Abstract
HIV/AIDS (Human immunodeficiency virus/ Acquired immuno deficiency syndrome) is a growing global problem, in terms of its incidence and mortality. Patients with HIV/AIDS are living much longer with HAART (Highly active antiretroviral therapy) therapy so much so that HIV/AIDS has now become a part of the chronic disease burden, like hypertension and diabetes. Patients with HIV/AIDS and symptoms suggestive of cardiac disease represent a diagnostic and therapeutic challenge in clinical practice; Cardiologists are more frequently encountering this problem. An algorithmic, anatomic approach to diagnosis, localizing disease to the endocardium, myocardium and pericardium can be useful. An intimate knowledge of opportunistic infections affecting the heart, effects of HAART therapy and therapy for opportunistic infections on the heart is needed to be able to formulate a differential diagnosis. Effects of HAART therapy, especially protease inhibitors on lipid and glucose metabolism, and their influence on progression to premature vascular disease require consideration. Treatment of cardiac disease, in HIV/AIDS patients can vary from non-HIV patients, based on drug interactions, differences in responsiveness, and other factors; and this area requires further research.
Keywords: HIV/AIDS, heart, cardiomyopathy, heart failure, anti-retroviral drugs, opportunistic infections.
INTRODUCTION
The growing global epidemic of HIV/AIDS has made it the fourth leading cause of death worldwide [1]. HIV/AIDS represents a growing concern to healthcare workers and physicians worldwide with a current estimated prevalence of close to 40 million worldwide with HIV/AIDS [1]; and approx 1 in 302 or 0.33% or 1.2 million [1] people in the United States [2]. Moreover, there is not only an increasing incidence of HIV/AIDS, but also greatly improved long term survival with HAART therapy in this group of patients [3].
Incidence of opportunistic infections has decreased in the HAART era in these patients and also the metabolic effects of the Highly Active Anti-Retroviral therapy (HAART), especially the protease inhibitors (PI) become more prevalent in this patient population. Patients with HIV/AIDS are not only on HAART therapy, but also on prophylaxis for opportunistic infections, depending on their level of immunity competence and prior infections, thereby complicating the picture.
All of the above problems contribute to the increasing sub-group of patients with HIV/AIDS who have heart disease, up to 24 % in one autopsy series [4]. Heart disease in HIV/AIDS patients poses numerous diagnostic and therapeutic challenges; challenges that are unique to this population. Of particular importance to cardiologists nationwide is the increasing numbers of patients referred by internists for a cardiac work up for patients with HIV. Hence, in this review article, we aim to provide an overview of the cardiac manifestations of HIV/AIDS, including an algorithmic approach, intended for the practicing cardiologist.
This review article focuses on an anatomic rather than etiologic (see Table 1) classification of heart disease in patients with HIV/AIDS. The three broad categories are pericardial disease, myocardial disease and endocardial disease. The fourth category includes arrhythmias, coronary artery disease, vascular disease, aneurysmal disease and pulmonary hypertension.
Table 1.
Etiology of Cardiac Effects of HIV/AIDS
| HIV directly affecting the heart |
| Opportunistic infections or treatment/prophylaxis of opportunistic infections |
| Effects of HAART on the Heart |
| Non-HIV cardiac risk factors (such as Diabetes Mellitus or Hypertension) |
| Mode of acquisition of HIV (Intravenous drug use related complications) |
The etiology for each of the above type of heart disease in patients with HIV/AIDS is summarized in Table 1.
In addition, a number of cardiac medications used for treatment of various cardiovascular conditions like arrhythmias and coronary artery disease interact with anti-retroviral therapy and an intimate knowledge of drug- drug interactions are needed in these patients (see Table 2).
Table 2.
