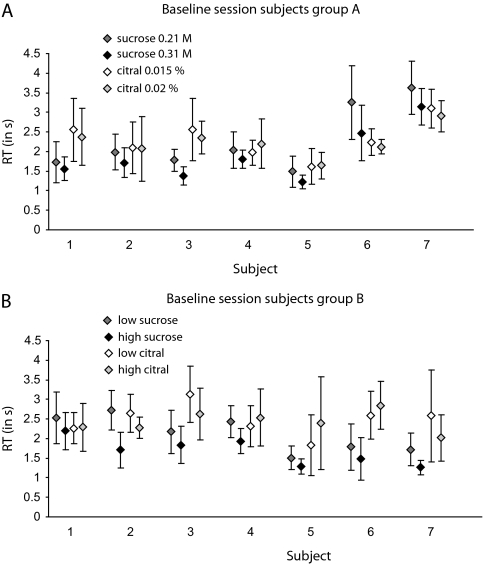
The study divided the subjects into 2 groups of 7 each, with one group assigned to condition A and the other to condition B. The 2 conditions differed only in the test stimuli that were used in the initial baseline measurements that preceded the main experiment. The procedure used in the main experiment was the same in both conditions. In both sets of subjects, the baseline test measured simple RTs to 2 concentrations each of citral and sucrose, within a single session containing 2 blocks of 60 trials each. We discarded the first 6 trials of each block, treating those trials as practice.
In the baseline of condition A (7 subjects), we measured simple RTs to 4 stimuli: 0.21 M sucrose, 0.31 M sucrose, 0.015% citral, and 0.02% citral. Deionized water was also presented as a stimulus, but subjects were instructed not to respond to water, only to a flavorant. Deionized water was used as a nontarget stimulus in all the experimental conditions, baseline, and main, to help ensure that the subject would not simply respond to the presentation of any solution in the mouth. Sucrose, citral, and water were each presented on approximately one-third of the trials in each block—that is, each concentration of sucrose and citral was presented on approximately one-sixth of the trials. The different stimuli were randomly interleaved within each session. At the end of the baseline test, a concentration of sucrose and a concentration of citral that produced the greatest overlap in RT distributions were selected for each subject and used in the main experiment.
The baseline of condition B (7 subjects) began, in the first block of 60 trials, with the same 4 stimuli used in the baseline measurements of condition A. For some of the subjects, however, the RT distributions for sucrose fell well below those for citral. Consequently, in the second block of 60 trials, we dropped the lower concentration of citral (0.015%) and added a higher concentration (0.03%). Note that responses generally become faster (RTs become smaller) with increasing stimulus strength, as reported for gustatory stimuli (Bonnet et al. 1999) and flavor stimuli (Veldhuizen et al. 2005)—an observation that we kept in mind as we sought to produce overlapping RT distributions to sucrose and citral by adjusting the stimulus concentrations. Even so, the resulting sucrose and citral distributions did not overlap fully for all subjects, with RTs to sucrose generally being smaller than RTs to citral (Figure 1B).
Figure 1.
Distribution of RTs (averaged over trials ± SD) to the single-component flavorants per subject in the baseline measurement session for condition A (panel A) and condition B (panel B).
We were somewhat constrained, however, in our attempts to match the RT distributions. First, slowing down the responses to sucrose by setting sucrose concentration too low caused the detection rates to fall below 90%. Second, speeding up the responses to citral by setting citral concentration too high caused the ethanol diluent to become detectable. These constraints made it impossible, for some subjects, to find concentrations of sucrose and citral that produced wholly comparable RTs. As a result, the distributions of RTs overlapped only in part, thereby limiting the sensitivity of the present paradigm. Fortunately, this did not have serious consequences for the results.
For the main experiment, we selected those concentrations of sucrose and citral for which: 1) the RT distributions overlapped fully or at least partially if we could not achieve full overlap, and 2) correct detection rates were above 90% (see Figure 1A and Table 1). The resulting concentrations for all subjects appear in Table 1.
Table 1.
Resulting nominal concentration of citral and sucrose for each subject after the baseline session
| Subject No. | Sucrose (M) | Citral in % |
| A1 | 0.021 | 0.02 |
| A2 | 0.021 | 0.02 |
| A3 | 0.021 | 0.02 |
| A4 | 0.021 | 0.02 |
| A5 | 0.021 | 0.02 |
| A6 | 0.031 | 0.015 |
| A7 | 0.031 | 0.015 |
| B1 | 0.049 | 0.03 |
| B2 | 0.031 | 0.02 |
| B3 | 0.031 | 0.03 |
| B4 | 0.031 | 0.03 |
| B5 | 0.031 | 0.03 |
| B6 | 0.021 | 0.03 |
| B7 | 0.031 | 0.03 |
Equipment
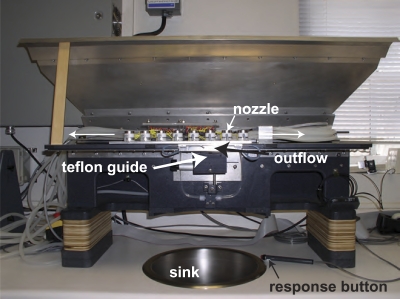
Temporally Automated System for Taste Experiments (TASTE) is a computer-operated, automated flow system that provides precise temporal control over a large number of possible stimuli (Ashkenazi et al. 2004, see Figure 2). The original system contained 16 lines, each terminating in a polyacetal misting nozzle. The system used here was modified to use up to 8 lines, fitted with titanium streaming nozzles. The use of titanium nozzles, together with shorter lengths of tubing, provides greater uniformity and consistency than did the original misting nozzles. Importantly, the streaming nozzles allow the system to operate at low pressure (1 psi), rather than the high pressure required by the misting nozzles (40 psi). Each solution was presented via its own line and nozzle, keeping it isolated from all the others. Because each line and nozzle was dedicated to a specific flavorant, rapid switching was possible without cross-contamination. Isolation also prevented the subjects from sniffing any of the stimuli before stimulus onset.
Figure 2.
