Abstract
Lipid rafts are sphingolipid and cholesterol rich micro-domains of the plasma membrane that coordinate and regulate varieties of signaling processes. Lipid rafts are also present in cardiac myocytes and are enriched in signaling molecules and ion channel regulatory proteins. Lipid rafts are receiving increasing attention as cellular organelles contributing to the pathogenesis of several structural and functional processes including cardiac hypertrophy and heart failure. At present, very little is known about the role of lipid rafts in cardiac function and dysfunction. This review will discuss the possible role of lipid rafts in cardiac health and disease.
INTRODUCTION
The traditional model of plasma membrane as a homogeneous fluid lipid bilayer, as demonstrated by Singer and Nicholson (1972), has been extensively modified in recent years, and it has become increasingly clear that plasma membrane consists of numerous lipids that constitute much more complex structure than previously thought. However, work over last decade has provided evidence that the plasma membrane is not a random ocean of lipids; rather, there is structure within this sea of lipids that in turn imposes organization on the distribution of proteins in the bilayer. The lipid “structures” within the membrane ocean are called lipid rafts [1]. The fatty-acid chains of lipids within the raft tend to be extended and so more tightly packed, creating domains with higher order. It is therefore thought that rafts exist in a separate ordered phase that floats in a sea of poorly ordered lipids.
Lipid rafts are sphingolipid and cholesterol-rich-domains of the plasma membrane which contain a variety of signaling and transport proteins. Different subtypes of lipid rafts can be distinguished according to their protein and lipid composition. Caveolae, a subset of lipid rafts, are flask-like invaginations of plasma membrane that contains proteins of caveolin family (Caveolin-1, caveolin-2 and caveolin-3) [1]. The presence within lipid rafts of a variety of membrane proteins involved in cell signaling has led to the consensus that these lipid domains play an important role in the process of signal transduction [2]. In some cases, preassembled signaling complexes are localized in this lipid raft domains [2].
LIPID RAFT AND SIGNALING COMPONENTS
A large number of GPCR (G-protein coupled receptor) have been reported to co-localize with lipid raft/ caveolae. In case of Angiotensin I receptor, GPCR-caveolin interaction is important for receptor sorting and delivery to plasma membrane [3]. According to the caveolin signaling hypothesis, caveolae bring downstream effectors in proximity to receptors (e.g., GPCRs) for initiating receptor, tissue and cell-specific signal transduction [4, 5]. These effectors are thought to reside within caveolae by direct interaction with caveolin. Palmitoylation may enhance caveolar localization of proteins [6, 7].
Among the different binding proteins of caveolin, its interaction with eNOS has been most extensively studied [8]. Binding of eNOS with caveolin inhibits enzyme activity [9] and loss of caveolin expression upregulates eNOS activity [10]. Like eNOS, caveolin is also thought to negatively regulate Adenylate Cyclase (AC) activity. Caveolin-1 and caveolin-3, but not caveolin-2 inhibits AC activity and this inhibition is AC isoform specific [11]. Like eNOS, protein kinases (PKA/PKC) can also interact with caveolin-1 and inhibit its activity [12]. The PKC family of enzymes translocate to the cellular compartment in response to the external stimuli [13]. The phosphatidylinositol-3-kinase/protein kinase B (PI3K/PKB, Akt) pathway is another protein kinase system that interacts with caveolin and this interaction may regulate cell survival. For example, caveolin retains Akt in activated form (phosphorylated form) in prostate cancer [14], presumably via interaction with caveolin scaffolding domain of caveolin and by inhibition of protein phosphatase 1 and 2A [15]. In muscle, we can also found a linear relationship between the expression of caveolin-3 and activation of PI3K/Akt pathway in the regulation of cell survival [16]. In addition, the phosphorylated form of caveolin is involved in EGF receptor transactivation, which is dependent on Src and Akt phosphorylation and for which caveolin helps integrate this signaling cascade [17].