Cardiovascular Drugs Interacting with Antiviral Therapy [49]
| Cardiovascular medications that interact with anti-retrovirals |
| Dihydropyridine calcium-channel blockers |
| Sildenafil |
| β-Blockers, digoxin, and non-dihydropyridine calcium-channel blockers |
| Statins Metabolized by CYP3A4: atorvastatin, lovastatin, simvastatin, Not metabolized by CYP3A4: fluvastatin, pravastatin |
| Anticoagulants- warfarin, antiarrhythmics- amiodarone, antiplatelets- ASA, clopidogrel |
| Drugs used in HIV positive individuals that interact with cardiovascular drugs |
| Protease Inhibitors (PI’s)- some act as substrates, CYP enzyme inhibitor/ inducers |
| Nucleoside reverse transcriptase inhibitors (NRTI)-some act as substrates. CYP enzyme inhibitor/ inducers |
| Non-nucleotide reverse transciptase inhibitors (NNRTI)- some act as substrates. CYP enzyme inhibitor/inducers |
| Antibiotics- Cotrimoxazole, anti-virals- class of acyclovir, anti-fungalsazoles |
| Anti-tuberculous therapy |
CARDIAC DISEASE IN PATIENTS WITH HIV/AIDS
Pericardial Disease
Diseases of the pericardium seen in patients with HIV/AIDS include pericarditis and pericardial effusions. Pericarditis in this patient population can result from bacterial pericarditis with tuberculosis being the most common, Kaposi’s sarcoma [5] or lymphomas [6]. These patients can also present with pericardial effusions, but very large effusions causing tamponade is rare. Up to 20 % of patients with AIDS have been shown to have pericardial effusions by echocardiography, and 4 % had large effusions [7]. Tuberculosis infrequently affects the pericardium, and is less common in developed countries, especially in the United States. Pericardial effusions, in patients with HIV/AIDS, even without tamponade, frequently require pericardiocentesis, since the etiology can be varied, and treatment depends on the specific etiology [8]. The only caveat of pericardiocentesis, in these patients, is the poor diagnostic yield for tuberculous pericarditis. In this setting, especially with a negative tuberculin skin test, pericardial biopsy may be more sensitive in the diagnosis. Treatment of tuberculous pericarditis requires special attention; includes anti-tuberculous therapy and corticosteroids [9, 10]. Empiric anti-tuberculous treatment should be considered in AIDS patients with undiagnosed pericardial effusions. Addition of steroids has been shown to have a significant mortality benefit in these patients [11]. And the tuberculous effusions may sometimes require pericardial fenestration. Presence of pericardial disease is a marker of poor prognosis in patients with AIDS [7].
Myocardial Disease
Diseases of the myocardium in patients with HIV/AIDS include cardiomyopathy, myocarditis, cardiac tumors and drug toxicity.
Left ventricular dysfunction associated with HIV/AIDS patients is most often clinically silent, and can progress to symptomatic left heart failure. The rate of progression from left ventricular dysfunction to heart failure can be considerably slowed by HAART therapy [12]. The pathogenesis of HIV- associated cardiomyopathy is exceedingly complex, and includes direct effects of the HIV virus on the heart [13], the inflammatory response of the host myocardium to the virus [14] and the presence of auto antibodies [13], as well as decreased immunity that makes them prone to infection. The myocardium lacks CD4 receptors and since this receptor is the portal of entry of HIV into the cell, the virus requires injury to the cardiac myocyte by other viruses as a pre-requisite for entry.
Myocarditis in HIV/AIDS patients is common, but identification of a specific cause can be difficult; only 20 % of myocarditis can be attributed to specific causes in HIV/AIDS patients. Causes of myocarditis can be extremely varied; and can vary from fungal-candidiasis, histoplasmosis, cryptococcosis, aspergillosis, viral-herpes simplex, cytomegalovirus, bacterial-tuberculosis or parasitic- toxoplasmosis [15]. Evaluation should include toxoplasma serology since it is a potentially treatable cause of myocarditis/cardiomyopathy in these patients. Added to the already long list is the fact that the HIV virus by itself can cause myocarditis. Drug use- cocaine and methamphetamine contributes to cardiac toxicity. Recently, selenium deficiency has been shown to be associated with cardiomyopathy [11, 16]. Reconstitution of the immune system by anti-retroviral therapy has been shown to trigger autoimmune responses that can contribute to myocardial dysfunction [17, 18].