The TASTE apparatus, showing its workings (hidden from the subjects). For a detailed description, see Ashkenazi et al. (2004). The hidden workings include a sliding nozzle array that allows for rapid switching between stimuli. Because each line and each nozzle was dedicated to a specific flavorant, cross-contamination and sniffing of the stimuli before stimulus onset was precluded. The subjects readied themselves for stimulus presentation by extending the tongue to the Teflon guide under the outflow point of the preselected nozzle. After stimulus presentation, the subject indicated, as quickly as possible, if a target stimulus was present (sucrose, citral or, sucrose–citral mixture) by pressing the response button. Between trials, subjects rinsed with deionized water and expectorated into the sink. This figure appears in color in the online version of Chemical Senses.
Calibrations were performed with a Thru-beam standard fiber set that was attached to a fiber-optic Thru-beam sensor, a National Instruments A/D card, and a data acquisition program (MATLAB). The Thru-beam sensor was placed as close as possible to the nozzles during calibration. Calibrations indicated small temporal variability in activating the nozzles (SDs around 2 ms). Solutions were stored in 2-L containers, housed inside canisters, and were carried via silicon tubing to the nozzles. The silicon tubing, coupled with a wider gauge of tubing, provided less resistance than the rigid Teflon used in the old system, allowing less surface area to come into contact with the solution during delivery. The 8 titanium streaming nozzles were attached to a linear slide of the device. The slide could move sideways, placing the preselected nozzle above the subject's mouth. Each concentration and stimulus had its designated line for the entire experiment. At the start of the experiment, the line (from the canister to the nozzle) was filled with the solution and remained full throughout the experiment. The tubes were flushed with deionized water at the end of each day. Delivery of the stimuli was controlled by MATLAB software, which operated the 2-way isolation valves, positioned the nozzle on the linear slide, determined the duration of each stimulus, and recorded the subject's responses.
It was not practical to change the tubing after each experimental session, so the tubing remained constant throughout the study. Consequently, it is possible that some of the flavorants sorbed onto the surface of the silicon tubes over the course of the study as well as over the course of individual test sessions and then desorbed into the stimuli. We did not attempt to measure any changes in stimulus concentration over time. Hence the values of concentration given in Table 1 should be considered nominal. We did, however, counterbalance the order of blocks and conditions over subjects and sessions, so any effects of sorption and desorption should average out across the whole experiment. Nevertheless, as described below, we made additional measurements at the end of the main study to determine whether changes in concentration resulting from sorbed and desorbed solute might have had any functional consequence to the study.
At the end of the main study, it was evident that citral did linger as deionized water run through a line used to present citral in the study had a weak but detectable citral flavor. By way of comparison, water run through a line used to present sucrose was not detectable. To assess the likelihood that the residual citral might have had a functional consequence in the main study, 4 of the subjects (23–33 years old, mean = 28 ± 5.77) from the main study (2 who originally participated in condition A and 2 who participated in condition B) returned for a follow-up experiment, which aimed to determine whether subjects could reliably and quickly detect residual citral presented by itself. For this follow-up study, we measured RTs (as in the main experiments) to 4 possible stimuli: 1) 0.03% citral (the same concentration presented to the 4 subjects in the main experiment), 2) 0.003% citral, 3) water presented through a line with residual citral, and 4) water presented through a line used only for water. The subjects were again paid to participate in this follow-up study and gave informed consent per protocols approved by Yale University's Human Subjects Committee. Each subject was instructed not to eat or drink anything except water for 1 h prior to the experiment.
One hour prior to each session, we ran 2 blocks of 60 pseudotrials each on the TASTE system to simulate the buildup of residue in the silicon tubing in the main experiment. To do this, we ran 1) 0.03% citral through the original citral line, 2) 0.03% citral through the original 0.03% citral + sucrose mixture line, 3) 0.003% citral through the original sucrose line, and 4) deionized water through the original water line. Stimulus parameters were otherwise identical to those of the main experiment (although no subject was present, the solution on each trial simply flowing into the sink). For these 60 pseudotrials, each of the 4 possible stimuli was run through the indicated line a total of 30 times (corresponding to the number of real trials per line in the main experiment), after which we flushed each line with deionized water at a high pressure (5 psi) for 220 s to clean the valves and silicon tubing (as we did at the end of each day during the main experiment). Much of the sorption, however, likely takes place at the start of each test session, once the lines are filled with solution.
During the follow-up experiment proper, each subject was presented with 2 blocks of 60 trials, each block containing a randomized sequence of 15 replicates of each of the 4 possible stimuli: 0.03% citral through the original citral line, 0.003% citral through the original sucrose line, deionized water through the line that had 0.03% citral in the pretest (originally, 0.03% citral + sucrose), and deionized water through the original water line. Each stimulus was presented a total of 30 times over the course of the session. Again, the subjects were instructed to respond as quickly as possible to any flavorant but not to water. Details of presentation and response were identical to those of the main experiment.
For each stimulus, we calculated, separately for each subject, the median RT and the proportion of presentations detected (proportion of responses made within the 5 s allotted to each trial). These values are listed in the Table 1 in the Supplementary Data. The results are informative: 2 of the 4 subjects (2 and 3) failed to detect residual citral in water within the allotted 5 s on more than half of the trials, and they failed to detect 0.003% citral on more than half of the trials—median RTs in these instances equaled 5 s and proportions of detection fell below 0.5. Subject 2 also did not respond on most trials to water, but subject 3 actually responded to pure water more readily than to either residual citral or 0.003% citral. Subject 1 did respond more rapidly to residual citral than to water, being the only subject of the 4 to do so, although subject 1, like subject 4, did detect the residual citral more often than water. Note that none of the 4 subjects responded more quickly to residual citral than to 0.003% citral (2 of the subjects had identical nominal RTs of 5 s, not detecting either stimulus on more than half of the trials).
To provide a global picture of the results, for each of the 4 stimuli, we also pooled RTs across subjects, then ranked, and cumulated the RTs to produce a cumulative (probability) density function (CDF) as shown in Figure 1 in the Supplementary Data. The figure shows considerable overlap between the CDFs for residual citral (water in the citral line) and deionized water and some overlap between these CDFs and the CDF for 0.003% citral. Importantly, the functional effectiveness of residual citral is undoubtedly greater when presented in water alone than it would be when added to 0.03% citral. Given that subjects perform poorly when trying to detect water containing desorbing citral, these results imply that the residual citral probably had little functional consequence in the main experiment.