Receptor tyrosine kinases also have been localized to caveolae [e.g., EGF, NGF, IGF and PGDF] and their downstream effectors MAP kinases, which regulate numerous cellular processes, are also regulated by caveolin [18, 19]. P42/44 MAPK localizes to caveolae and is negatively regulated by interaction with caveolin 1 [20]. Overexpression of caveolin-1 also inhibits the MEK/ERK signaling pathways [21]. Consistent with this action, caveolin-1 and-3 knock out mice showed increased activation of p42/44 MAPK [22]. Ischemia reperfusion showed differential activation of p42/44 ERK and p38MAPK in cavaeolar and noncaveolar fraction, indicating differential regulation of these kinases by caveolin [23]. Certain nonreceptor tyrosine kinases such as members of src family (c-Src, Fyn, lyn) are enriched in caveolae and interactions with caveolin-1 also suppress the kinases activities [24, 25]. Tyrosine phosphorylation of caveolin itself makes phospho caveolin, which acts as a key site of tyrosine kinase signaling [26].
CAVEOLIN KNOCKOUT AND PHENOTYPE
The most appropriate approach for the study of caveolin is the use of knock out (KO) mice. Caveolin-KO mice (Cav-1,-2, -3) and caveolin 1/3 double KO mice have already been developed. Although they are viable, they are fertile but display numerous phenotypes. Caveolin-1 knockout mice develop progressive cardiac hypertrophy as demonstrated by transthoracic echocardiography (TTE) and magnetic resonance imaging (MRI) [22]. In contrast, caveolin-3 knockout mice develop cardiomyopathy characterized by hypertrophy, vasodilatation and reduced contractility as well [27]. Caveolin-1 and caveolin-3 double knockout mice completely lacking caveolae are deficient in all three caveolin proteins because caveolin-2 is degraded in absence of caveolin-1. The double knockout mice developed severe cardiomyopathic phenotype with cardiac hypertrophy and decreased contractility [28]. Additionally, Cav-1 KO mice exhibited myocardial hypertrophy, pulmonary hypertension and alveolar cell hyper proliferation caused by constitutive activation of p42/44 mitogen activated protein kinase and Akt [29] Interestingly, in Cav-1-reconstituted mice, cardiac hypertrophy and pulmonary hypertension were completely rescued [29]. Again, genetic ablation of Cav-1 leads to a striking biventricular hypertrophy and to a sustained eNOS hyper-activation yielding increased systemic NO levels [30]. Furthermore, a diminished ATP content and reduced level of cyclic AMP in hearts of knockout mice was also reported [30]. Taken together, these results indicate that genetic disruption of caveolin-1 is sufficient to induce severe biventricular hypertrophy with signs of systolic and diastolic heart failure [30].
Apart from its ability to degrade extracellular matrix proteins, matrix metalloproteinase-2 (MMP-2) was recently revealed to have targets and actions within the cardiac myocyte. MMP-2 (gelatinase A) has been localized to the thin and thick myofilaments of the cardiac sarcomere, as well as to the nucleus [31, 32]. The intracellular proteins troponin I and myosin light chain-1 are proteolyzed by MMP-2 in ischemia/reperfusion injury [31, 32]. The tissue inhibitors of metalloproteinase (TIMPs) control MMP activities [33], but other mechanisms of regulation are less well elucidated. In endothelial cells, MMP-2 has been localized to the caveolae [34] yet its function there is unknown. Disruption of caveolae activates MMP-2 in fibrosarcoma cells [35] while Cav-1 overexpression in tumor cells causes decreased MMP-2 activity [36] suggesting that Cav-1 may participate in the regulation of MMP-2. Whether the role of MMP-2 activity in the heart is affected by caveolin still remains unknown. Here we present evidence that MMP-2 localizes with Cav-1 in the mouse heart, and that CSD inhibits MMP-2 activity and that hearts of mice deficient in Cav-1 have increased MMP-2 activity.
Interestingly, Cav-3 KO mice show a number of myopathic changes, consistent with a mild to moderate muscular dystrophy phenotype. However, it remains unknown whether a loss of cav-3 affects the phenotypic behavior of cardiac myocytes in vivo. Cav-3 knockout hearts display significant hypertrophy, dilation and reduced fractional shortening as revealed by gated cardiac MRI and transthoracic echocardiography. Histological analysis reveals marked cardiac myocyte hypertrophy, with accompanying cellular infiltrates and progressive interstitial/ peri-vascular fibrosis. It has also demonstrated that p42/44MAPK (ERK1/2) is hyperactivated in heart derived from caveolin-3 knockout mice, which can lead to cardiac hypertrophy [37].