Cardiac tumors affecting the heart in patients with HIV/AIDS are more frequently secondary than primary, as in the general population. Kaposi’s sarcoma is usually a part of disseminated mucocutaneous involvement, only rarely is the heart the sole site of involvement. Lesions are frequently asymptomatic. Lymphomas can affect the heart; lymphomas in patients with HIV/AIDS are predominantly non-Hodgkin’s [19]- they can be secondary or primary, the former being much more common than the latter. Primary Non-Hodgkin’s lymphoma originating in the heart is exceedingly rare. Primary cardiac lymphomas, usually B-cell lymphomas usually involve the right atrium, and can present with conduction disturbances, secondary to infiltration of the conduction system [20], arrythmias, superior vena cava obstruction or heart failure. Chemotherapy and radiation therapy have shown mixed results in these patients [21-23].
Drug toxicity can cause clinically significant myocardial dysfunction; drugs specific to the HIV/AIDS patients that can cause heart failure include zidovudine [24], interferon alpha, foscarnet, doxorubicin, pentamidine and amphotericin B.
Endocardial Disease
Endocardial/ valvular disease in patients with HIV/AIDS can be secondary to bacterial or non-bacterial (marantic) endocarditis [4]. Bacterial endocarditis is usually secondary to intravenous drug abuse in this patient population [25], making Staphylococcus aureus and Streptococcus viridans the most common organisms and the tricuspid valve, the most common valve involved. Unlike in the myocardium, the HIV virus does not affect the endocardium directly. Non-bacterial (marantic) endocarditis is usually clinically silent, affects the tricuspid valve and can lead to embolism into the pulmonary artery, which is also clinically silent. The CD4 count has implications on the risk of developing heart disease, as well as on the prognosis. Patients with lower CD4 count, especially less than 200, have a higher risk of endocarditis, and more importantly, patients with endocarditis and lower CD4 counts have a much poorer prognosis [26]. Treatment of infective endocarditis in HIV-infected patients does not differ from those who are HIV-negative.
Others- Arrhythmias, Coronary Artery Disease, Vascular Disease, Aneurysmal Disease, Pulmonary Hypertension, Venous Thrombosis
A. Arrhythmias
Arrhythmias in patients with HIV/AIDS can be the result of drug toxicity or the secondary manifestation of myocardial disease. Pentamidine/ Pyrimethamine and TMP-SMZ (Trimethoprim- Sulfamethoxazole) used in the treatment of toxoplasmosis and PCP (Pneumocystis jirovecii) pneumonia respectively, can cause significant Q-T prolongation, and therefore torsades de pointes, that can sometimes be fatal. 29 % of hospitalized patients had QT prolongation [27] and torsades de pointes has been described in the absence of drug therapy. Ganciclovir, used in the treatment of CMV infections, can cause ventricular tachycardia. As discussed above, myocardial disease, including heart failure, and myocarditis can cause arrhythmias in patients with HIV/AIDS. Interferon alpha therapy can predispose patients to develop heart blocks and sudden cardiac death.
B. Coronary Artery Disease and Vascular Disease (Cerebral and Peripheral)
On one hand, although HAART therapy slows the progression to HIV- associated cardiomyopathy, HAART therapy, especially the protease inhibitors, have clinically significant effects on metabolism; causing hyperlipidemia, insulin resistance, lipodystrophy and hyperglycemia [28-30]. Different classes of HAART appear to have varying effects on the lipid profile, notably, PIs raising low density lipoproteins (LDL) [31, 32] and NNRTIs raising HDL cholesterol [31]. Accelerated atherosclerosis appears to be one of the unexpected side-effects of HAART. The relationship between anti-retroviral therapy and coronary artery disease is a topic of much debate and uncertainty. Suffice, to say, the current literature suggests that HAART therapy decreases cardiovascular risk in the short term, but prolonged use of HAART therapy, especially protease inhibitors has been shown to be associated with increased risk of CAD/ MI [28, 33-35].