Procedure
Each session began with a rinse of deionized water. At the beginning of each trial, the prompt “Ready” was displayed on the computer monitor, cueing the subject to place her or his nose against the gauze pad, place her or his tongue onto the Teflon guide located underneath the metal casing of the apparatus, and place her or his right thumb over the handheld response button in preparation for the next trial. After 3–4 s (randomized), the system released 0.5 ml of solution (1 ml/s with duration of 0.5 s) onto the tip of the subject's tongue. The subject pulled her/his tongue back into the mouth and responded, by pressing the trigger as quickly as possible, if a target stimulus was present (sucrose, citral, or sucrose–citral mixture). Subjects were instructed to refrain from pushing the button if there was no flavor (water presented). Thus, in the present design, subjects should not respond on a substantial fraction (25%) of trials. Because of this, we set a 5-s limit, from stimulus onset, on the time within which the subject had to make each response. Because the volume of solution presented was so small, the subjects were permitted to swallow the solution or expectorate into the sink after responding. After the subject responded (or after 5 s had elapsed and the subject did not respond), the word “Rinse” appeared on the computer monitor. This cued the subject to sip from a glass of deionized water, swirl the water in her or his mouth, and then expectorate the rinse into the sink. The next trial began after 30 s and the procedure was repeated. After a few practice trials, this task became “automatic.”
Every subject participated in 6 experimental sessions, each of which lasted about 1.25 h. In each session, the subject ran in 2 conditions (experimental and control). In the experimental condition, trials presenting each of the 3 flavorants and water were randomly interleaved during the session. Each stimulus was presented 15 times within the 60-trial block. Control conditions consisted of 3 separate blocks, each block containing one of the 3 possible target stimuli, randomly interleaved with only the nontarget water. Observers made speeded responses to the flavorants and were instructed to ignore the water. Twenty trials (15 target and 5 nontarget) were included in each block in the control condition. The order of the conditions was counterbalanced so that the experimental condition was run before the control condition every other session. The blocks in the control condition were run separately from the experimental condition, and the order of the blocks was counterbalanced across observers.
Data analysis
For each stimulus (sucrose, citral, sucrose–citral mixture) and for each of the 14 subjects, the RTs were sorted in ascending order and divided into 20 bins, each containing 5% of the RTs, in order to create a CDF. It is then possible to use the distributions of RTs to the individual components to predict the CDF of RTs to the mixture under the null hypothesis (H0) of independent activation and probability summation. If we assume stochastically independent activations, then the predicted RT to the mixture equals the smaller of the RTs to the 2 separate components. To construct this CDF, we took (for each subject) the value of the RT in each bin of sucrose and paired it with the value of the RT in every bin of citral, making 400 pairs of RTs in all. From each pair, we then selected the faster RT, thereby simulating the prediction from a model of stochastic independence. The resulting predicted RTs were then ranked and cumulated to produce the predicted CDF, which will be referred to as RTsumSC. This predicted RT distribution serves as the yardstick (H0) to compare to the CDF of observed RTs to the sucrose–citral mixture, referred to as RTinterleavedSC. The CDFs of RTs in the control conditions were analyzed the same way. The CDFs of observed RTs for the sucrose, citral, and sucrose–citral mixture trials obtained in the interleaved conditions will be referred to as RTinterleavedS, RTinterleavedC, and RTinterleavedSC, respectively, and the CDFs obtained in the control conditions (during which the each flavorant was presented, with water, in a separate block of trials) as RTcontrolS, RTcontrolC, and RTcontrolSC.
Results
Response times to flavor mixture
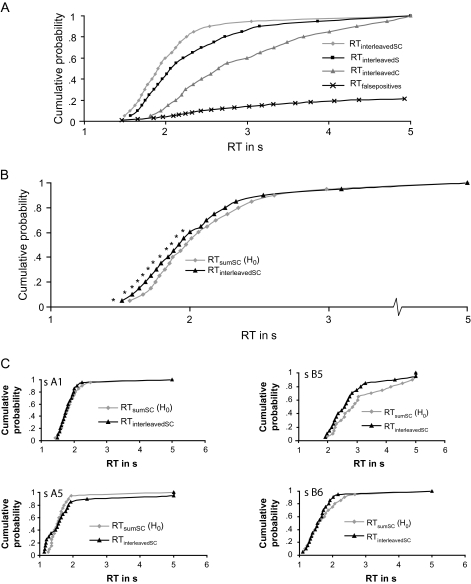
Figure 3A depicts the CDFs for the 4 different stimuli presented in the interleaved condition: mixture (RTinterleavedSC), sucrose (RTinterleavedS), citral (RTinterleavedC), and water (RTfalsepositives), the last constituting false-positive responses to the nontarget. Note that the distributions of sucrose and citral do not fully overlap. Despite the lack of overlap, RTs to the mixture are clearly smaller (responses faster) than RTs to either component on its own. If the flavor system integrates signals from gustation and olfaction, then the time needed to respond to the citral–sucrose mixture should be smaller than the time predicted by a statistical model of independent activation. As can be seen in Figure 3B, RTs (RTinterleavedSC) to the sucrose–citral mixtures are smaller across all bins in the CDF compared with the predicted yardstick response time distribution (RTsumSC), with the exception of the last 2 bins (RTs > 3 s). Figure 3C gives examples of CDFs obtained from 4 individual subjects, illustrating the large interindividual variation. Some subjects do not show shorter RTinterleavedSC than RTsumSC (subject A1), whereas others show shorter RTs across all bins (subject B5) and others only in the later bins (subject B6). For some subjects, the effect reverses, so that the first few bins show RTinterleavedSC < RTsumSC, whereas later ones show RTinterleavedSC > RTsumSC (subject A5).
Figure 3.
Cumulative probability density functions for the observed sucrose–citral mixtures (gray diamonds), the sucrose stimulus (black squares), and the citral stimulus (gray triangles) averaged over subjects (in panel A). In panel A, we also plotted the pooled (across subjects) false-positive responses to the water stimulus (black crosses). In panel B, we reproduced the observed sucrose–citral mixtures (black triangles) and added the predicted model of independent activation and probability summation (gray diamonds) averaged over subjects. Panel C gives a sample of data of individual subjects (averaged over trials) CDFs. Significant differences (at α = 0.05) are indicated with an asterisk.
