Summary
Background
Associative memory formation requires that animals choose predictors for experiences they need to remember. When an artificial odor is paired with an aversive experience, that odor becomes the predictor. In more natural settings, however, animals can have multiple salient experiences that need to be remembered and prioritized. The mechanisms by which animals deal with multiple experiences are incompletely understood.
Results
Here we show that Drosophila males can be trained to discriminate between different types of female pheromones; they suppress courtship specifically to the type of female that was associated with unsuccessful courtship. Such “trainer-specific” learning is mediated by hydrocarbon olfactory cues and modifies the male’s processing of those cues. Animals that are unable to use olfactory cues can still learn by using other sensory modalities, but memory in this case is not specific to the trainer female’s maturation state. Concurrent and serial presentation of different pheromones demonstrates that the ability to consolidate memory of pheromonal cues can be modified by the temporal order in which they appear.
Conclusion
Suppression of memory by new learning demonstrates that the dynamics of memory consolidation are subject to plasticity in Drosophila. This type of metaplasticity is essential for navigation of experience-rich natural environments.
Introduction
Predicting an outcome based on previous experience has huge survival value for an organism in the wild, and most species that have been examined demonstrate some form of learning. Associative learning has been elegantly demonstrated for Drosophila melanogaster. Association of a defined conditioned stimulus (CS) with a controlled unconditioned stimulus (US) in the odor-shock paradigm allowed the first genetic dissections of the molecular basis of learning and memory [1, 2]. In the natural environment, however, cues are rarely so clear cut. Animals are presented with complex chemosensory stimuli that they must deconvolute to extract salient information. Mammals, birds, and honeybees are able to process and assign varying levels of salience to multiple cues, and interactions between components of complex cues are commonly observed (for review, see [3]). The ability to evaluate and prioritize multiple experiences over time requires neural mechanisms that allow plasticity to be modulated. Mechanisms in this class have been broadly termed “metaplasticity” [4] and are common to all organisms, including humans. To investigate these issues in Drosophila, we have explored the ability of male flies to use and discriminate complex pheromonal signals in an associative-learning paradigm.
Male courtship in Drosophila is a stereotyped set of behaviors that are stimulated by chemical, visual, tactile, and auditory signals given off by female flies [5]. Although courtship appears to be a hard-wired aspect of the male nervous system, the gating of the behavior is plastic [6]. Exposure to a mated female has been shown to suppress subsequent courtship via an associative mechanism [7]. The US is believed to be an aversive substance that females produce after mating, whereas the CS is a courtship-stimulating chemical cue, or pheromone [8]. Learning in this paradigm must be driven primarily by chemosensory cues because visual input is not necessary [9]. How the learning-relevant substances are detected, via gustatory or olfactory pathways, is unknown (for review, see [10]).
Suppression of courtship after mated female training can be demonstrated with mature, immature, and mated female testers (e.g., [6, 11, 12]), suggesting that males are capable of extracting general information about the sex of another fly by using chemosensory input. The pheromones involved are thought to be cuticular hydrocarbons [13], which change in amount and type as adult animals mature [14–16]. Males can also extract more than just information about the sex of the courtship object from her pheromonal profile. Observations of basic courtship have shown that immature females generally elicit a higher level of initial courtship than do mature virgin females [17, 18]. Immature females and mature virgin females, therefore, do not present identical chemosensory cues, although males avoid them equally after training with a mated female.
Can males use their ability to discriminate between females of different ages in a learning situation? In this study we train males to specifically avoid females of a particular age and show that this associative learning is based on olfactory, and not gustatory, cues. Learning involves a specific modulation of the male’s processing of these olfactory cues. Presentation of compound cues and sequential training experiments further suggest that males can modulate memory consolidation of a specific pheromonal cue based on its temporal relationship with the US and the association of other cues with the same US. The flexible processing of multiple stimuli demonstrated by male flies in courtship learning implies the existence of metaplasticity mechanisms that gate memory consolidation in Drosophila.
Results
Trainer-Type-Specific Conditioning with Virgin Females
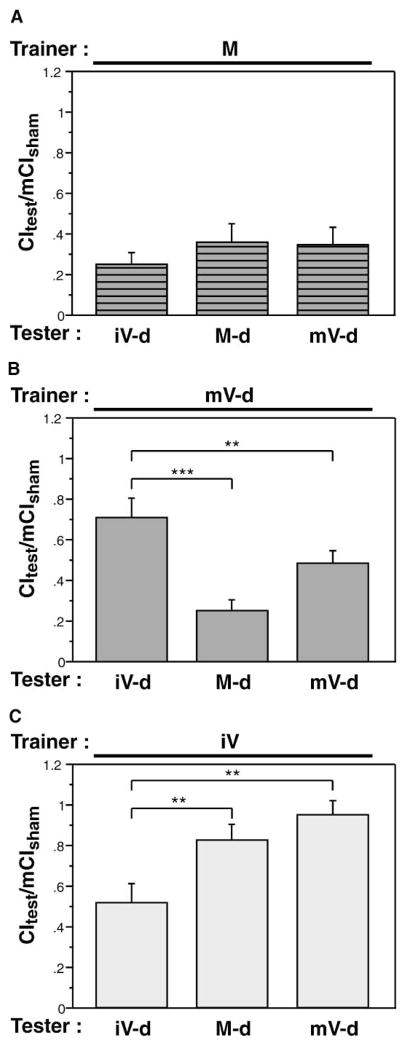
Exposure of a male to a mature mated female reduces the intensity of subsequent courtship toward mature virgins (4 days old) [6], mated females (4 days old, 24 hr post-mating) [12] and immature virgins (0–24 hr old) [11]. Different types of females are not, however, courted with identical vigor by naive males [17, 19], suggesting that the stimulatory chemosensory properties of these three types of females differ. The disparity in courtship toward virgins of different maturities is not understood at a chemical level, but it implies that maturation induces qualitative and/or quantitative changes in stimulatory pheromones. To determine if the suppression of courtship activity after training with a mated female might be biased toward mature females, we trained males with mature mated females and then tested those males with all three female types (Figure 1A). Memory was expressed as a ratio of the courtship index (CI) during the 10 min test period to the mean CI of a sham-trained male (mCIsham; sham males have spent an hour alone in a courtship chamber) tested with the same type of female. The use of a ratio allows direct comparison of the strength of memory between conditions, with a value of CItest/mCIsham = 1 indicating no memory. The magnitude of courtship suppression for all female testers was statistically indistinguishable, showing that experience with a mated female causes an equal reduction in courtship of all types of females. This indicates that mated female training is generalized to pheromonally distinct female types.
Figure 1. Differential Memory Formation with Trainers of Different Maturity.
Males were trained for 1 hr with the indicated female type (M, mated female; mV, mature virgin; iV, immature virgin; -d, decapitated), and memory was assessed immediately after training. In all experiments, memory is expressed as the courtship index during the 10 min test period over the mean courtship index of sham-trained males of the same genotype (CItest/mCIsham). A value of 1 indicates no memory. Trainer type is indicated above each panel, and tester type is indicated below each bar. Data are presented as mean ± SEM.
(A) Mated female trainer. No statistical difference was seen for memory scores between tester types (p > 0.05).
(B) Decapitated mature virgin trainer. Testing with mature females (mated or virgin) revealed significantly stronger memory than if testing was done with an immature virgin (***p < 0.001, **p < 0.05).
(C) Immature virgin trainer. Memory with an immature virgin tester was significantly greater than with mature female testers (**p < 0.05).
Because males can distinguish mature and immature virgins, we tested the effects of training with these female types, neither of which is believed to give off an aversive chemical cue. Previous reports on courtship conditioning have claimed that virgin female trainers are not able to produce courtship suppression. In all cases [7, 8, 20–23], training was done with immature virgin females, which rarely copulate [24], and testing was performed with mature virgin females. No experiments in which the maturation of the tester was the same as the trainer were reported. To revisit this issue, we trained with either decapitated mature virgins or immature virgins. After the training, males were paired with decapitated immature virgins, mature virgins, or mated females. Decapitation serves to lower the probability of copulation with mature virgin trainers [25] and to eliminate maturation-specific differences in female rejection behavior during testing. No copulation was observed with decapitated females in our experiments.