In the endoplasmic reticulum, Cav-3 initiates the biogenesis of caveolae organelles by forming homooligomers and hetero-oligomers with Cav-1 [38]. At the plasmalemma, Cav-3 interact with dystrophin and its associated glycoproteins [39, 40]. Cav-3 and dystrophin competitively bind to the same site of β-dystroglycan, suggesting that Cav-3 may regulate the membrane recruitment of dystrophin and the assembly of the dystrophin glycoprotein complex (DGC) [41]. At the cell surface, Cav-3 colocalizes also with signaling molecules such as Gi2α, Gβ γ, c-Src, other Src kinases as well as nitric oxide synthases (neuronal and inducible NOS), indicating that muscle caveolae might be involved in the modulation of these signaling processes [42, 43]. In addition, Cav-3 plays a role in the regulation of energy metabolism of muscle cells as it is required for the cell membrane targeting of phosphofructokinase, an enzyme that catalyzes a rate-limiting reaction in glycolysis [44].
In vitro studies have shown that Cav-3 plays a critical role in myoblast cell differentiation and survival and in myotube formation [45]. The relevance of Cav-3 in muscle physiology was further confirmed by the findings that mutations in the CAV3 gene result in distinct neuromuscular and cardiac disorders, such as limb girdle muscular dystrophy (LGMD) 1-C, idiopathic persistent elevation of serum creatine kinase (hyperCKemia), inherited rippling muscle disease (RMD), distal myopathy and familial hypertrophic cardiomyopathy (HCM) [46-48].
The CAV3 gene (OMIM no. 601253) spans 12 kb of genomic DNA on chromosome 3p25 and contains two exons. At present, 20 different point mutations, 2 base-pair deletions and 1 novel splice site mutation have been reported [49]. More recently, four novel CAV3 mutations have been identified in patients affected by congenital long-QT syndrome (LQTS) in the absence of signs of primary cardiomyopathy, suggesting a possible role for Cav-3 in the regulation of cardiac ion channels [49, 50].
CAVEOLAE AND CARDIAC ION CHANNELS
Modulation of ion channel activity plays a critical role in regulating cardiovascular function. Recently, it has become apparent that the regulation of channel function is not the only means of controlling excitability, the trafficking and localization of ion channels with signaling molecules also play a significant role. Most cells in the cardiovascular system express multiple channel types (e.g., voltage-gated Na+, K+ and Ca2+ channels) and even multiple isoforms of a particular channel, with each channel uniquely contributing to excitability [51, 52]. Voltage gated Na+ channels are responsible for the initial depolarization of the cardiac sarcolemma, to permit the opening of voltage-gated L-type Ca2+ channels, resulting in Ca 2+ influx and contraction. Membrane repolarization is controlled by K+ channels. Therefore, altering the number of channels and/or their function can have significant impact on both resting membrane potential and the cardiac action potential wave form. Defects in either of these processes can have life-threatening implications [51, 52].
In several cell types, including smooth muscle and endothelial cells, mediators of calcium signaling, such as Ca2+-ATPase, inositol-triphosphate receptor (IP3R), Ca2+ pumps and L-type Ca 2+ channels, large conductance Ca2+ activated K+ channel, calmodulin and transient receptor potential (TRP) channels, localize in cholestetrol-rich membrane domains. Such localization suggest that membrane raft and/or caveolae have a role in calcium handling and Ca2+ entry that control excitation-contraction of heart muscle [53-55]. TRP channels, in particular TRPC1, -3 and -4 are enriched in caveolae and caveolin-1 regulates the plasma membrane localization and function of TRP channels [56]. Current evidence indicates that caveolae regulate calcium entry and depletion of cholesterol by methyl-β-cyclodextrin reduces colocalization of caveolin-1 and TRPC1 and redistribution of TRPC1, thus preventing Ca2+ influx [57]. Moreover, Na+ pump, Na/K-ATPase, contains two caveolin binding motifs and resides in caveolae in a number of cells, including smooth muscle cells and cardiomyocytes, thereby helping to maintain Na+ gradient [58]. Voltage gated K+ channels are also localized in caveolae and play an important role to maintaining cellular excitability. In fibroblast, the Kv 1.5 subunit colocalizes with caveolin-1, Kv 2.5 localizes with membrane raft and depletion of cholesterol with MβCD redistributes and alters the function of K+ channel [59]. These findings imply that alteration of caveolae and/or caveolin by any disease or drug treatments can shift the localization of the channels, thereby altering cellular excitability and functional activity.