Given these large variations, we compared subjects more directly by standardizing (z-transforming) all the binned RTs for each subject. The averages and SDs of the standardized RTs across subjects are given in Table 2. We performed a paired t-test (2 tailed, α = 0.05) in each bin of the CDF to compare RTsumSC to RTinterleavedSC. Out of 19 tests performed, we observed significantly shorter RTinterleavedSC than RTsumSC in the first 12 bins, these bins accounting for the 60% shortest RTs (RTs < 2.1 s, see Table 2 and Figure 3). Because we performed 19 t-tests, we should expect some false rejections of the null hypothesis due to inflation of alpha. A family-wise correction of the P values (e.g., Bonferroni correction) would be too conservative because the observations in different bins of the CDF are not independent. False discovery rate methods offer a more powerful solution to the problem of multiple comparisons (Benjamini et al. 2001). Consequently, we adjusted P values for the expected number of false H0 rejections based on 19 multiple comparisons, and we observed significantly faster RTinterleavedSC compared with RTsumSC in the first 8 bins, accounting for the 40% shortest RTs (RTs < 1.9 s, see Table 3).
Table 2.
Average standardized RT and SD over subjects for the citral and sucrose mixtures versus the summed single components of citral and sucrose
| Bin | MsumCS | SDsumCS | MmixCS | SDmixCS | P value |
| 0.05 | −0.9275 | 0.1581 | −0.9874 | 0.1743 | 0.004* |
| 0.1 | −0.8244 | 0.1187 | −0.9127 | 0.1685 | 0.004* |
| 0.15 | −0.7653 | 0.1234 | −0.8570 | 0.1592 | 0.004* |
| 0.2 | −0.7304 | 0.1168 | −0.8100 | 0.1446 | 0.007* |
| 0.25 | −0.7034 | 0.1136 | −0.7723 | 0.1361 | 0.007* |
| 0.3 | −0.6664 | 0.0993 | −0.7313 | 0.1281 | 0.007* |
| 0.35 | −0.6323 | 0.0918 | −0.6962 | 0.1216 | 0.008* |
| 0.4 | −0.6060 | 0.0865 | −0.6445 | 0.1181 | 0.016* |
| 0.45 | −0.5480 | 0.0829 | −0.6027 | 0.1085 | 0.030 |
| 0.5 | −0.5132 | 0.0798 | −0.5655 | 0.1065 | 0.032 |
| 0.55 | −0.4717 | 0.0891 | −0.5261 | 0.1034 | 0.035 |
| 0.6 | −0.4181 | 0.0929 | −0.4834 | 0.1170 | 0.035 |
| 0.65 | −0.3560 | 0.0979 | −0.4003 | 0.1228 | 0.052 |
| 0.7 | −0.2945 | 0.1498 | −0.3569 | 0.1287 | 0.074 |
| 0.75 | −0.2093 | 0.2139 | −0.3031 | 0.1268 | 0.079 |
| 0.8 | −0.1317 | 0.2646 | −0.2213 | 0.1410 | 0.094 |
| 0.85 | −0.0320 | 0.3391 | −0.1411 | 0.1770 | 0.101 |
| 0.9 | 0.1433 | 0.4522 | 0.0560 | 0.3736 | 0.181 |
| 0.95 | 0.5548 | 0.8133 | 0.5893 | 1.0322 | 0.450 |
| 1.0 | 2.5952 | 0.5196 | 2.5952 | 0.5196 | — |
Bold font indicates significance at α = 0.05.
Significant with false discovery rate–corrected P value.
Table 3.
Average standardized RT and SD over subjects for the interleaved condition versus the control conditions for the sucrose solution
| Bin | MinterleavedS | SDinterleavedS | McontrolS | SDcontrolS | P value |
| 0.05 | −0.9087 | 0.1626 | −0.9472 | 0.1853 | 0.311 |
| 0.1 | −0.8175 | 0.1235 | −0.8561 | 0.1515 | 0.303 |
| 0.15 | −0.7604 | 0.1270 | −0.7759 | 0.1683 | 0.722 |
| 0.2 | −0.7188 | 0.1266 | −0.7237 | 0.1667 | 0.904 |
| 0.25 | −0.6739 | 0.1398 | −0.6668 | 0.1544 | 0.865 |
| 0.3 | −0.6117 | 0.1409 | −0.6126 | 0.1457 | 0.971 |
| 0.35 | −0.5592 | 0.1491 | −0.5578 | 0.1391 | 0.954 |
| 0.4 | −0.5117 | 0.1598 | −0.5173 | 0.1387 | 0.814 |
| 0.45 | −0.4549 | 0.1720 | −0.4829 | 0.1339 | 0.296 |
| 0.5 | −0.4078 | 0.1849 | −0.4338 | 0.1406 | 0.386 |
| 0.55 | −0.3455 | 0.1956 | −0.3786 | 0.1479 | 0.275 |
| 0.6 | −0.2599 | 0.2268 | −0.3196 | 0.1476 | 0.122 |
| 0.65 | −0.1451 | 0.3297 | −0.2762 | 0.1549 | 0.040 |
| 0.7 | −0.0377 | 0.4311 | −0.2136 | 0.1677 | 0.040 |
| 0.75 | 0.1172 | 0.5374 | −0.1372 | 0.2125 | 0.027 |
| 0.8 | 0.2567 | 0.6433 | −0.0123 | 0.3141 | 0.053 |
| 0.85 | 0.4778 | 0.8095 | 0.1360 | 0.4068 | 0.034 |
| 0.9 | 0.7558 | 0.8042 | 0.4643 | 0.8127 | 0.005 |
| 0.95 | 1.6007 | 1.1108 | 0.9745 | 1.0213 | 0.006 |
| 1.0 | 2.5952 | 0.5196 | 2.5952 | 0.5196 | — |
Bold font indicates significance at α = 0.05.
Decisional strategies—experimental versus control conditions
Besides predicting coactivation, we predicted that there would be no difference between the distributions of RTs to sucrose, citral, or the sucrose–citral mixture in the fully interleaved experimental conditions and in the blocked control conditions. This prediction derived from the hypothesis that attentional processes and decisional strategies do not affect the coactivation of gustatory and olfactory flavor signals. Consequently, the distributions of RTs should not be affected by subjects selectively directing their attention to just one of the 3 possible target stimuli rather than attending to all of them.