Patients on HAART therapy have a 26% increased relative risk of a myocardial infarction, per year of treatment [36]. More recently, it has also been shown that NNRTIs have a low to no increased risk of myocardial infarction compared to protease inhibitors [37]. It has also been shown that Ritonavir, protease inhibitors, is associated with increase in carotid intimal wall thickness [38]. The incidence of peripheral arterial disease appears to be increased in this patient population, independent of traditional cardiovascular risk factors. Atherosclerosis and vascular disease in patients with HIV/AIDS and HAART is a topic of great interest and a complete discussion of this topic is beyond the scope of this article.
C. Aneurysmal Disease
Patients with HIV/AIDS are more predisposed to aneurysmal disease, especially that of the aortic and cerebral blood vessels; at a higher incidence than the general population. Aneurysms can be due to vasculitis, either by the HIV virus itself [39] or secondary infections with CMV or tuberculosis [35]. The aneurysms are usually atypical and multiple when caused by the HIV virus itself.
D. Pulmonary Hypertension
Patients with HIV/AIDS can develop pulmonary hypertension that is believed to be secondary to a combination of inflammation and genetic factors [40, 41]. Plexogenic arteriopathy has been described in this patient population [42, 43].
Primary pulmonary hypertension, occurs in less than 0.5% of patients with HIV infection [42], the prognosis is usually poor. Echocardiography is useful for the diagnosis of pulmonary hypertension and to rule out secondary forms. Right heart catheterization remains the gold standard for diagnosis. Histologically, plexogenic arteriopathy is found most commonly, similar to the findings in immunocompetent patients [42, 43]. Thrombotic arterial lesions and veno-occlusive disease occur far more rarely [43]. Intravenous drug users are prone to the development of pulmonary hypertension, which maybe related to intravenous injection of foreign material [44]. This pulmonary hypertension is usually worsened by poor compliance [44]. Treatment of pulmonary hypertension includes calcium-channel blockers, diuretics, anticoagulation, and prostacyclin analogues [45]. The latter, specifically epoprostenol, efficiently reduces pulmonary artery pressure both acutely and in the long term in patients with HIV infection [46]. The effect of HAART therapy on slowing the progression of pulmonary hypertension is a topic of current research. Zuber et al. showed improvement of pulmonary arterial pressures with long term HAART therapy [42]. Pulmonary hypertension, associated with HIV/AIDS, differs from idiopathic/ primary pulmonary hypertension in terms of rapidity of progression, is unrelated to CD4 count and is associated with a worse prognosis compared to non- AIDS patients. Bosentan/ PDE inhibitors and heart-lung transplant are usually the only treatment options that work in these sub-groups of patients [42].
E. Venous Thrombosis
The incidence of deep venous thrombosis has been shown to be approximately ten times more than the general population [47]. The pathogenesis of the prothrombotic state appears to be a combination of factors: increase in plasminogen activator inhibitor (PAI)- type 1, heparin cofactor II, protein S and d-dimer values [48].
CLINICAL APPROACH TO HEART DISEASE IN HIV/AIDS PATIENTS
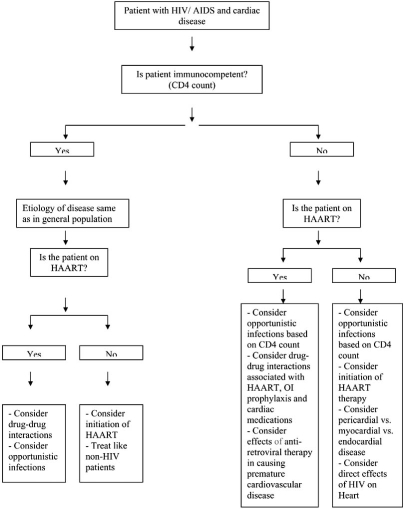