CAVEOLAE AND CARDIOVASCULAR DISEASE
There is a vast literature about the roles of caveolae and caveolin in the regulation of many cellular processes in cultured cells and many investigators considered them as an essential platform of signaling molecules. However, in the past few years, development of animal models and usage of genetically altered mice have been instrumental in deciphering their physiological functions in vivo. Transgenic over expression of caveolin-1 or caveolin-3 in mice or targeted disruption of each of the caveolin gene locus in mice (Cav-1, Cav-2 and Cav-3 genes) has provided significant insight into the roles of caveolin and caveolae [60]. The potential role of caveolin in cardiovascular physiology has become apparent by the discovery of cavelin-1 and caveolin-3 KO mice and double knockout mice, which have cardiomyopathic phenotype. Caveolin-1 KO mice show complete ablation of the presence of the caveolae, cellular organelle, in the endothelium and fat. Similarly, caveolin-3 KO mice lack caveolae in cells that normally express this protein such as skeletal muscle, heart and diaphragm. Heart tissue is made up of different types of cells. Differentiated cardiomyocytes surrounded by a network of cardiac fibroblasts and endothelial cells and less abundant vascular smooth muscle cells. There is also a controversy regarding expression of caveolin isoforms in the heart muscle. It is well known that cardiac myocytes express caveolin-3 and other cell types in the heart express caveolin-1 and caveolin-2. But recent studies provided the evidence of the existence of caveolin-1 in cardiomyocytes [61].
Caveolin and Atherosclerosis
Experimental evidence indicates that caveolae and caveolins have the possibility to influencing atherogenesis in many ways. Caveolin-1 is a cholesterol-binding protein that can transport cholesterol from the endoplasmic reticulum (ER) to the plasma membrane. The major receptors for high-density lipoprotein, SR-B1, and a scavenger receptor for modified forms of LDL, CD36, can also reside in and signal in caveolae-type microdomains [62]. In addition, oxidized LDL can extract caveolae cholesterol, unlocalize eNOS, and impair NO release [63]. Conversely, blockade of HMG CoA reductase with statin-based drugs reduces caveolin levels and promotes eNOS activation [64]. This concept has been validated in apolipoprotein E-deficient (ApoE–/–) mice where statin treatment decreases caveolin-1 expression and promotes NOS function in vivo [65]. However, to date, there are no data showing changes in caveolin-1 levels in atherosclerotic lesions from humans [60].
To verify, if caveolin-1 influenced lesion progression in mice, Lisanti and his coworkers crossbred caveolin-1–/– mice with ApoE–/– mice that develop atheromas. Interestingly, the loss of caveolin-1 in the ApoE–/– mice resulted in a proatherogenic lipid profile, similar to that seen in CD36–/– mice bred to an ApoE background [66, 67]. Surprisingly, despite a pro-atherogenic lipid profile, the loss of caveolin-1 reduced lesion burden by 80%, suggesting caveolin-1 regulated LDL-mediated vascular dysfunction, inflammation, and lesion progression. The authors suggested this may be caused by a decrease in stability of the scavenger receptor for oxidized or modified LDL, CD36 in macrophages, and an increase in endothelium-derived NO production, which would reduce vascular inflammation. These remarkable findings unequivocally support the importance of caveolin-1/caveolae in the pathogenesis of atherosclerosis [60].
Caveolin and Cardiac Hypertrophy
Cardiac hypertrophy is the consequence of an increase in cardiac myocyte size and/or mass. Since cardiac myocytes have no capacity for cellular proliferation, their only means of growth is by cellular enlargement. Given that cardiac failure is the most common result of insufficiency of myocardium, it is not surprising that cardiomyocyte hypertrophy is the dominant cellular response to virtually all forms of hemodynamic overload [68]. However, long-term adaptive/compensatory hypertrophy is associated with progressive ventricular dilation. As a consequence of cardiac enlargement and wall thinning, stress on the wall also increases, despite constant intracavitary pressure. This mathematical increase in wall stress generates its own hemodynamic stress on the heart, further stimulating overloaded hypertrophy signaling pathway and thereby altering the balance from cell growth response to cell death. Once these processes have progressed to this stage (decompensation, loss of cardiac myocytes), irreversible functional deterioration develops, which leads to heart failure and, ultimately, death [69, 70].