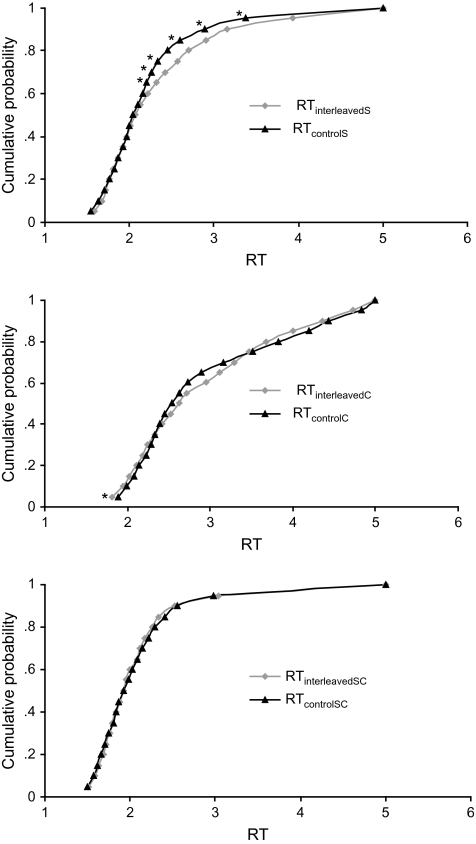
Figure 4 depicts the CDF of each target stimulus in the interleaved and control conditions. As can be seen in Figure 4 and in Table 3, subjects did respond slightly faster to sucrose in the RTcontrolS condition compared with the RTinterleavedS condition but only so in later bins of the CDF (where the RTs were longer than 2.3 s). This suggests that attention did slightly affect speed of response to sucrose but only on those trials on which the subjects responded very slowly—implying that when subjects could not decide quickly whether they perceived the tastant, attention may induce a small strategic shift in behavior. Note that the difference between interleaved and control conditions never survives the correction of P values for multiple comparisons mentioned earlier. Even more importantly, note that the small effect of attention/decision is not evident in the regions of the CDF that display coactivation of gustatory and olfactory signals, consistent with our prediction. Subjects responded faster to citral when the citral trials were interleaved with the others rather than being blocked but only in very first bin of the CDF (Figure 4 and Table 4). This difference also does not survive correction for multiple comparisons. There was no difference between CDFs obtained for the citral–sucrose mixture in the interleaved versus control conditions (see Figure 4 and Table 5).
Figure 4.
Cumulative probability density functions in the interleaved (black triangles) and control blocks (gray diamonds) for sucrose, citral, and sucrose–citral mixtures (averaged over subjects). Significant differences (at α = 0.05) are indicated with an asterisk.
Table 4.
Average standardized RT and SD over subjects for the interleaved condition versus the control conditions for the citral solution
| Bin | MinterleavedC | SDinterleavedC | McontrolC | SDcontrolC | P value |
| 0.05 | −0.6999 | 0.2088 | −0.6171 | 0.2459 | 0.037 |
| 0.1 | −0.5674 | 0.2035 | −0.5105 | 0.2696 | 0.163 |
| 0.15 | −0.4833 | 0.2044 | −0.4231 | 0.2835 | 0.131 |
| 0.2 | −0.3988 | 0.2286 | −0.3687 | 0.2910 | 0.482 |
| 0.25 | −0.3228 | 0.2290 | −0.2764 | 0.2934 | 0.278 |
| 0.3 | −0.2768 | 0.2295 | −0.2198 | 0.2927 | 0.200 |
| 0.35 | −0.1913 | 0.2444 | −0.1709 | 0.2914 | 0.736 |
| 0.4 | −0.0983 | 0.2943 | −0.1170 | 0.3035 | 0.809 |
| 0.45 | 0.0020 | 0.3366 | −0.0510 | 0.2908 | 0.559 |
| 0.5 | 0.0903 | 0.3872 | 0.0301 | 0.3141 | 0.556 |
| 0.55 | 0.1823 | 0.4642 | 0.1200 | 0.3226 | 0.582 |
| 0.6 | 0.3884 | 0.6817 | 0.2298 | 0.3365 | 0.365 |
| 0.65 | 0.5372 | 0.7841 | 0.3852 | 0.3998 | 0.460 |
| 0.7 | 0.7197 | 0.7946 | 0.6420 | 0.5567 | 0.676 |
| 0.75 | 0.9051 | 0.7796 | 0.9611 | 0.6743 | 0.656 |
| 0.8 | 1.1189 | 0.7209 | 1.2898 | 0.7324 | 0.166 |
| 0.85 | 1.4881 | 0.7098 | 1.6742 | 0.7874 | 0.428 |
| 0.9 | 1.8686 | 0.6766 | 1.9432 | 0.6297 | 0.647 |
| 0.95 | 2.2837 | 0.5555 | 2.4027 | 0.5094 | 0.497 |
| 1.0 | 2.5952 | 0.5196 | 2.5952 | 0.5196 | — |
Bold font indicates significance at α = 0.05.
Table 5.
Average standardized RT and SD over subjects for the interleaved condition versus the control conditions for the citral and sucrose mixture
| Bin | MinterleavedSC | SDinterleavedSC | McontrolSC | SDcontrolSC | P value |
| 0.05 | −0.9874 | 0.1743 | −0.9950 | 0.1913 | 0.766 |
| 0.1 | −0.9127 | 0.1685 | −0.9248 | 0.1823 | 0.663 |
| 0.15 | −0.8570 | 0.1592 | −0.8760 | 0.1794 | 0.386 |
| 0.2 | −0.8100 | 0.1446 | −0.8297 | 0.1751 | 0.424 |
| 0.25 | −0.7723 | 0.1361 | −0.7852 | 0.1697 | 0.534 |
| 0.3 | −0.7313 | 0.1281 | −0.7357 | 0.1712 | 0.850 |
| 0.35 | −0.6962 | 0.1216 | −0.6878 | 0.1658 | 0.718 |
| 0.4 | −0.6445 | 0.1181 | −0.6484 | 0.1431 | 0.867 |
| 0.45 | −0.6027 | 0.1085 | −0.6170 | 0.1324 | 0.566 |
| 0.5 | −0.5655 | 0.1065 | −0.5559 | 0.1295 | 0.702 |
| 0.55 | −0.5261 | 0.1034 | −0.5040 | 0.1169 | 0.430 |
| 0.6 | −0.4834 | 0.1170 | −0.4584 | 0.1136 | 0.415 |
| 0.65 | −0.4003 | 0.1228 | −0.4046 | 0.1043 | 0.896 |
| 0.7 | −0.3569 | 0.1287 | −0.3404 | 0.1052 | 0.621 |
| 0.75 | −0.3031 | 0.1268 | −0.2690 | 0.1208 | 0.303 |
| 0.8 | −0.2213 | 0.1410 | −0.1937 | 0.1451 | 0.420 |
| 0.85 | −0.1411 | 0.1770 | −0.0675 | 0.2221 | 0.167 |
| 0.9 | 0.0560 | 0.3736 | 0.0709 | 0.3167 | 0.870 |
| 0.95 | 0.5893 | 1.0322 | 0.4895 | 0.7640 | 0.744 |
| 1.0 | 2.5952 | 0.5196 | 2.5952 | 0.5196 | — |
Bold font indicates significance at α = 0.05.