Figures 1B and 1C show the effects of training with virgins. Training with mature virgins produced a significantly greater suppression of subsequent courtship toward mature females (virgin and mated) compared with immature females (p < 0.01 for both virgin and mated females). Conversely, training with immature virgins caused a greater decrease in courtship toward immature virgins than toward mated females or mature virgins (p < 0.01 for both virgin and mated females). These data are consistent with previously published studies that failed to find modification of behavior toward mature virgins after training with immature virgins [7, 8, 20–23]. When the tester is of the same maturity (Figure 1B and 1C), however, learning is revealed. Our data indicate that males can use maturation-specific cues to learn about different females and that an aversive pheromone is not required for all types of courtship learning.
Maturation Alters the Female Cuticular Pheromone Profile
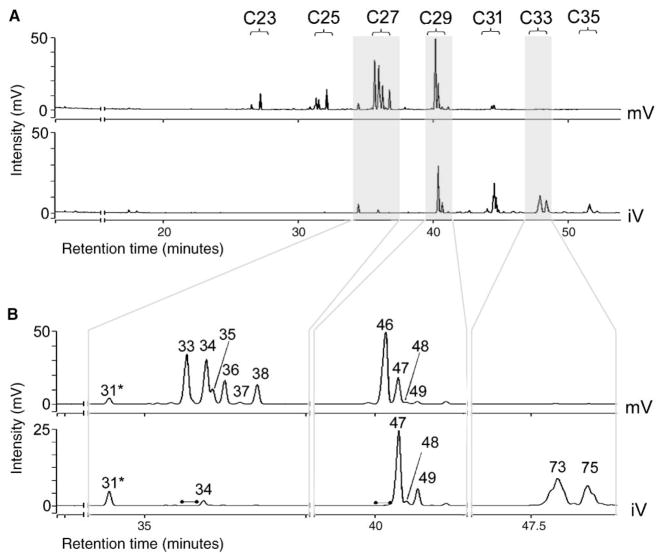
Previous work on a number of Drosophilid species [14, 15, 26], houseflies [27], blowflies [28], and grasshoppers [29, 30] has suggested that maturation affects the type and quantity of cuticular hydrocarbons. To determine whether discrimination between immature and mature virgins might reflect differences in cuticular hydrocarbons, we qualitatively and quantitatively compared hexane washes of 0- to 1-day-old virgins and 4- to 5-day-old virgins by using gas chromatography-flame ionization detection and mass spectrometry. Five replicate washes, each from 20 flies, were analyzed (Figure 2; Table S1 in the supplemental data available with this article online). Comparison of the profiles shows that 63 peaks are found in common. Mature virgins contain 10 compounds that are not found in immature virgins, and immature virgins have 12 peaks, including a number of high-molecular-weight, complex peaks that are not found for mature females. In addition to these qualitative differences, the total amount of hydrocarbons significantly increased in mature virgins (mean amount hydrocarbons/fly = 1885.5 ± 52.9 ng) compared to immature virgins (mean amount hydrocarbons/fly = 896.7 ± 50.4 ng) (p ≤ 0.01).
Figure 2. Hydrocarbon Profiles of Individual Extracts from Mature and Immature Virgins.
The chromatogram plots the area of each peak associated with a compound in units of millivolts (mV) with column retention time on the x axis.
(A) Total hydrocarbon profiles for mature virgins (mV; top) and immature virgins (iV; immediately below). Bracketed peaks show areas of these chromatograms that contain compounds in the range of C23–C35. Shaded areas indicate regions of comparison shown in (B) below.
(B) Magnified details from the chromatograms in (A) illustrate the absence of the major dienes such as 7,11-nC27:2 (peak 33) and 7,11-nC29:2 (peak 46) in immature virgins (represented by bulleted lines) and the presence of large-chain alkenes in immature virgins (Xi-nC33:2, peak 73; and Xi-nC33:1, peak 75). These examples illustrate the more general developmental delay in the appearance of unsaturated compounds in female Drosophila. Immature (iV) samples typically show lower amounts of total hydrocarbons, and therefore the scale has been adjusted for better peak identification. Peak 31, indicated by an asterisk, is the reference standard nC26 [10 ng].
Immature and mature virgins differ in their proportion of saturated and unsaturated hydrocarbons, as shown in the Supplemental Data. A key difference is that unsaturated hydrocarbons are increased in mature virgins. We and others believe that such differences in hydrocarbon class correspond to differences in function on the cuticle (e.g., [31]). Due to their higher melting temperatures and more efficient organization on the cuticle, n-alkanes and externally branched monomethylalkanes are believed to prevent water loss [32–34]. In contrast, unsaturation lowers the melting point of hydrocarbons and consequently increases their fluidity and volatility [33], making them more likely candidates for olfactory signals [31].
Pheromonal activity has been attributed to many of the unsaturated hydrocarbons. C27 dienes on mature females have been shown to stimulate wing extension, a late courtship behavior [35]. Immature virgins express only trace amounts, if any, of this compound on their cuticle, implying that their ability to stimulate courtship is due to the presence of different pheromones. Interestingly, younger virgins express high levels of C33 and C35 dienes, which may act as an identifier of immaturity [36]. As will be demonstrated below, these hexane extracts contain molecules that are able to act as a maturation-specific CS, suggesting that the maturation level of Drosophila females can be identified by distinct compounds or sets of compounds on the cuticle.
Trainer-Type-Specific Conditioning Is Not Habituation
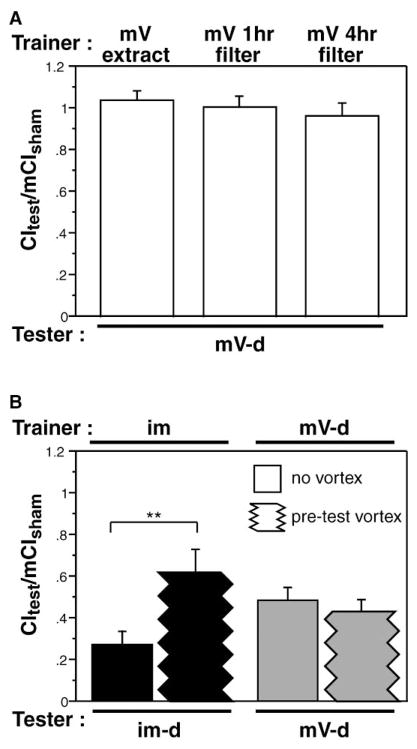
Courtship learning can be either associative, as in the case of training with mated females [7, 8], or nonassociative, as in habituation after exposure to immature males [19, 37, 38]. Reduced courtship of immature males after prior experience was shown to be habituation based on the ability of pheromones themselves, either transferred to filter paper by the immature male [37] or obtained by hexane extraction of immature males ([38] and A.E., unpublished data], to be an effective trainer in the absence of a courtship object. To determine whether learning with virgin females is habituation, we tested the ability of pheromones transferred to wet filter paper and the hexane extracts characterized above to produce trainer-type specific courtship suppression (Figure 3A). A 1 hr training session with filters exposed for either 1 or 4 hr to a mature virgin or spotted with a hexane extract equivalent of 1.25 mature virgins failed to modify courtship of a mature virgin tester. These results are consistent with this type of learning requiring additional associative cues.
Figure 3. Trainer-Specific Learning Is Not Habituation.
Trainer condition is indicated above the panel, and tester type is indicated below each set of bars (mV, mature virgin; im, immature male; -d, decapitated). Data are presented as means ± SEM.
(A) The ability of a 1 hr exposure to a filter on which mature virgins had been stored (for 1 or 4 hr) or a filter containing a hexane extract of mature virgins (1.25 fly equivalents) to produce trainer-specific memory was assessed. Males exposed to pheromone-containing filters in the absence of a courtship object failed to form memory when tested with mature virgins (p > 0.05).
(B) Male flies trained with either immature males or mature virgins were subjected to a 1 min dishabituating stimulus (vortexing) before the memory test. Habituation of the response to immature males was significantly reversed by vortexing (**p < 0.05). Memory for training with a mature virgin was unaffected by the dishabituating stimulus (p > 0.05).
Another way in which habituation can be distinguished from associative learning is via dishabituation: the ability of nonspecific noxious stimuli to reset the response level. To determine if trainer-type-specific learning could be dishabituated, we subjected males to a 1 min vortexing immediately after training. As a positive control, we carried out the same protocol on males that had been habituated to immature males. Figure 3B demonstrates that immature-male habituation can be significantly reversed by vortexing (p < 0.01 in a comparison of vortexed with non-vortexed trained males), but mature virgin training is unaffected (p > 0.05). This is inconsistent with trainer-type-specific learning being simple habituation.