Overexpression of caveolin-3 in neonatal cardiac myocytes decreases the ability of the adrenergic agonist phenylephrine or endothelin-1 to increase cell size [71]. A similar kind of effect is seen in cardiac myoblasts (H9C2) in which cav-3 reduces angiotensin II–promoted hypertrophy [72]. Other studies indicate that cardiac hypertrophy results in decreased expression of cav-3 [73, 74] and that right heart [73] left heart [75] hypertrophy is enhanced in caveolin-1 KO and caveolin-1/3 double KO mice. Down regulation of growth signals are the most likely cause of expressed caveolin induced inhibition of cardiomyocyte gowth. Cav-1 and -3 KO mice show hyperactivation of p42/44 MAPK [76] and upregulation of eNOS activity and nitrosative stress [74, 75, 61]. By contrast, increased caveolin expression down regulates activity of those entities [71, 77]. Chronic myocardial hypoxia increases eNOS expression while decreasing the expression of cav-3, consistent with the idea that the expression and activity of eNOS is dependent on caveolin [78]. Alterations in caveolin expression almost certainly change the ability of the hypertrophied heart to respond to a variety of physiologic and pharmacologic agonists/ stimulus [61].
Caveolin and Myocardial Ischemia
Ischemic heart disease is major problem in Western society and a major cause of death and disability. Precondition (PC) is the phenomenon whereby brief episodes of ischemia and reperfusion render the heart resistant to ischemic injury from a subsequent ischemic insult. Thus, ischemic PC is a protective and adaptive mechanism produced by short periods of ischemic stress rendering the heart more protected against another similar or greater stress. Early preconditioning depends on adenosine, opioids and to a lesser degree, on bradykinin and prostaglandins, released during ischemia. This molecule activate G-protein coupled receptor, initiates activation of KATP channel and generate oxygen free radicals, and stimulate a series of protein kinases, which include protein kinase C, tyrosine kinase and members of MAP kinase family. Late preconditioning is triggered by a similar sequence of events, but in addition essentially depends on newly synthesized proteins, which comprise iNOS, COX-2, manganese superoxide dismutase and possibly heat shock proteins. The final mechanism of PC is still not very clear. However, evidence is rapidly accumulating about the involvement of caveolin or caveolae in cardioprotection against myocardial ischemia and ischemia/reperfusion injury [79].
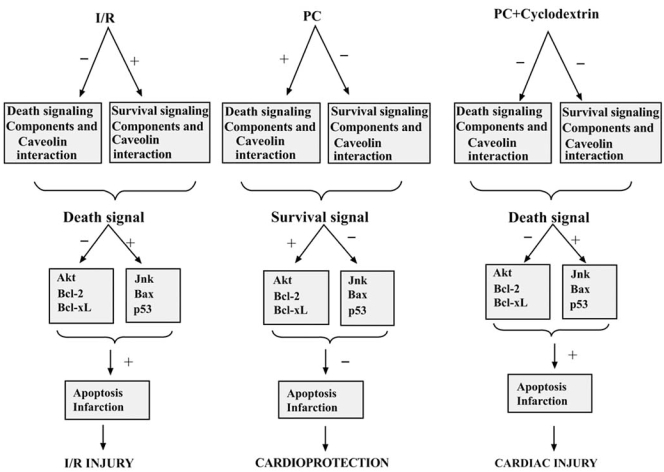
Ischemia/reperfusion injury activates p42/44 and p38MAPK, redistributes caveolin-3 and downregulates expression of caveolin-1 [80]. Disruption of caveolae using MβCD eliminates the ability of ischemia and pharmacological preconditioning to protect the cardiac myocyte from injury [81]. This is also supported by the decreased ability of Cav-1 KO mice to undergo pharmacological preconditioning [82]. Recent investigations also showed that pro-survival signaling components (e.g., ERK1/ 2, HO-1, eNOS and p38MAPKβ) translocate and/or interact with caveolin in ischemia/reperfusion heart and render the heart less abundance to pro-survival signal and induces myocardial injury. Similarly, in preconditioned heart death signaling components (e.g., p38MAPKα, JNK and Src) translocates and/or interact with caveolin in preconditioned heart and rendering the heart less exposed to death signaling components and more abundant to pro-survival signaling components [83, 84]. Although detail mechanism of action of caveolin is not very clear, but evidence indicates that proteasomes play a very important role in the interaction between caveolin and signaling components. However, overall observation indicates that caveolin plays a pivotal role in cardioprotection against ischemic injury (Fig. 1).