Probability summation: accounting for false positives
In the probability summation model we used here, we assumed that noise, or the absence of a flavorant, never produces detection. However, this assumption is not tenable as we observed ∼20% false-positive responses (responses to water). Signal detection theory proposes that noise itself, in the absence of an external signal, can produce internal events that are indistinguishable from those produced by signals. Given a mixture containing gustatory and olfactory stimulus components and given noise in the flavor system, this means that a detection response may arise from any one of 3 sources—the gustatory signal, the olfactory signal, or noise. The probability summation model should take into account the false-positive responses that result from noise. The corrected probability of detecting a signal in each of the single components is then given by:
| (1) |
| (2) |
where POS, and POC stand for the probability of detecting sucrose and citral, respectively, and PFP for the probability of a response to water alone (noise in the flavor system). The corrected probability of detecting a signal under the H0 of probability summation then becomes:
| (3) |
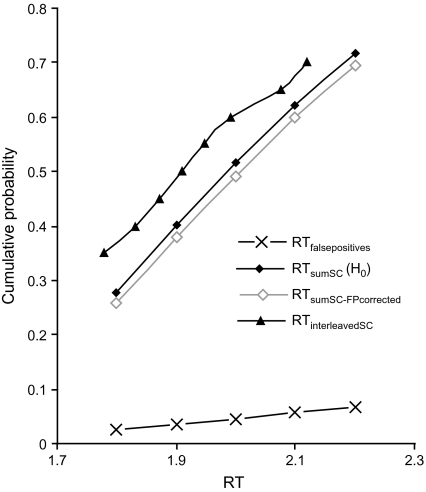
We used these equations to calculate corrected values of detection based on the false alarm rates observed in the interleaved blocks for the CDF of RTsumSC. Because there were proportionally few observations of false positives (∼20% of all water presentations) per subject, we pooled the observations of false positives across subjects, sorted the RTs in ascending order, and divided the sorted RTs into 20 bins, each containing 5% of the values. The resulting CDF will be referred to as RTfalsepositives. We observed overlap between the CDF for RTinterleavedS, RTinterleavedC, and RTfalsepositives in the approximate range of RTs longer than 1.8 s and shorter than 2.2 s (see also Figure 3A). Thus, we limited calculating the correction of RTsumSC to this range of RTs. As each of the bins in the CDFs has different RT values, we interpolated by means of a linear function (with a goodness of fit [R2] of > .98). By applying equations (1) and (2), we calculated the CDF of RTsumSC corrected for false positives, referred to as RTsumSC−FPcorrected.
The CDFs of RTfalsepositives, RTsumSC, and RTsumSC−FPcorrected are shown in Figure 5. As the figure shows, the cumulative probability for RTsumSC−FPcorrected falls below that for RTsumSC, indicating that the H0 corrected for false positive predicts longer RTs than does the uncorrected H0. This means that the initial predictions, which did not take false positives into consideration, actually overestimated probability summation and thereby underestimated the degree of interaction (coactivation) of gustatory and olfactory flavorants.
Figure 5.
Cumulative probability density functions in a selected window of time for the observed sucrose–citral mixtures (black triangles), the predicted model of independent activation and probability summation (black diamonds), and the predicted model of independent activation and probability summation including the probability from false positives (gray diamonds), averaged over subjects. The black crosses depict the cumulative probability density function of the pooled observed false positives.
Discussion
We hypothesized that the flavor system integrates signals from gustation and olfaction and, therefore, predicted that the time needed to detect and respond to a gustatory–olfactory flavor (mixture) would be smaller than the time computed on the basis of a statistical model of independent activation of the 2 components (probability summation). Indeed, RTs to flavor mixtures were faster than those predicted by probability summation over the fastest 40–60% of the RT distribution, suggesting that the coactivation of taste and odor signals is rapid and implying that gustatory and olfactory information combines at an early stage of flavor processing, largely or wholly prior to any decisional or attentional strategies.
To examine the role of attentional processes or decisional strategies more explicitly, we included blocks of trials that contained only one of the 3 possible target stimuli (sucrose, citral, or sucrose–citral mixture) in addition to water trials (nontarget stimulus), allowing subjects to develop stimulus-specific attentional or decisional strategies (focus attention on a single stimulus). Under the assumption that gustatory and olfactory signals combine automatically, we predicted that RTs would be the same in the control and in the experimental condition, in which different flavorants were interleaved within the block of trials. Largely as predicted, we observed only a modest effect that was significant only if uncorrected for multiple comparisons. Further, the effect appeared only on responses to the sucrose presented alone and then only when the RTs were very large, over the slowest 35% of the RT distribution. Importantly, this modest effect did not appear over the region of the RT distribution that gives evidence of coactivation, suggesting again that coactivation arises largely before the ontogenesis of attentional or decisional processes.
To the best of our knowledge, this report is the first to examine coactivation of gustatory and retronasal olfactory signals in speeded responses. Our evidence of coactivation of gustatory and olfactory signals in RTs to flavors agrees with previous observations of integration in the detection of subthreshold gustatory and olfactory flavorants (Delwiche and Heffelfinger 2005). Coactivation is also consistent with linear addition of intensity of gustatory and olfactory components of flavors (Murphy et al. 1977; Murphy and Cain 1980; McBride 1993).