Trainer-Type-Specific Learning Is Associative
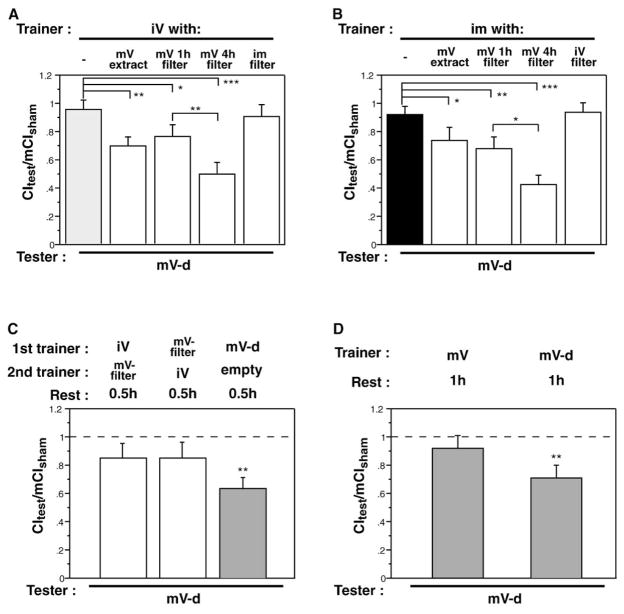
Associative learning requires concurrent exposure to the two stimuli being associated. To test whether trainer-type-specific conditioning is a form of associative learning, we attempted to reconstitute learning by pairing pheromone filters with courtship objects. Filters that had been exposed to a mature virgin for 1 or 4 hr or which contained a hexane extract equivalent of 1.25 mature virgins were paired with either an immature virgin (Figure 4A) or an immature male (Figure 4B) as a courtship object. As controls, immature virgin courtship objects were paired with hexane extract from an immature male and vice versa. All trained males were tested with a mature virgin. As with immature female trainers (Figure 1C), immature male trainers failed to produce courtship suppression with mature female testers (Figure 4B, filled bar). Pairing of mature virgin pheromone with a courtship object produced suppression of mature virgin courtship (Figures 4A and 4B). No modification of mature virgin courtship was observed after training with object/filter combinations that did not include mature virgin pheromones, showing that conditioning depends on the type of pheromone. The 4 hr pheromone filters were more effective modifiers of male behavior, indicating that the effect was correlated with the amount of substance on the filter (p < 0.06).
Figure 4. Trainer-Specific Learning Is Associative.
Males were trained for 1 hr. Trainer condition is indicated above the panel, and tester type is indicated below each set of bars (mV, mature virgin; iV, immature virgin; im, immature male; -d, decapitated). Data are presented as means ± SEM.
(A) Learning requires a trainer-specific pheromone signal. Males were trained with an immature virgin courtship object in the presence of a pheromone-laced or control filter. Compared to males trained with a control filter, males that had been exposed to mature virgin pheromones in the context of an unsuccessful courtship suppressed subsequent courtship of mature virgins (*p < 0.1, **p < 0.05, ***p < 0.001). Filters that had been exposed to a mature virgin for 4 hr were more effective trainers than filters that had only been exposed for 1 hr (**p < 0.05). Pairing of the courtship object with an irrelevant pheromone (immature male) did not produce memory when tested with a mature virgin.
(B) Substitution of an immature male for the immature virgin courtship object produced the same results, indicating that the nature of the courtship object does not influence the specificity of the learning.
(C) Sequential presentation of cues does not support memory formation. Males were trained for 1 hr with an immature virgin, then exposed for 1 hr to a filter containing mature-virgin pheromone (left bar) or to the same cues in reversed order (middle bar). No memory was formed in comparison to time-matched control males who were trained for 1 hr with a mature virgin and tested after a 1.5 hr delay (right bar, **p < 0.05 for comparison with the null hypothesis).
(D) Copulation during training blocks learning. Males were exposed to a mobile mature virgin (left bar) for 1 hr and copulation was confirmed visually. Control males were trained for 1 hr with a decapitated mature virgin and did not copulate. Males were tested for memory 1 hr after training to allow copulated males to recover from fatigue. Courtship vigor of males that had copulated was unaffected after training and rest (p > 0.1), whereas males that did not copulate showed significant suppression of courtship (**p < 0.05 for comparison to the null hypothesis).
To probe the temporal requirements for learning, we exposed males to the two cues sequentially. Males who spent 1 hr with an immature virgin courtship object followed by 1 hr with a mature virgin filter and 30 min rest, failed to demonstrate learning when tested with mature virgins. Reversing the order also failed to generate memory (Figure 4C). Memory decay over the course of the training protocol cannot account for the failure of these males to avoid mature virgins because training with a mature virgin and a subsequent 1.5 hr in an empty chamber produced robust memory (p < 0.05, for comparison with the null hypothesis, Figure 4C).
These experiments demonstrate that learning with virgin females requires association of a maturation-state-specific cuticular hydrocarbon pheromone with unsuccessful courtship and sheds light on the nature of the cues required to produce memory. The pheromone becomes a CS when paired with a courtship object. The US in this task must differ from that involved in mated-female courtship conditioning because virgins are not known to produce an aversive pheromone. We reasoned that in the case of virgin-female training, the failure of the male to complete the courtship program, i.e., to copulate, might be the US. In a number of learning situations, the failure to receive an expected reward is aversive [39–41]. To test this possibility, we exposed males either to an intact mature virgin (with whom they copulated) or to a decapitated mature virgin for 1 hr and assessed memory with a mature virgin tester 1 hr later. Males that had been allowed to copulate did not form memory (Figure 4D, p > 0.1), whereas males that had been paired with decapitated females and failed to copulate showed an obvious suppression of courtship (p < 0.01 for comparison with the null hypothesis). These data suggest that lack of copulation is a critical feature of the US that is provided by the courtship object.
The Conditioned Stimulus Is Sensed by the Olfactory System
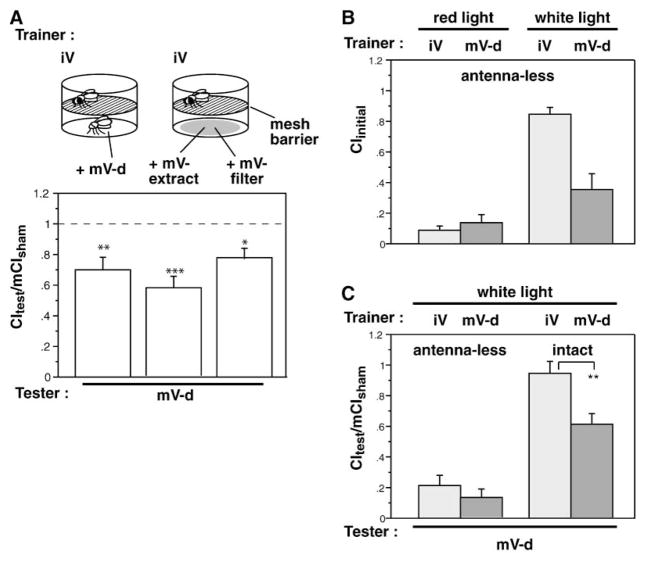
Early studies found that both olfactory [42] and gustatory [25] pathways provide courtship-relevant information to the male fly. Which sensory pathway provides the CS used for courtship suppression? In experiments shown in Figure 4, the CS was delivered on a filter that was paired with a courtship object. These filters contain both volatile and nonvolatile hydrocarbons (Figure 2). Males were allowed to touch the pheromone-containing filter, and cues could therefore have been detected by either the olfactory or the gustatory sensory system. To assess if the chemical cue used was volatile, we placed a mesh barrier between the male and the pheromone filter or decapitated mature female providing the CS. An immature virgin-female courtship object was placed with the male to elicit courtship behavior during training. Lack of direct contact with pheromone did not disturb the production of mature-female-specific courtship suppression (Figure 5A). Without a courtship object, presentation of a pheromone filter or a decapitated mature virgin over the mesh did not elicit any courtship behavior (data not shown). These results indicate the pheromones used to discriminate between mature and immature virgins, and to provide the trainer-specific CS, are volatile. This is consistent with our hypothesis that alkenes, due to their greater fluidity and volatility, are the important signaling molecules.
Figure 5. The Conditioned Stimulus for Trainer-Specific Learning Is a Volatile Odorant.