Fig. (1).
Proposed model of the role of lipid raft in the ischemic preconditioning of the heart. In I/R heart, anti-death signaling components (p38MAPKβ and ERK 1/2) remain bound (+) with caveolin, whereas there was reduced association (-) of death signaling components (p38MAPKα, JNK and caspase-3) with caveolin. These unbound death signaling components induces reperfusion injury in the heart by expressing (+) JNK, BAX and p53 in the myocardium. In PC heart, death signaling components remain bound (+) with caveolin, whereas there was reduced association (-) of anti-death signaling components with caveolin. These unbound anti-death/survival signaling components induced cardioprotection by expressing (+) AKT, Bcl-2 and Bcl-xl in the myocardium. When precondition was performed in presence of cyclodextrin, lipid raft disintegrator, there was no particular strong interaction of survival signaling components or death signaling components with caveolin. Due to the loss of fine control on the availability death and survival signals, heart can not generate survival signal (cardioprotection) in the PC heart in presence of lipid raft disintegrator, which was further confirmed by the expression (+) of JNK, BAX and p53 in myocardium of cyclodextrin treated heart. [I/R= ischemia reperfusion, PC= precondition]. [Reproduced from Fig. (8) of Cell Physiol Biochem 2008; 21: 325-334 with permission from Karger].
CONCLUSION
Caveolae and caveolins are undoubtedly regulating various aspects of cardiovascular system. Clearly loss of caveolin-1 has profound effect on the eNOS pathway, indicating the importance of this interaction, whereas the loss of caveolin-3 impacts NOS as well as MAPK activation. Although detail mechanisms of actions are not very clear, experimental evidences demonstrate the predominant role of caveolin in cardiac hypertrophy, atherosclerosis, ischemic injury and different myocardial functions. Recent investigations are disentangling the complex processes of caveolin regulated signaling systems in the myocardium and developing novel approaches, aimed at counteracting cardiomyocyte apoptosis in heart failure and/or cardiovascular diseases.
REFERENCES
- 1.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–7. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–40. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 3.Wyse BD, Prior IA, Qian H, et al. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem. 2003;278:23738–46. doi: 10.1074/jbc.M212892200. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 5.Insel PA, Patel HH. Do studies in caveolin-knockouts teach us about physiology and pharmacology or instead the ways mice compensate for ‘lost proteins’? Br J Pharmacol. 2007;150:251–54. doi: 10.1038/sj.bjp.0706981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Woodman SE, Engelman JA, et al. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14) J Biol Chem. 2001;276:35150–58. doi: 10.1074/jbc.M104530200. [DOI] [PubMed] [Google Scholar]
- 7.Parat MO, Fox PL. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J Biol Chem. 2001;276:15776–82. doi: 10.1074/jbc.M006722200. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–40. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 9.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272:28187–90. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 10.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 11.Toya Y, Schwencke C, Couet J, Lisanti MP, Ishikawa Y. Inhibition of adenylyl cyclase by caveolin peptides. Endocrinology. 1998;139:2025–31. doi: 10.1210/endo.139.4.5957. [DOI] [PubMed] [Google Scholar]
- 12.Razani B, Lisanti MP. Two distinct caveolin-1 domains mediate the functional interaction of caveolin-1 with protein kinase A. Am J Physiol Cell Physiol. 2001;281:C1241–50. doi: 10.1152/ajpcell.2001.281.4.C1241. [DOI] [PubMed] [Google Scholar]
- 13.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–21. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–31. [PubMed] [Google Scholar]
- 15.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smythe GM, Rando TA. Altered caveolin-3 expression disrupts PI(3) kinase signaling leading to death of cultured muscle cells. Exp Cell Res. 2006;312:2816–25. doi: 10.1016/j.yexcr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–38. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- 19.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–73. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Galbiati F, Volonte D, Engelman JA, et al. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–48. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelman JA, Chu C, Lin A, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–11. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AW, Park DS, Woodman SE, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–74. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 23.Ballard-Croft C, Locklar AC, Kristo G, Lasley RD. Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol. 2006;291:H658–67. doi: 10.1152/ajpheart.01354.2005. [DOI] [PubMed] [Google Scholar]