In this experiment, we assumed that any orthonasal perception of those stimuli containing citral could not have contributed significantly to the coactivation of gustatory and olfactory signals. The TASTE system temporally controlled the onset of the stimulus, and subjects were trained to close their mouth and swallow as soon as they received the stimulus on the tongue. These mouth movements generally ensure a vacuum or outward air streams allowing for retronasal olfaction only. Consequently, it is unlikely that orthonasal olfaction contributed to the observed coactivation.
The probability summation model we tested here assumes that there is no correlation between olfactory and gustatory signals. Negative correlations between gustation and retronasal olfaction per se seem unlikely as they would imply “inhibitory cross-talk.” But negative correlations could result from shifts in selective attention (e.g., attention to one channel might lower the signal in the other channel). The present results indicate that any effects of attention occurred only in those (relatively late) responses that do not show coactivation. A negative correlation is therefore unlikely.
Positive correlations, however, are possible. Central noise, for example, would produce a positive correlation, and the presence of some central noise, as well as peripheral noise, is plausible—although the evidence marshaled by Marks et al. (2007) is consistent with the hypothesis that most of the noise in the flavor system comes from separate, stochastically independent, gustatory and olfactory sources. To the extent that some of the noise in the flavor system might arise centrally, however, the resulting positive correlation would reduce the magnitude of probability summation, strengthening even further our conclusion that gustatory and olfactory signals integrate (coactivate).
Coactivation is a widespread property of multisensory processing, previously reported, for example, with vision and hearing (Miller 1982, 1991) and with vision and touch (Forster et al. 2002). The present results add coactivation of the gustatory and olfactory systems to these findings. Coactivation of gustatory and olfactory systems may bear some liking to preattentional visual–auditory integration in the ventriloquist illusion. In the ventriloquist illusion, a speech signal tends to be localized toward the site of a synchronously moving mouth (Jack and Thurlow 1973; Thurlow and Jack 1973; Driver 1996). That is, the auditory percept is often said to be “spatially captured” by the visual stimulus. Such multisensory illusions are thought to be beneficial for perceiving coherent events in the external world. Flavor perception also shows spatial capture; retronasal odors, like tastes, are typically localized in the oral cavity (Murphy et al. 1977; Rozin 1982; Heilmann and Hummel 2004). As with ventriloquism, the perception of olfactory flavors in the mouth may be illusory in terms of the site of receptor stimulation, but it is not illusory from the vantage point of the flavor stimulus—which indeed is presented to the mouth.
It is tempting to suggest a connection between the integration (coactivation) of gustatory and olfactory flavor signals and the tendency to localize olfactory as well as gustatory flavors in the mouth—a connection that may relate to the coherence of flavor perception, and to the ability to detect and recognize “food objects” in the mouth. Three decades ago, Welch and Warren (1980) suggested that people unconsciously assume that spatially and temporally coincident signals from different modalities reflect a common, unitary source of perceptual information. Integration and interaction are most likely to occur, according to Welch and Warren, under conditions that encourage people to make this unconscious “assumption of unity.” By this hypothesis, the “assumption of unity” underlies integration and interaction (as it does the assumption that interactions reflect the operation of Bayesian statistical rules for optimizing performance: e.g., Alais and Burr 2004); alternatively, it is possible that integration and interaction are primary, contributing to the perception of unity and coherence.
Results of the present study suggest that olfactory and gustatory flavor signals integrate automatically, relatively early in flavor processing. This is most likely to occur when the olfactory signals overlap temporally with the gustatory and oral somatosensory signals (see Bartoshuk et al. 2004), that is, under conditions conducive to the perception of coherence. It is not certain, however, that coherence is conducive to gustatory–olfactory interaction. Indeed, the evidence of Dalton et al. (2000) and, especially, Pfeiffer et al. (2005) imply smaller interaction (enhancement of olfactory detection) under conditions that maximize flavor coherence (gustation plus retronasal olfaction) compared with conditions that presumably reduce coherence (gustation plus orthonasal olfaction). Welch and Warren's (1980) “unity assumption” came from considering interactions of visual, auditory, tactile, and proprioceptive signals. Multisensory interactions in the flavor system may operate under at least somewhat different rules from those operating in other perceptual systems.
In general, cross-modal interactions such as capture and coactivation seem to be characterized by a lack of voluntary control; one cannot help perceiving speech as coming from the ventriloquist's dummy or olfactory flavors as originating from the mouth. Here, we show that directing attention to just one of the 3 possible target stimuli, rather than attending to all of them, hardly improves the speed of detection, evident only in slow responses, which do not show coactivation of signals. This outcome differs from those in other perceptual systems, where performance is (modestly) better when subjects attend to just one modality rather than 2 (Tulving and Lindsay 1967; Long 1976). Our findings suggest that it is hard to selectively attend to the gustatory and olfactory components of a flavor, consistent with the observation that attention to vanillin does not improve its detectability in flavors (Ashkenazi and Marks 2004). Instead, it may be relatively easy to attend to flavor as a whole, easier than to attend to at least some kinds of multisensory auditory and visual stimuli. One exception to this generality may be speech, where it is relatively easy to integrate information across modalities and it can be hard to attend selectively to one component. For example, in the McGurk effect, phonetically different auditory stimuli (spoken speech) and visual stimuli (moving mouth) can blend to a phonetic intermediary (McGurk and MacDonald 1976). “Blending” of signals from gustation and olfaction is consistent with an ecological model of flavor perception. Such a model argues that flavors are unified percepts that arise from a unique system, responsive to stimuli presented to the mouth, even when components of the flavor stimuli activate different sensory systems: gustation and olfaction (Gibson 1966; Auvray and Spence 2008; Small 2008).
Supplementary material
Supplementary Table 1 and Figure 1 can be found at http://www.chemse.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health (R01 DC009021-02 to L.E.M.).
Supplementary Material
Acknowledgments
We thank International Flavors and Fragrances for providing the citral.