Males were trained for 1 hr. Trainer condition is indicated above the panel, and tester type is indicated below each set of bars (mV, mature virgin; iV, immature virgin; -d, decapitated). Data are presented as means ± SEM. (A) Males can sense trainer-specific pheromones without contacting them. Males were trained in a two-part chamber separated by a mesh. The courtship object was placed in the same chamber as the male, and the CS (either a mature virgin or a pheromone filter that had been in contact with a mature virgin or that contained a hexane extract equivalent to 1.25 mature virgins) was placed in the lower chamber. Volatile CS was an effective cue (*p < 0.1,**p < 0.05, or ***p < 0.001, for comparison with the null hypothesis).
(B) Males require olfaction to initiate courtship in the absence of visual cues. Antennae and maxillary palps were amputated at eclosion, and males were allowed to recover for 4 days. In dim red lights (a condition in which males cannot use visual cues [9]), males failed to initiate significant levels of courtship when presented with either an immature virgin or a mature virgin trainer (left bars). Observation of behavior under white lights shows that these males can court when allowed to see the female (right bars). Courtship of immature virgins is more vigorous than courtship of older females, as has been previously observed [17].
(C) Males with surgically ablated olfactory organs were trained with either immature or mature virgins and tested with mature virgins. Olfactory impairment suppressed courtship to both types of females to the same degree (p > 0.05 for comparison of immature virgin and mature virgin training), whereas intact males trained and tested under white lights showed trainer-specific memory (**p < 0.05, for comparison of immature virgin and mature virgin training).
Olfactory Input Is Necessary for Learning of Trainer Specificity
To determine whether olfaction is required for formation of trainer-specific memory, we prepared olfaction-impaired males by amputating the second and third antennal segments and the maxillary palps [43]. Males lacking these sensory organs were almost completely unable to initiate courtship under dim red lights, showing very low initial CIs during training (Figure 5B, left) due to a greatly increased latency to first courtship (406 ± 57 s versus 10 ± 4 s for intact males, both tested with decapitated virgins, p < 0.0001). Observing behavior under white lights (allowing these males to use visual cues to find the female) produced more normal levels of initial courtship (Figure 5B, right). Surprisingly, when trained with a mature virgin, these males were able to robustly suppress courtship (Figure 5C, left). The suppression produced, however, was not specific to the trainer type. Males trained with an immature virgin also suppressed courtship with a mature virgin tester. Intact males under the same white-light conditions were still able to form trainer-type-specific memory (Figure 5C, right). A two-way ANOVA shows that both trainer type (p < 0.01) and the presence of antennae (p < 0.0001) contribute to performance. Antenna-less males showed significantly lower courtship compared to intact males in both training conditions (p ≤ 0.0001 for both). These data suggest that in the absence of information from volatile cues, males associate some other, more general, cue with unsuccessful courtship. This cue is likely to be visual because learning in the absence of olfaction is still expressed as an increased latency to initiation (data not shown), but the cue could also be a general chemical one sensed by receptors in other organs. The ability of males to switch to a non-olfactory cue as a predictor of the US suggests that unsuccessful courtship is a potent negative experience for males.
Trainer-Type Learning Modulates Olfactory Processing
How does learning alter the courtship-behavior circuit? Male courtship in Drosophila is a series of innate behaviors that are triggered by external cues. Initiation and orientation toward the female are limited by the ability of the male to detect the female and are believed to be primarily under control of vision and olfaction ([44]; Figure 5). Subsequent courtship steps are enhanced by gustatory information the male receives by touching the female’s abdomen and genitals [45]. Sub-behaviors are done in an ordered fashion, although the male may return to earlier stages multiple times before copulation. This results in courtship behaviors occurring in clusters, or “bouts” during the observation period.
Memory in courtship conditioning paradigms is expressed as CI decrease, which reflects total courtship, initiation through attempted copulation. A decreased CI could therefore result from either fewer initiations or decreased bout duration. To investigate these possibilities, we looked at the latency to first courtship activity (Figure 6). For males trained with an immature virgin and tested with a mature virgin, latency was very short. Males trained with a mature virgin and tested with the same type of female had a much longer latency, and latency was negatively correlated with memory index (CItest/CIsham) for individual animals. Average latency for males tested with a mature virgin after training with an immature virgin was 12.4 ± 2.7 s, compared with 128.7 ± 28.7 s after training with a mature virgin (p < 0.001). The 10-fold effect on latency suggests that change in initiation rate is the primary behavioral manifestation of learning.
Figure 6. Trainer-Specific Learning Is Expressed by Modulation of Courtship Initiation.
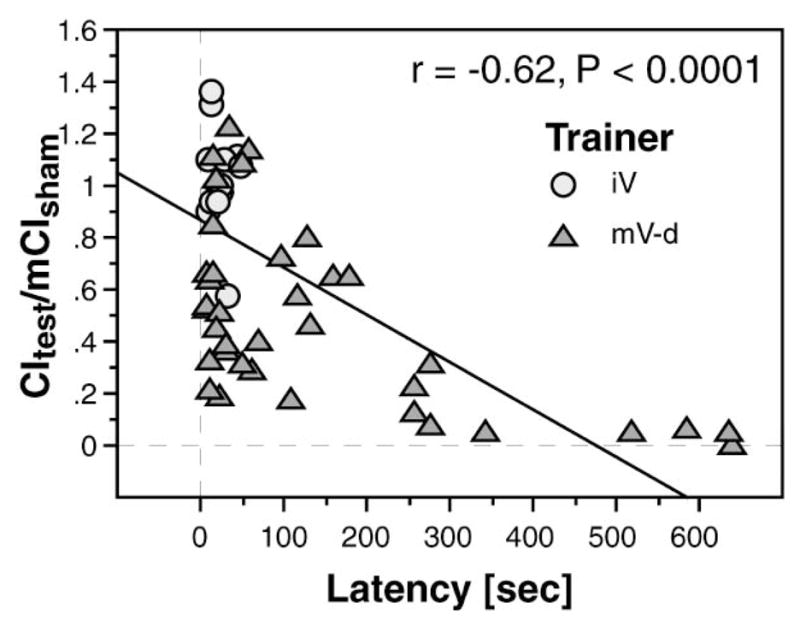
Memory scores (CItest/mCIsham) for individual animals tested with a mature virgin immediately after training were plotted against their latency to first courtship. Males trained with an immature virgin showed low latency and poor memory scores (light gray circles). Males trained with mature virgins (dark gray triangles) had longer latency, and length of latency was negatively correlated with memory score (r = −0.62, p < 0.0001).
Analysis of courtship bout length (the CItest/number of bouts) showed that, for trained flies, bout length with a tester female of the same maturity as the trainer was 2-fold shorter than if the tester was of different maturity (for males tested with a mature virgin, 15.0 ± 1.4 s after training with an immature virgin versus 6.9 ± 1.2 s after training with a mature virgin, p < 0.005). This indicates that in addition to the substantial effect on latency, there is also a smaller effect on the intensity of courtship. In the absence of visual cues, olfactory input is the critical determinant of latency (see Figure 5). Our data support the idea that learning changes the ability of the male to respond to maturation-specific volatile pheromones but leaves the gustatory pathways that stimulate courtship, once it has started, largely intact.
cAMP-Pathway Mutants Have Abnormal Trainer-Type-Specific Memory
Odor-shock learning has been used to dissect the molecular basis of associative memory. Two of the first mutants that were isolated with this paradigm are dunce (dnc) [46] and amnesiac (amn) [47], both of which encode proteins (a phosphodiesterase and a family of neuropeptides, respectively) believed to modulate cAMP signaling pathways. These mutants are also abnormal in mated-female courtship conditioning [6, 21]. To determine whether trainer-type-specific memory formation utilized similar biochemical machinery, we trained dnc1 and amn28A males with both mature and immature virgins and tested them with mature virgins.
In contrast to results with odor-shock or mated-female training, dnc1 males were able to learn to suppress courtship after training with a virgin. Interestingly, however, dnc1 males failed to show trainer-specific suppression (Figure 7A), decreasing courtship toward a mature virgin after training with either mature or immature females. This defect is similar to the behavior of olfaction-impaired males in white light (Figure 5C). It is important to note that the ability to discriminate between mated and virgin females is intact in dnc1 mutant males [21, 48], suggesting that the primary sensory neurons that process pheromonal signals are intact. The discrimination defect after training is consistent with these males having an olfactory-processing impairment. This has been demonstrated for the dnc1 allele in other paradigms [49–51]. The ability to successfully suppress courtship suggests either that the dnc-encoded phosphodiesterase is not involved in initial association in this paradigm or that the anatomical locus of memory formation for trainer-type-specific learning is distinct from that of mated female and odor-shock learning.