The history and physical examination must be used to detect symptoms and signs of cardiovascular disease in patients with HIV/AIDS. The history must include details of previous opportunistic infections, traditional risk factors for atherosclerosis, details regarding present and prior anti-retroviral therapy. One of the important questions clinicians should ask themselves is whether an HIV-positive individual is immunocompetent or immunodeficient- on the basis of a recent CD4 count and if not available, this would be necessary for further diagnostic evaluation and decisions regarding treatment and prognosis (see Fig. (1) for algorithm). If the patient is not already on anti-retroviral therapy and presents with cardiac symptoms, this may require referral to an infectious disease specialist for decision making regarding anti-retroviral therapy. Co-ordination of care between infectious disease and cardiology can improve the quality of care and aid in developing an individualized treatment plan based on all of the above factors. Routine use of electrocardiography or echocardiography in these patients is discouraged, especially because of the lack of evidence for finding sub-clinical disease. Shortness of breath is a common complaint, and in patients with HIV/AIDS, requires consideration of cardiomyopathy and pulmonary hypertension as possible etiologies. Transthoracic echocardiography is required for further evaluation. Drug therapy of for heart failure is not different from HIV-negative individuals, except for consideration of drug-drug interactions, especially with anti-retroviral therapy. Endomyocardial biopsy may be needed in HIV/AIDS patients with ventricular dysfunction on echocardiography to identify potentially treatable causes of myocarditis/cardiomyopathy. Lastly, cardiotoxic medications may need to be stopped in patients who have pre-existing or those who have developed significant cardiovascular disease. Management of pericardial disease, especially tuberculous effusions, is different in this patient population: addition of steroids is indicated and pericardiocentesis is needed even in the absence of tamponade. Treatment of endocarditis does not differ from HIV negative individuals. In this new era of significantly improved prognosis in patients with HIV/AIDS, both cardiac procedures and cardiovascular surgery, including valve replacement and coronary artery bypass grafting should be done in these patients, except in the setting of advanced immunosuppression or high risk of mortality from AIDS- related complications. Increased incidence of coronary artery disease, peripheral vascular disease and deep venous thrombosis has been shown in this patient population and requires careful consideration of the adverse effects of the different classes of anti-retroviral therapy.
Fig. (1).
An algorithmic approach to cardiac problems in HIV/AIDS.
CONCLUSION
A patient with HIV/AIDS and symptoms suggestive of cardiac disease, a growing problem, represents a diagnostic and therapeutic challenge in clinical practice. An intimate knowledge of opportunistic infections affecting the heart, effects of HAART therapy and therapy for opportunistic infections on the heart need to be considered in the differential diagnosis. Effects of HAART therapy, especially protease inhibitors on lipid and glucose metabolism, and their influence on progression to premature vascular disease require consideration. Finally, management of these patients can vary from non-infected patients, based on drug interactions, differences in responsiveness, and other factors; and this area requires further research.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) WHO. AIDS epidemic update: December 2004 Geneva: UNAIDS 2004 Available at http://www.unaids.org . 2004.
- 2.Fauci AS. The AIDS epidemic—considerations for the 21st century. N Engl J Med. 1999;341:1046–50. doi: 10.1056/NEJM199909303411406. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Cammarosano C, Lewis W. Cardiac lesions in acquired immune deficiency syndrome (AIDS) J Am Coll Cardiol. 1985;5:703. doi: 10.1016/s0735-1097(85)80397-1. [DOI] [PubMed] [Google Scholar]
- 5.Stotka JL, Good CB, Downer WR, et al. Pericardial effusion and tamponade due to Kaposi’s sarcoma in acquired immunodeficiency syndrome. Chest. 1989;95:1359–61. doi: 10.1378/chest.95.6.1359. [DOI] [PubMed] [Google Scholar]