- 24.Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a Gα subunit (Gi1α) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol. 1997;43:293–303. [PubMed] [Google Scholar]
- 25.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–90. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–75. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 27.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J. Caveolin-3 knockout mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 28.Park DS, Woodman SE, Schubert W, Cohen AW, Frank PG, Chandra M. Caveolin 1/3 double knockout mice are viable but lack both muscle and non-muscle caveolae and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–17. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204(10):2373–82. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunderlich C, Schober K, Lange SA, et al. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340(2):702–8. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543–49. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- 32.Sawicki H, Leon H, Sawicka J, et al. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112:544–52. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 33.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res. 2001;262:28–36. doi: 10.1006/excr.2000.5069. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson SJ, English JL, Holway N, Murphy G. Cellular cholesterol regulates MT1 MMP dependent activation of MMP 2 via MEK-1 in HT1080 fibrosarcoma cells. FEBS Lett. 2004;566:65–70. doi: 10.1016/j.febslet.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–75. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 37.Woodman SE, Park DS, Cohen AW, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277(41):38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 38.Capozza F, Cohen AW, Cheung MW, et al. Muscle-specific interaction of caveolin isoforms: differential complex formation between caveolins in fibroblastic vs. muscle cells. Am J Physiol Cell Physiol. 2005;288:C677–C691. doi: 10.1152/ajpcell.00232.2004. [DOI] [PubMed] [Google Scholar]
- 39.Song KS, Scherer PE, Tang Z, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–5. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 40.Crosbie RH, Yamada H, Venzke DP, et al. Caveolin-3 is not an integral component of the dystrophin glycoprotein complex. FEBS Lett. 1998;427:279–82. doi: 10.1016/s0014-5793(98)00442-6. [DOI] [PubMed] [Google Scholar]
- 41.Sotgia F, Lee JK, Das K, et al. Caveolin-3 directly interacts with the C-terminal tail of beta—dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem. 2000;275:38048–58. doi: 10.1074/jbc.M005321200. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Cardena G, Fan R, Stern DF, et al. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biol Chem. 1996;271:27237–40. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 43.Smythe GM, Eby JC, Disatnik MH, et al. A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes. J Cell Sci. 2003;116(Part 23):4739–49. doi: 10.1242/jcs.00806. [DOI] [PubMed] [Google Scholar]
- 44.Sotgia F, Bonuccelli G, Minetti C, et al. Phosphofructokinase muscle-specific isoform requires caveolin-3 expression for plasma membrane recruitment and caveolar targeting: implications for the pathogenesis of caveolin-related muscle diseases. Am J Pathol. 2003;163:2619–34. doi: 10.1016/S0002-9440(10)63616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galbiati F, Volonte D, Engelman JA, et al. Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J Biol Chem. 1999;274:30315–21. doi: 10.1074/jbc.274.42.30315. [DOI] [PubMed] [Google Scholar]
- 46.Minetti C, Sotgia F, Bruno C, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–8. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 47.Carbone I, Bruno C, Sotgia F, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology. 2000;54:1373–6. doi: 10.1212/wnl.54.6.1373. [DOI] [PubMed] [Google Scholar]
- 48.Galbiati F, Razani B, Lisanti MP. Caveolae and caveolin-3 in muscular dystrophy. Trends Mol Med. 2001;7:435–41. doi: 10.1016/s1471-4914(01)02105-0. [DOI] [PubMed] [Google Scholar]
- 49.Fulizio L, Nascimbeni AC, Fanin M, et al. Molecular and muscle pathology in a series of caveolinopathy patients. Hum Mutat. 2005;25:82–9. doi: 10.1002/humu.20119. [DOI] [PubMed] [Google Scholar]
- 50.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 51.O'Connell KM, Martens JR, Tamkun MM. Localization of ion channels to lipid Raft domains within the cardiovascular system. Trends Cardiovasc Med. 2004;14(2):37–42. doi: 10.1016/j.tcm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res. 2006;69(4):798–807. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Fujimoto T, Miyawaki A, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-like protein in plasmalemma caveolae is linked to actin filaments. J Cell Sci. 1995;108((Pt. 1)):7–15. doi: 10.1242/jcs.108.1.7. [DOI] [PubMed] [Google Scholar]