Figure 7. Trainer-Specific Learning Requires Intact cAMP Signaling Pathways.
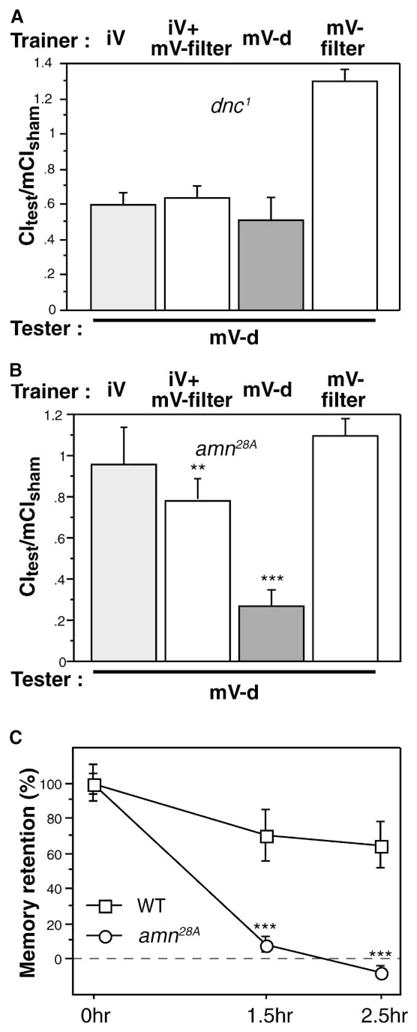
Males of the indicated genotypes were trained for 1 hr and tested at varyious times after training. The training condition is indicated above each panel, and the tester type is indicated below each panel (mV, mature virgin; iV, immature virgin; -d, decapitated). Data are presented as means ± SEM.
(A) dnc1 males were trained and tested for memory immediately after training. These males failed to form trainer-specific memory and suppressed courtship of a mature virgin tester equally regardless of training condition (p > 0.05).
(B) amn28A males were trained and tested for memory immediately after training. They were able to form trainer-specific memory with either a mature virgin trainer (***p < 0.001, for comparison to the null hypothesis) or a pheromone filter and immature-virgin courtship object (**p < 0.05, for comparison to the null hypothesis).
(C) amn28A males failed to maintain memory. Memory retention [(1 − CItest/mCIsham)/m(1 − CItest/mCIsham at t = 0)] is plotted against time after training. Mutant male memory decayed to zero by 1.5 hr, whereas wild-type memory was robust out to 2.5 hr (***p < 0.001 for comparison between wild-type and amn28A).
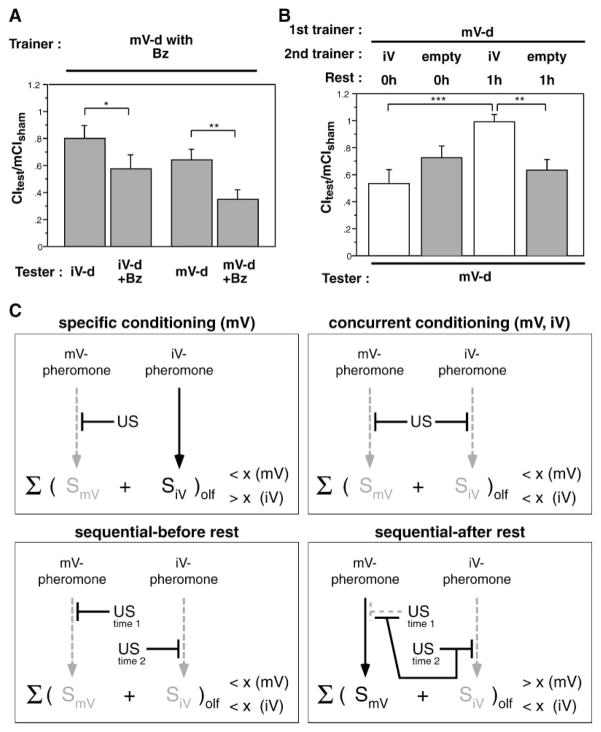
amn28A males learned normally if tested immediately after training with a mature virgin and did not avoid mature virgins after training with an immature female (Figure 7B). Training with a pheromone filter paired with a courtship object produced memory, but it was significantly weaker. Introducing a delay between training with a mature virgin and testing uncovered a more rapid decay of memory in amn28A males (Figure 7C), consistent with their phenotype in other behavioral assays, including mated-female courtship conditioning [6]. By 1.5 hr after training, amn28A memory had decayed to baseline, whereas wild-type memory was at 65% of its initial level after 2.5 hr. This experiment indicates that trainer-type-specific associative learning requires amn peptide for consolidation.
Males Can Learn Multiple Cues
In experiments shown in Figure 4A, we trained males with an immature female courtship object paired with a filter containing mature virgin pheromone and found that males could learn to avoid mature virgins. Had these males concurrently learned to avoid immature females? Training in the presence of two maturation-specific pheromones resulted in the male learning to avoid both types of females. Memory measured with an immature virgin tester for males trained with an immature virgin in the presence of a mature female filter (CItest/mCIsham = 0.33 ± 0.07) was equivalent to that measured for males trained with an immature virgin alone (p > 0.1).
Does this ability to learn two simultaneously presented cues generalize to non-pheromonal odors? We trained males with a mature virgin in the presence of benzaldehyde (Bz). Using immature and mature virgin testers, we observed males in the presence or absence of Bz. We kept sham-trained males in a chamber with Bz for 1 hr as a control to test for nonspecific effects of Bz on courtship. Bz during training did not affect the ability of males to form trainer-type-specific memory; when trained with a mature virgin in the presence of Bz, males failed to suppress courtship toward Bz-free immature virgins but showed some suppression with a Bz-free mature virgin tester. Additionally, males tested in the presence of Bz could discriminate between tester types (Figure 8A, p < 0.06 for comparison of immature and mature virgin testers in the presence of Bz). Testing in the presence of Bz enhanced avoidance of the mature virgin (p < 0.06 for comparison ± Bz) and produced suppression of immature-virgin courtship (p <0.1 for comparison ± Bz). These data are consistent with the male associating both mature virgin pheromone and Bz with unsuccessful courtship, such that the effects are roughly additive. Simultaneous memory formation for two distinct stimuli (either two pheromones or a pheromone and an odorant) suggests that each cue was of similar salience to the male. In situations where salience is unequal, the high salience cue can “overshadow” the low salience cue and block memory formation. This type of cue competition has been seen in many organisms (for review, see [3]).
Figure 8. Multiple Associations and Cue Interactions in Trainer-Specific Learning.
Cue interactions were investigated by concurrent and sequential presentation of multiple conditioned stimuli. Trainer conditions are indicated above each panel, and tester type is indicated below each panel (mV, mature virgin; iV, immature virgin; -d, decapitated; Bz, benzaldehyde). Data are presented as means ± SEM.
(A) Male flies can make multiple associations when trained in the presence of a non-pheromonal odorant. Males were trained for 1 hr with a mature virgin in the presence of Bz and tested ± Bz with the indicated female types. Males formed trainer-specific memory and additional Bz-specific memory that could be seen when testers were presented in the presence of Bz (*p < 0.1 and **p < 0.05, for comparisons ± Bz).
(B) Sequential training modulates memory retention. Males received two training sessions. In the first they were trained for 1 hr with a mature virgin. The second training session consisted of 30 min with an immature virgin or an empty chamber. Males were tested immediately after the second training session or after a 1 hr delay. Males who spent the second session in an empty chamber formed memory against mature virgins, and this memory was evident even if testing was delayed. Males that received a second training session with an immature virgin had memory when tested with immature virgins, even after a 1 hr delay (data not shown). When tested with a mature virgin, however, these males had normal memory immediately after training, but by 1 hr after the second session memory was gone (***p < 0.001 for comparison of memory tests for double-trained males 1 hr after the second session with double-trained males tested immediately after training and **p < 0.05 for comparison with males receiving an empty-chamber second session and a 1 hr delay before testing).