- 6.Gowda RM, Khan IA, Mehta NJ, Gowda MR, Sacchi TJ, Vasavada BC. Cardiac tamponade in patients with human immunodeficiency virus disease. Angiology. 2003;54(4):469–74. doi: 10.1177/000331970305400411. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Eisenberg MJ, Kee LL, et al. Pericardial effusion in AIDS. Incidence and survival. Circulation. 1995;92:3229. doi: 10.1161/01.cir.92.11.3229. [DOI] [PubMed] [Google Scholar]
- 8.Lepori M, Tinguely F, Erard V, et al. Pericardial involvement in an HIV-infected patient. Schweiz MedWochenschr. 1999;129:736–40. [PubMed] [Google Scholar]
- 9.Trautner BW, Darouiche RO. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis. 2001;33:954–61. doi: 10.1086/322621. [DOI] [PubMed] [Google Scholar]
- 10.Hakim JG, Ternouth I, Mushangi E, et al. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart. 2000;84(2):183–8. doi: 10.1136/heart.84.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dworkin BM, Antonecchia PP, Smith F, et al. Reduced cardiac selenium content in the acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr. 1989;13:644. doi: 10.1177/0148607189013006644. [DOI] [PubMed] [Google Scholar]
- 12.Sudano I, Spieker LE, Noll G, Corti R, Weber R, Lüscher TF. Cardiovascular disease in HIV infection. Am Heart J. 2006;151(6):1147–55. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Malnick S, Goland S. Dilated cardiomyopathy in HIV-infected patients. N Engl J Med. 1998;339(16):1093–9. [PubMed] [Google Scholar]
- 14.Lewis W. Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis. 2000;43(2):151–70. doi: 10.1053/pcad.2000.9031. [DOI] [PubMed] [Google Scholar]
- 15.Magula NP, Mayosi BM. Cardiac involvement in HIV-infected people living in Africa: a review. Cardiovasc J S Afr. 2003;14(5):231–7. [PubMed] [Google Scholar]
- 16.Kavanaugh-McHugh AL, Ruff A, Perlman E, et al. Selenium deficiency and cardiomyopathy in acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr. 1991;15:347. doi: 10.1177/0148607191015003347. [DOI] [PubMed] [Google Scholar]
- 17.Herskowitz A, Willoughby S, Wu TC, et al. Immunopatho-genesis of HIV-1-associated cardiomyopathy. Clin Immunol Immunopathol. 1993;68:234. doi: 10.1006/clin.1993.1124. [DOI] [PubMed] [Google Scholar]
- 18.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 19.Ioachim HL, Cooper MC, Hellman GC. Lymphomas in men at high risk for acquired immune deficiency syndrome (AIDS). A study of 21 cases. Cancer. 1985;56(12):2831–42. doi: 10.1002/1097-0142(19851215)56:12<2831::aid-cncr2820561220>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa Y, Akaishi M, Handa S, et al. A case of malignant lymphoma simulating acute myocardial infarction. Cardiology. 1991;78:357–62. doi: 10.1159/000174817. [DOI] [PubMed] [Google Scholar]
- 21.Little RF, Gutierrez M, Jaffe ES, et al. HIV-associated non-Hodgkin lymphoma: incidence, presentation, and prognosis. JAMA. 2001;285:1880. doi: 10.1001/jama.285.14.1880. [DOI] [PubMed] [Google Scholar]
- 22.Montalbetti L, Della Volpe A, Airaghi ML, et al. Primary cardiac lymphoma. A case report and review. Minerva Cardioangiol. 1999;47:175. [PubMed] [Google Scholar]
- 23.Sturm A, Noppeney R, Reimer J, et al. AIDS and non-Hodgkin's lymphoma: initial cardiac manifestations of highly malignant B-cell lymphoma 18 years after HIV infection. Dtsch Med Wochenschr. 2001;126:364. doi: 10.1055/s-2001-12430. [DOI] [PubMed] [Google Scholar]
- 24.Frerichs FC, Dingemans KP, Brinkman K. Cardiomyopathy with mitochondrial damage associated with nucleoside reversetranscriptase inhibitors. N Engl J Med. 2002;347:1895–6. doi: 10.1056/NEJM200212053472320. [DOI] [PubMed] [Google Scholar]
- 25.Miro JM, del Rio A, Mestres CA. Infective endocarditis and cardiac surgery in intravenous drug abusers and HIV-1 infected patients. Cardiol Clin. 2003;21:167–84. doi: 10.1016/s0733-8651(03)00025-0. v-vi. [DOI] [PubMed] [Google Scholar]
- 26.Pulvirenti JJ, Kerns E, Benson C, et al. Infective endocarditis in injection drug users: importance of human immunodeficiency virus serostatus and degree of immunosuppression. Clin Infect Dis. 1996;22:40–5. doi: 10.1093/clinids/22.1.40. [DOI] [PubMed] [Google Scholar]