References
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr Biol. 2004;14:257–262. doi: 10.1016/j.cub.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Fritz M, Buckley J, Marks LE. The Temporal Automated System for Taste Experiments (TASTE) Behav Res Methods Instrum Comput. 2004;36:83–88. doi: 10.3758/bf03195552. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Marks LE. Effect of endogenous attention on detection of weak gustatory and olfactory flavors. Percept Psychophys. 2004;66:596–608. doi: 10.3758/bf03194904. [DOI] [PubMed] [Google Scholar]
- Auvray M, Spence C. The multisensory perception of flavor. Conscious Cogn. 2008;17:1016–1031. doi: 10.1016/j.concog.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Chapo AK, Fast K, Yiee JH, Hoffman HJ, Ko C-W, Snyder DJ. From psychophysics to the clinic: missteps and advances. Food Qual Pref. 2004;15:617–632. [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Zamora MC, Buratti F, Guirao M. Group and individual gustatory reaction times and Pieron's law. Physiol Behav. 1999;66:549–558. doi: 10.1016/s0031-9384(98)00209-1. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses. 2001;26:625–637. doi: 10.1093/chemse/26.6.625. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Validation of a stimulation protocol suited to the investigation of odor-taste interactions with fMRI. Physiol Behav. 2004;81:389–396. doi: 10.1016/j.physbeh.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses. 1994;19:583–594. doi: 10.1093/chemse/19.6.583. [DOI] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS. Thresholds for odor and nasal pungency. Physiol Behav. 1990;48:719–725. doi: 10.1016/0031-9384(90)90217-r. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PAS. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci. 2000;3:431–432. doi: 10.1038/74797. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Delwiche JF, Heffelfinger AL. Cross-modal additivity of taste and smell. J Sens Stud. 2005;20:512–525. [Google Scholar]
- Driver J. Enhancement of selective listening by illusory mislocation of speech sounds due to lip-reading. Nature. 1996;381:66–68. doi: 10.1038/381066a0. [DOI] [PubMed] [Google Scholar]
- Forster B, Cavina-Pratesi C, Aglioti S, Berlucchi G. Redundant target effect and intersensory facilitation from visual-tactile interactions in simple reaction time. Exp Brain Res. 2002;143:480–487. doi: 10.1007/s00221-002-1017-9. [DOI] [PubMed] [Google Scholar]
- Frank RA, Shaffer G, Smith DV. Taste-odor similarities predict taste enhancement and suppression in taste-odor mixtures. Chem Senses. 1991;16:523. [Google Scholar]
- Frank RA, van der Klaauw NJ, Schifferstein HNJ. Both perceptual and conceptual factors influence taste-odor and taste-taste interactions. Percept Psychophys. 1993;54:343–354. doi: 10.3758/bf03205269. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The senses considered as perceptual systems. Boston (MA): Houghton Mifflin; 1966. [Google Scholar]
- Heilmann S, Hummel T. A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci. 2004;118:412–419. doi: 10.1037/0735-7044.118.2.412. [DOI] [PubMed] [Google Scholar]
- Jack CE, Thurlow WR. Effects of degree of visual association and angle of displacement on the “ventriloquism” effect. Percept Mot Skills. 1973;37:967–979. doi: 10.1177/003151257303700360. [DOI] [PubMed] [Google Scholar]
- Long J. Divided attention, order of report and responses to immediately successive signals. Q J Exp Psychol. 1976;28:339–353. doi: 10.1080/14640747608400561. [DOI] [PubMed] [Google Scholar]
- Marks LE, Elgart BZ, Burger K, Chakwin EM. Human flavor perception: application of information integration theory. Teor model. 2007;1:121–132. [PMC free article] [PubMed] [Google Scholar]
- McBride RL. Integration psychophysics: the use of functional measurement in the study of mixtures. Chem Senses. 1993;18:83–92. [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Miller J. Divided attention: evidence for coactivation with redundant signals. Cogn Psychol. 1982;14:247–279. doi: 10.1016/0010-0285(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Miller J. Channel interaction and the redundant-targets effect in bimodal divided attention. J Exp Psychol Hum Percept Perform. 1991;17:160–169. doi: 10.1037//0096-1523.17.1.160. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS. Taste and olfaction: independence vs interaction. Physiol Behav. 1980;24:601–605. doi: 10.1016/0031-9384(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. Mutual action of taste and olfaction. Sens Processes. 1977;1:204–211. [PubMed] [Google Scholar]
- Pfeiffer JC, Hollowood TA, Hort J, Taylor AJ. Temporal synchrony and integration of sub-threshold taste and smell signals. Chem Senses. 2005;30:539–545. doi: 10.1093/chemse/bji047. [DOI] [PubMed] [Google Scholar]
- Rozin P. Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982;31:397–401. doi: 10.3758/bf03202667. [DOI] [PubMed] [Google Scholar]
- Schifferstein HNJ, Verlegh PWJ. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol. 1996;94:87–105. doi: 10.1016/0001-6918(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Small DM. Flavor and the formation of category-specific processing in olfaction. Chemosens Percept. 2008;1:136–146. [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Experience-dependent neural integration of taste and smell in the human brain. J Neurophys. 2004;92:1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Thurlow WR, Jack CE. Certain determinants of the “ventriloquism effect. Percept Mot Skills. 1973;36:1171–1184. doi: 10.2466/pms.1973.36.3c.1171. [DOI] [PubMed] [Google Scholar]
- Tulving E, Lindsay PH. Identification of simultaneously presented simple visual and auditory stimuli. Acta Psychol. 1967;27:101–109. doi: 10.1016/0001-6918(67)90050-9. [DOI] [PubMed] [Google Scholar]
- van der Klaauw NJ, Frank RA. Scaling component intensities of complex stimuli: the influence of response alternatives. Environ Int. 1996;22:21–31. [Google Scholar]
- Veldhuizen MG, Vessaz MN, Kroeze JHA. Comparison times are longer for hedonic than for intensity judgements of taste stimuli. Physiol Behav. 2005;84:489–495. doi: 10.1016/j.physbeh.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Welch RB, Warren DH. Immediate perceptual response to intersensory discrepancy. Psychol Bull. 1980;88:638–667. [PubMed] [Google Scholar]
- Wilson CWM, O’ Brien C, MacAirt JG. The effect of metronidazole on the human taste threshold to alcohol. Addiction. 1973;68:99–110. doi: 10.1111/j.1360-0443.1973.tb01230.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.