(C) Models of trainer-specific memory formation and consolidation. The decision to initiate courtship, in the absence of visual cues, is based on the strength of stimulatory olfactory cues that the female presents to the male. If the strength of the stimulatory cue exceeds some threshold, x, courtship will ensue. If the strength of stimulation is less than x, the male will not initiate courtship toward that female type. Solid lines indicate intact pathways, and dashed lines indicate blocked pathways. If the male is trained with a single type of female, e.g., a mature virgin, he will not initiate courtship when presented with that female type [summed stimulus < x (mV)], but he will initiate with the other female type [summed stimulus > x (iV)], in this example an immature virgin (top left panel). If both pheromonal cues are paired concurrently with the US, the male will fail to initiate with either female (top right panel). Sequential training (bottom panels) has time-dependent effects. The male initially forms memory for the cue that is trained first, in this case the mature-virgin pheromone, as can be seen if memory is assessed immediately after the second training session. The second training actively blocks retention of the memory for the first cue, resulting in apparently accelerated decay. If a rest is imposed after the two training sessions, memory for the first cue is lost. Memory for the cue that was trained second is seen both before and after the delay.
Memory Consolidation Can Be Altered by New Learning
What happens when training sessions with distinct cues are separated in time? We trained males with a mature virgin for 1 hr and then gave the male a second 30 min training session with either an empty chamber or an immature virgin. Testing memory with a mature virgin immediately after the second training session demonstrated that males formed mature-virgin memory regardless of whether there was another female presented during the second training session (Figure 8B). If a 1 hr delay was imposed before testing, memory in males that had been trained with immature females during the second training period was undetectable (p > 0.05 for the comparison of sham and immature virgin second session training following 1 hr delay). This suggests that the temporal dissociation of the two learning experiences allowed the second experience to become dominant and block maintenance of the first memory. Because the memory of the first pheromone can be demonstrated immediately after training, the effect of the second training session must entail some active process that disrupts the consolidation of the first memory.
Discussion
Memory Consolidation in Drosophila Is Plastic
Consolidation of memory in Drosophila has been shown to occur in multiple, mechanistically discrete stages that occur in the hours following initial learning. Separation of these stages can be accomplished by physical and genetic manipulation for classical [52], operant [53, 54], and courtship conditioning [6, 55]. The ordered nature and slow time course of consolidation imposes a delay between learning and the time a memory achieves its ultimate long-term form. This delay provides a temporal window in which the animal can revise its initial assessment of the importance of an experience and reconcile it with subsequent experiences. The ability to alter priorities on the fly is important for organisms, including humans, in complex environments and is likely to have strong survival value.
The trainer-specific learning paradigm has given us an opportunity to catch this reordering occurring in a laboratory setting. The ability of males to use maturation-specific pheromones to discriminate between female types allowed us to investigate the mechanisms by which males can deal with multiple simultaneous or temporally separated experiences. The experiments lead to several conclusions. First, learning one cue does not prohibit learning of a second cue. In many learning paradigms, concurrent presentation of cues of unequal salience is associated with suppression of response to the weaker cue, a phenomenon termed “blocking” or “overshadowing” [3]. Males trained in the presence of both mature- and immature-virgin pheromone can remember both stimuli when they are individually presented during testing, implying that the two pheromone cues are of equivalent salience. More importantly, initial memory for cues trained sequentially is also normal; having learned one cue does not prevent the animal from learning another. We conclude that initial learning in this paradigm does not appear to be subject to metaplastic regulation.
Second, memories for simultaneously learned cues can coexist and do not appear to interact. The ability of concurrent training with either multiple pheromones or both pheromone and odorant to produce normal memory to both cues argues that the animal is capable of holding multiple memories at one time. Concurrent training with cues of equivalent salience should not invoke any sort of competitive mechanism because the animal has no reason to value one memory over another.
Third, new learning with the same US can block consolidation of old learning. Sequential presentation of each pheromonal CS in the context of unsuccessful courtship revealed that the maintenance of memory of a pheromone can be modified by its temporal relationship to the US. A model of how this might occur is shown in Figure 8C. Animals that are trained with a mature virgin and then with an immature virgin have memory for both if tested immediately after the last training session. If a 1 hr delay is imposed before testing, the second memory is still intact, but the first memory is completely gone. This loss cannot be due to normal decay over time because time-matched animals trained with a mature virgin have normal levels of memory. This type of phenomenon has been seen with conditioned taste aversion in rats for which the CS most closely associated in time with illness is preferentially remembered [56]. Modulation of consolidation is a form of behavioral metaplasticity in which new learning alters the rules by which the subsequent processing of an older memory is handled [57].
Identification of Conditioned and Unconditioned Stimuli for Trainer-Specific Learning
The trainer-specific conditioning paradigm has also allowed us to define both conditioned and unconditioned stimuli relevant for courtship learning. The inability to copulate with a trainer female provides a very strong negative stimulus that can produce learning even in the absence of an aversive chemical substance such as the one postulated to be the US in mated-female conditioning. The ability of lack of copulation to act as a US implies that once the male has begun to court, he has an expectation that the behavior will end with mating. Furthermore, the culmination of this behavioral cycle must be associated with reward; preventing completion is aversive. Consistent with this hypothesis, we have found that the efficacy of learning can be modulated by dopaminergic transmission (A.E., unpublished results), which has been associated with both reward and aversive US pathways in Drosophila [58, 59].
Males can associate failure to copulate with several CS cues, but the dominant association in males that possess an intact olfactory system is with maturation-specific cuticular pheromones. A number of compounds are expressed only by one female type; most of these are dienes. These compounds are all candidates to be the maturation-specific CS, and given that the learning-relevant cue is volatile, it is likely that unsaturated compounds are playing this role. Previous studies of mature female compounds [35] have focused on C27 dienes such as 7,11-heptacosadiene and have used wing vibration as an assay. Wing vibration, or courtship song, occurs at a late stage of courtship and is under control of compounds sensed by the gustatory system [45]. In addition, 7,11-heptacosadiene may act in this role; it has been reported to be incapable of stimulating courtship initiation; blind males were not stimulated to sing by its presence [35]. It is therefore unlikely that 7,11-heptaco-sadiene is the mature-virgin CS of trainer-specific learning. Our results suggest a more extensive role for hydrocarbons in recognizing the trainer. Precise identification of the candidate compounds that are behaviorally relevant will require substantial additional experimentation.
Mechanisms of Trainer-Specific Learning
Manipulation of identified associative cues has also allowed us to define neural and molecular mechanisms for trainer-specific conditioning and its plasticity. Both smell and taste are important sensory modalities for courtship [25, 42]. Gustatory input is important for the progression of courtship once it has begun [45], but olfaction is used to initiate the behavior ([44] and Figure 5B). Our results demonstrate that olfaction is the predominant chemosensory system involved in plasticity of courtship behavior. The CS is a volatile hydrocarbon, and males require an intact olfactory system to learn to discriminate between young and old females.
The olfactory system is not just the driver of trainer-specific plasticity; it is also the target. The main effect of learning is to modify olfactory courtship drive. The courtship suppression exhibited after training is due mostly to a change in courtship initiation rate, with only small effects on the intensity of courtship, implicating the processing of olfactory information as a central focus of initial plasticity. The unique effects of the dnc1 mutation in this assay, a loss of pheromone specificity, supports this idea because dnc1 has previously been shown to have defects in olfactory processing [49–51].
Mechanisms of Consolidation and Metaplasticity
Newly formed trainer-specific memory is likely resident in antennal lobes [60]. In Drosophila antennal lobes, as in the mammalian olfactory bulb, odor quality is encoded initially in local activity patterns [61]. For odor-shock learning, the CS and US can converge in projection neurons to alter odor representation immediately after training. These local changes do not last long, and information regarding association may be only transiently present in this structure before being moved up to lateral protocerebrum/mushroom bodies during consolidation [62].
Studies of mated female courtship conditioning also suggest that there are initial transient sites of memory formation that feed into mushroom bodies in this behavior. Memory recall immediately after training does not require intact mushroom bodies, but 30–60 min after training, recall is impaired in animals with damage in that region [55]. The involvement of amn in retention of mated-female [6] and trainer-specific memory is also consistent with late involvement of the mushroom body. The amn gene product, which is believed to be a PACAP-like neuropeptide [63], is expressed most predominantly in the dorsal paired medial neurons that innervate the mushroom body neuropil [64]. The fast memory decay in amn mutants may not reflect enhanced forgetting but rather may reflect failure of the memory trace to be transferred to the mushroom body [65].