- 27.Gebo KA, Burkey MD, Lucas GM, et al. Incidence of Risk Factors for Clinical Presentation and 1-Year Outcomes of Infective Endocarditis in an Urban HIV Cohort. J Acquir Immun Defic Syndr. 2006;43:426. doi: 10.1097/01.qai.0000243120.67529.78. [DOI] [PubMed] [Google Scholar]
- 28.Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 29.Vittecoq D, Escaut L, Chironi G, et al. Coronary heart disease in HIV-infected patients in the highly active antiretroviral treatment era. AIDS. 2003;17(Suppl 1):S70–6. doi: 10.1097/00002030-200304001-00010. [DOI] [PubMed] [Google Scholar]
- 30.Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor–associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 31.van Leth F, Phanuphak P, Stroes E, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1:e19–e19. doi: 10.1371/journal.pmed.0010019. [Erratum, PLoS Med 2004; 1: e73] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fontas E, van Leth F, Sabin CA, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–74. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 33.Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–8. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 34.Iloeje UH, Yuan Y, L'Italien G, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 35.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17(17):2529–31. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 36.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [Erratum, N Engl J Med 2004; 350: 955] [DOI] [PubMed] [Google Scholar]
- 37.The DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 38.Johnsen S, Dolan SE, Fitch KV, et al. Carotid Intimal Medial Thickness in Human Immunodeficiency Virus-Infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4916–24. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair R, Robbs JV, Naidoo NG, et al. Clinical profile of HIV-related aneurysms. Eur J Vasc Endovasc Surg. 2000;20:235– 40. doi: 10.1053/ejvs.2000.1169. [DOI] [PubMed] [Google Scholar]
- 40.Pellicelli AM, Palmieri F, Cicalini S, et al. Pathogenesis of HIV-related pulmonary hypertension. Ann N Y Acad Sci. 2001;946:82–94. doi: 10.1111/j.1749-6632.2001.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 41.Pellicelli AM, Palmieri F, D’Ambrosio C, et al. Role of human immunodeficiency virus in primary pulmonary hypertension—case reports. Angiology. 1998;49:1005–11. doi: 10.1177/000331979804901206. [DOI] [PubMed] [Google Scholar]
- 42.Le Houssine P, Karmochkine M, Ledru F, et al. Primary pulmonary hypertension in human immunodeficiency virus infection. Study of 9 cases and review of the literature. Rev Med Interne. 2001;22:1196–203. doi: 10.1016/s0248-8663(01)00491-x. [DOI] [PubMed] [Google Scholar]
- 43.Mesa RA, Edell ES, Dunn WF, et al. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clin Proc. 1998;73:37–45. doi: 10.1016/S0025-6196(11)63616-1. [DOI] [PubMed] [Google Scholar]
- 44.Petureau F, Escamilla R, Hermant C, et al. Pulmonary artery hypertension in HIV seropositive drug addicts. Apropos of 10 cases. Rev Mal Respir. 1998;15:97–102. [PubMed] [Google Scholar]
- 45.Klings ES, Farber HW. Current management of primary pulmonary hypertension. Drugs. 2001;61:1945–56. doi: 10.2165/00003495-200161130-00005. [DOI] [PubMed] [Google Scholar]
- 46.Aguilar RV, Farber HW. Epoprostenol (prostacyclin) therapy in HIVassociated pulmonary hypertension. Am J Respir Crit Care Med. 2000;162:1846–50. doi: 10.1164/ajrccm.162.5.2004042. [DOI] [PubMed] [Google Scholar]
- 47.Saber AA, Aboolian A, LaRaja RD, et al. HIV/AIDS and the risk of deep vein thrombosis: a study of 45 patients with lower extremity involvement. Am Surg. 2001;67:645. [PubMed] [Google Scholar]
- 48.Toulon P. Hemostasis and human immunodeficiency virus (HIV) infection. Ann Biol Clin (Paris) 1998;56:153. [PubMed] [Google Scholar]
- 49.Egger S, Drewe J. Interactions of cardiac and antiretroviral medication. Herz. 2005;30(6):493–503. doi: 10.1007/s00059-005-2723-4. [DOI] [PubMed] [Google Scholar]