- 54.Lohn M, Furstenau M, Sagach V, et al. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–39. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 55.Wang XL, Ye D, Peterson TE, et al. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–64. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 56.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–83. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 57.Bergdahl A, Gomez MF, Dreja K, et al. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–47. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 58.Fagan KA, Smith KE, Cooper DM. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem. 2000;275:26530–37. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- 59.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkun MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv1.5 to caveolae. J Biol Chem. 2001;276:8409–14. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 60.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94(11):1408–17. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 61.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graf GA, Matveev SV, Smart EJ. Class B scavenger receptors, caveolae and cholesterol homeostasis. Trends Cardiovasc Med. 1999;9:221–5. doi: 10.1016/s1050-1738(00)00031-1. [DOI] [PubMed] [Google Scholar]
- 63.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–9. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 64.Feron O, Dessy C, Desager JP, Balligand JL. Hydroxymethylglutaryl-coenzyme a reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–8. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- 65.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E-/- mice in vivo. Circulation. 2003;107:2480–6. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 66.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–56. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diwan A, Dorn GW. Decompensation of cardiac hypertrophy: cellular mechanism and novel therapeutic target. Physiology. 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 69.Hill JA, Karimi M, Kutschke W, et al. Cardiac hypertrophy is not a required compensatory response to short term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 70.Sano M, Schneider MD. Still stressed out but doing fine: normalization of wall stress is superfluous to maintain cardiac function in chronic pressure overload. Circulation. 2002;105:8–10. [PubMed] [Google Scholar]
- 71.Koga A, Oka N, Kikuchi T, Miyazaki H, Kato S, Imaizumi T. Adenovirus-mediated overexpression of caveolin-3 inhibits rat cardiomyocyte hypertrophy. Hypertension. 2003;42:213–9. doi: 10.1161/01.HYP.0000082926.08268.5D. [DOI] [PubMed] [Google Scholar]
- 72.Fujita T, Otsu K, Oshikawa J, et al. Caveolin-3 inhibits growth signal in cardiac myoblasts in a Ca2+-dependent manner. J Cell Mol Med. 2006;10:216–24. doi: 10.1111/j.1582-4934.2006.tb00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Souza AP, Cohen AW, Park DS, et al. MR imaging of caveolin gene-specific alterations in right ventricular wall thickness. Magn Reson Imaging. 2005;23:61–8. doi: 10.1016/j.mri.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Ohsawa Y, Toko H, Katsura M, et al. Overexpression of P104L mutant caveolin-3 in mice develops hypertrophic cardiomyopathy with enhanced contractility in association with increased endothelial nitric oxide synthase activity. Hum Mol Genet. 2004;13:151–57. doi: 10.1093/hmg/ddh014. [DOI] [PubMed] [Google Scholar]
- 75.Wunderlich C, Schober K, Lange SA, et al. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–8. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 76.Woodman SE, Park DS, Cohen AW, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 77.Feron O, Balligand JL. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res. 2006;69:788–97. doi: 10.1016/j.cardiores.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Cardena G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 79.Patel HH, Tsutsumi YM, Head BP, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21(7):1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 80.Ballard-Croft C, Locklar AC, Kristo G, Lasley RD. Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol. 2006;291:H658–67. doi: 10.1152/ajpheart.01354.2005. [DOI] [PubMed] [Google Scholar]
- 81.Das M, Gherghiceanu M, Lekli I, Mukherjee S, Popescu LM, Das DK. Essential role of lipid raft in ischemic preconditioning. Cell Physiol Biochem. 2008;21(4):325–34. doi: 10.1159/000129391. [DOI] [PubMed] [Google Scholar]
- 82.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 83.Das M, Das S, Das DK. Caveolin and MAP kinase interaction in angiotensin II preconditioning of the myocardium. J Cell Mol Med. 2007;11(4):788–97. doi: 10.1111/j.1582-4934.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Das M, Cui J, Das DK. Generation of survival signal by differential interaction of p38MAPKalpha and p38MAPKbeta with caveolin-1 and caveolin-3 in the adapted heart. J Mol Cell Cardiol. 2007;42(1):206–13. doi: 10.1016/j.yjmcc.2006.08.118. [DOI] [PMC free article] [PubMed] [Google Scholar]