The time delay between the end of the second training session and the loss of the first memory implies that loss of the initial memory is an active process: deletion of an already formed memory. The time course is remarkably close to that of the memory loss seen in amn mutants. One interpretation of these data is that formation of a second association with the US prevents the normal transfer of the memory trace for the first association to the mushroom body. Accordingly, the initial interaction of the two cues is probably occurring proximal to the mushroom body, perhaps in antennal lobes. These data suggest that the consolidation process is itself plastic, and new associations with a common US are preferentially maintained. At the cellular level, this may reflect alterations in antennal-lobe activity patterns generated by the second associative event [66]. Similar behavioral phenomena in mammals remain poorly understood, and the detailed mechanisms of this higher-order associative behavior will be approachable in flies if available genetic, electrophysiological, and functional imaging tools are used.
Learning from experience is crucial to survival, and animals have developed a multitude of well-characterized plasticity mechanisms to cope with the external world. Dealing with multiple related experiences demands that an animal prioritize its responses to external cues based on either their importance or their temporal proximity. Plasticity in the dynamics of memory formation in response to new learning provides one type of solution to this problem. We find that in Drosophila, this type of metaplasticity exists at the level of modulation of consolidation of newly formed memories.
Experimental Procedures
Fly Strains
Flies were raised on autoclaved cornmeal-yeast-sucrose-agar food in a 12 hr light/dark cycle at 25°C. Males and females were anesthetized with CO2 and separated on the day of eclosion, then used immediately as immature flies or aged for 4–5 days. Experimental males were housed in individual tubes. For mated-female preparation, 3-day-old females were placed with males. Only females that copulated for ≥14 min were used the next day. For preparation of decapitated flies, heads were cut off with fine scissors just before use. Amputation of olfactory organs (second and third antennal segments and maxillary palps) was done with fine forceps at eclosion. Males were allowed to recover from surgery in a humidified food vial until testing. Canton-S was used as the wild-type strain. dnc1 is described in Lindsley and Zimm (1992) [67]. amn28A is described in Moore et al. [68].
Behavioral Assays
All behavior was done under dim red lights (except where noted) in a Harris environmental room (25°C, 80% humidity). A 4- or 5-day-old male was placed with a trainer in a single-pair-mating chamber (8 mm in diameter, 3 mm deep) for 1 hr. Wet filter paper (Whatman, 42 ashless) was put in each chamber to maintain humidity. So that direct contact with pheromone filters would be prevented (experiments in Figure 5), a fine nylon mesh (Tetko, 3-180/43) was introduced into a two-part chamber (8 mm diameter, 6 mm deep). The first 10 min of the training period were videotaped with a digital camcorder (SONY, DSR-PD150). Pairs that copulated during training or had initial CI < 0.1 were eliminated from further analysis. Immediately after training, males were transferred into a clean chamber and paired with a decapitated tester and videotaped for 10 min. In some experiments, additional training or delays were imposed before testing. Sham-trained males were are kept alone in the mating chamber for the first hour and then paired with a tester for 10 min.
For each of the 10 min periods, a courtship index (CI) was calculated. CI is the fraction of time a male spent in courtship activity in the 10 min observation period (CI = courtship [s]/observation [s]). Memory index is calculated by dividing CI at test (CItest) by the mean of sham CIs (CIsham): CItest/mCIsham. If CItest/mCIsham= 1, it indicates that there has been no learning because the courtship level of trained males is equivalent to that of sham-trained males. At least 20 males were tested for each condition. Latency was scored as the time lag (in sec) to the first display of courtship after pairing with the courtship object. Males that failed to initiate courtship during the 10 min observation period received a latency score of 600 s.
Statistics and Data Presentation
Each CI was subjected to arcsine-square-root transformation to effect an approximation of normal distribution; Statview software version 4.5 for the Macintosh was used. ANOVA with each indicated condition as the main effect was performed on the transformed data. Posthoc analysis was done with Fisher’s PLSD test for behavioral data and with a 2-tailed Steel’s rank test for hydrocarbon data. Bars in figures represent means ± SEM, with levels of significance indicated by *** p significant = α < 0.001, ** p significant = α < 0.05, and * p significant = α < 0.1. Trainer and tester types are abbreviated as follows: M, mated female; mV, mature virgin; iV, immature virgin; im, immature male; -d, decapitated; Bz, benzaldehyde. Histogram bars for data in which males were trained with mated females are dark gray with stripes; with mature virgins, dark gray; immature virgins, light gray; and immature males, black. For more complex protocols involving pairing of filters and courtship objects, bars are white.
Pheromone Extraction
For pheromone collection, a fly was put on a wet filter paper in a mating chamber for 1 or 4 hr to transfer odors to the filter. For preparation of hexane extracts, the bodies of 20 flies were washed with 80 μl of hexane (ALDRICH). For training, 5 μl of extract was applied to the filter paper in a mating chamber and evaporated for 2 min, after which 7 μl of water was added to the filter to add humidity. For chemistry, the extract was evaporated (passive evaporation at room temperature in a dust-free environment) and stored at −80°C until analyzed.
Benzaldehyde Training
Benzaldehyde (0.1%, 10 μl; ALDRICH) was applied to each filter paper in a mating chamber immediately prior to training.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant P01 NS44232 and a McKnight Technical Innovation Award to L.C.G., and by a Fyssen Foundation Fellowship to C.L. This research was undertaken, in part, thanks to funding from the Canada Research Chairs Program to J.D.L. We thank Don Katz and Jeff Hall for useful discussions. We thank Gina Turrigiano for reading this paper.
Footnotes
Supplemental Data
Supplemental Results, Experimental Procedures, and a table are available with this article online at http://www.current-biology.com/cgi/content/full/15/3/194/DC1/.
References
- 1.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 2.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasserman EA, Miller RR. What’s elementary about associative learning? Annu Rev Psychol. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WC, Tate WP. Metaplasticity: A new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 5.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci USA. 1979;76:565–578. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerman SL, Siegel RW. Chemically reinforced conditioned courtship in Drosophila: Responses to wild-type and the dunce, amnesiac and don giovanni mutants. J Neurogenet. 1986;3:111–123. doi: 10.3109/01677068609106898. [DOI] [PubMed] [Google Scholar]
- 8.Tompkins L, Siegel RW, Gailey DA, Hall JC. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Behav Genet. 1983;13:565–578. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- 9.Joiner MA, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J Neurosci. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siwicki KK, Ladewski L. Associative learning and memory in Drosophila: Beyond olfactory conditioning. Behav Processes. 2003;64:225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 11.Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 12.Kane NS, Robichon A, Dickinson JA, Greenspan RJ. Learning without performance in PKC-deficient Drosophila. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- 13.Ferveur JF. The pheromonal role of cuticular hydrocarbons in Drosophila melanogaster. Bioessays. 1997;19:353–358. doi: 10.1002/bies.950190413. [DOI] [PubMed] [Google Scholar]
- 14.Savarit F, Ferveur JF. Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J Exp Biol. 2002;205:3241–3249. doi: 10.1242/jeb.205.20.3241. [DOI] [PubMed] [Google Scholar]
- 15.Wicker C, Jallon JM. Hormonal control of sex pheromone biosynthesis in Drosophila melanogaster. J Insect Physiol. 1995;41:65–70. [Google Scholar]
- 16.Jallon JM. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984;14:441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- 17.Cook R, Cook A. The attractiveness to males of female Drosophila melanogaster: Effects of mating, age and diet. Anim Behav. 1975;23:521–526. doi: 10.1016/0003-3472(75)90129-3. [DOI] [PubMed] [Google Scholar]
- 18.Eastwood L, Burnet B. Courtship latency in male Drosophila melanogaster. Behav Genet. 1977;7:359–372. doi: 10.1007/BF01077449. [DOI] [PubMed] [Google Scholar]
- 19.Gailey DA, Lacaillade RC, Hall JC. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav Genet. 1986;16:375–405. doi: 10.1007/BF01071319. [DOI] [PubMed] [Google Scholar]
- 20.Jackson FR. The isolation of biological rhythm mutations on the autosomes of Drosophila melanogaster. J Neurogenet. 1983;1:3–15. doi: 10.3109/01677068309107068. [DOI] [PubMed] [Google Scholar]
- 21.Gailey DA, Jackson FR, Siegel RW. Conditioning mutations in Drosophila melanogaster affect an experience-dependent behavioral modification in courting males. Genetics. 1984;106:613–623. doi: 10.1093/genetics/106.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gailey DA, Hall JC, Siegel RW. Reduced reproductive success for a conditioning mutant in experimental populations of Drosophila melanogaster. Genetics. 1985;111:795–804. doi: 10.1093/genetics/111.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan TM, Siegel RW. Mutational and pharmacological alterations of neuronal membrane function disrupt conditioning in Drosophila. J Neurogenet. 1984;1:333–344. doi: 10.3109/01677068409107095. [DOI] [PubMed] [Google Scholar]
- 24.Manning A. The control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 25.Spieth HT. Drosophilid mating behavior: The behaviour of decapitated females. Anim Behav. 1966;14:226–235. doi: 10.1016/s0003-3472(66)80076-3. [DOI] [PubMed] [Google Scholar]
- 26.Doi M, Tomaru M, Matsubayashi H, Yamanoi K, Oguma Y. Genetic analysis of Drosophila virilis sex pheromone: genetic mapping of the locus producing Z-(11)-pentacosene. Genet Res. 1996;68:17–21. doi: 10.1017/s001667230003384x. [DOI] [PubMed] [Google Scholar]
- 27.Trabalon M, Campan M, Clement JL, Lange C, Miquel MT. Cuticular hydrocarbons of Calliphora vomitoria (Diptera): Relation to age and sex. Gen Comp Endocrinol. 1992;85:208–216. doi: 10.1016/0016-6480(92)90004-4. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs A, Kuenzli M, Blomquist GJ. Sex- and age-related changes in the biophysical properties of cuticular lipids of the housefly, Musca domestica. Arch Insect Biochem Physiol. 1995;29:87–97. doi: 10.1002/arch.940290108. [DOI] [PubMed] [Google Scholar]
- 29.Chapman RF, Espelie KE, Sword GA. Use of cuticular lipids in grasshopper taxonomy. Biochem Syst Ecol. 1995;23:383–395. [Google Scholar]
- 30.Tregenza T, Buckley SH, Pritchard VL, Butlin RK. Inter- and intra-population effects of sex and age on epicuticular composition of meadow grasshopper Clorthippus parallelus. J Chem Ecol. 2000;26:259–278. [Google Scholar]
- 31.Lucas C, Pho DB, Fresneau D, Jallon JM. Hydrocarbon circulation and colonial signature in Pachycondyla villosa. J Insect Physiol. 2004;50:595–607. doi: 10.1016/j.jinsphys.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Toolson EC, Kuper-Simbron R. Laboratory evolution of epicuticular lipid composition and cuticular permeability in Drosophila pseudoobscura: Effects on sexual dimorphism and thermal acclimation ability. Evolution Int J Org Evolution. 1989;43:468–473. doi: 10.1111/j.1558-5646.1989.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs AG. Water-proofing properties of cuticular lipids. Am Zool. 1998;38:471–482. [Google Scholar]
- 34.Gibbs AG, Pomonis JG. Physical properties of insect cuticular hydrocarbons: The effects of chain length, methyl-branching and unsaturation. Comp Biochem Physiol. 1995;112B:243–249. [Google Scholar]
- 35.Antony C, Jallon JM. The chemical basis for sex recognition in Drosophila melanogaster. J Insect Physiol. 1982;28:873–880. [Google Scholar]
- 36.Pechine JM, Jallon JM. Precise characterization of cuticular compounds in young Drosophila by mass spectrometry. J Chem Ecol. 1988;14:1071–1085. doi: 10.1007/BF01019336. [DOI] [PubMed] [Google Scholar]
- 37.Gailey DA, Jackson FR, Siegel RW. Male courtship in Drosophila: The conditioned response to immature males and its genetic control. Genetics. 1982;102:771–782. doi: 10.1093/genetics/102.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaias LJ, Napolitano LM, Tompkins L. Identification of stimuli that mediate experience-dependent modification of homosexual courtship in Drosophila melanogaster. Behav Genet. 1993;23:91–97. doi: 10.1007/BF01067558. [DOI] [PubMed] [Google Scholar]
- 39.Reilly S, Trifunovic R. Gustatory thalamus lesions eliminate successive negative contrast in rats: evidence against a memory deficit. Behav Neurosci. 2003;117:606–615. doi: 10.1037/0735-7044.117.3.606. [DOI] [PubMed] [Google Scholar]
- 40.Grigson PS, Flaherty CF. Cyproheptadine prevents the initial occurrence of successive negative contrast. Pharmacol Biochem Behav. 1991;40:433–442. doi: 10.1016/0091-3057(91)90576-n. [DOI] [PubMed] [Google Scholar]
- 41.Hellstern F, Malaka R, Hammer M. Backward inhibitory learning in honeybees: A behavioral analysis of reinforcement processing. Learn Mem. 1998;4:429–444. doi: 10.1101/lm.4.5.429. [DOI] [PubMed] [Google Scholar]
- 42.Shorey HH, Bartell RJ. Role of a volatile female sex pheromone in stimulating male courtship behaviour in Drosophila melanogaster. Anim Behav. 1970;18:159–164. doi: 10.1016/0003-3472(70)90085-0. [DOI] [PubMed] [Google Scholar]
- 43.Ayer RK, Jr, Carlson J. Olfactory physiology in the Drosophila antenna and maxillary palp: acj6 distinguishes two classes of odorant pathways. J Neurobiol. 1992;23:965–982. doi: 10.1002/neu.480230804. [DOI] [PubMed] [Google Scholar]
- 44.Markow TA. Behavioral and sensory basis of courtship success in Drosophila melanogaster. Proc Natl Acad Sci USA. 1987;84:6200–6204. doi: 10.1073/pnas.84.17.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 46.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- 48.O’Dell KM, Jamieson D, Goodwin SF, Kaiser K. Abnormal courtship conditioning in males mutant for the RI regulatory subunit of Drosophila protein kinase A. J Neurogenet. 1999;13:105–118. doi: 10.3109/01677069909083469. [DOI] [PubMed] [Google Scholar]
- 49.Devaud JM, Keane J, Ferrus A. Blocking sensory inputs to identified antennal glomeruli selectively modifies odorant perception in Drosophila. J Neurobiol. 2003;56:1–12. doi: 10.1002/neu.10216. [DOI] [PubMed] [Google Scholar]
- 50.Martin F, Charro MJ, Alcorta E. Mutations affecting the cAMP transduction pathway modify olfaction in Drosophila. J Comp Physiol [A] 2001;187:359–370. doi: 10.1007/s003590100208. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Diaz C, Martin F, Alcorta E. The cAMP transduction cascade mediates olfactory reception in Drosophila melanogaster. Behav Genet. 2004;34:395–406. doi: 10.1023/B:BEGE.0000023645.02710.fe. [DOI] [PubMed] [Google Scholar]
- 52.DeZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- 53.Xia SZ, Feng CH, Guo AK. Multiple-phase model of memory consolidation confirmed by behavioral and pharmacological analyses of operant conditioning in Drosophila. Pharmacol Biochem Behav. 1998;60:809–816. doi: 10.1016/s0091-3057(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 54.Gong Z, Xia S, Liu L, Feng C, Guo A. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. J Insect Physiol. 1998;44:1149–1158. doi: 10.1016/s0022-1910(98)00076-6. [DOI] [PubMed] [Google Scholar]
- 55.McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 56.Kucharski D, Spear NE. Potentiation and overshadowing in preweanling and adult rats. J Exp Psychol Anim Behav Process. 1985;11:15–34. doi: 10.1037//0097-7403.11.1.15. [DOI] [PubMed] [Google Scholar]
- 57.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 58.Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 59.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joiner MA, Griffith LC. Mapping of the anatomical circuit of CaM kinase-dependent courtship conditioning in Drosophila. Learn Mem. 1999;6:177–192. [PMC free article] [PubMed] [Google Scholar]
- 61.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 62.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning: Memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 63.Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- 64.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 65.Davis RL. Mushroom bodies, Ca2+ oscillations, and the memory gene amnesiac. Neuron. 2001;30:653–656. doi: 10.1016/s0896-6273(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Q, Poo MM. Reversal and consolidation of activity-induced synaptic modifications. Trends Neurosci. 2004;27:378–383. doi: 10.1016/j.tins.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- 68.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.