Numerous influenza pandemics in the last century resulted in the death of millions of people worldwide. With the current and future threats of influenza pandemics development of new antiviral compounds remains a great demand. However, currently only a limited number of drugs are available for the treatment and prophylaxis of influenza. A neuraminidase (NA) inhibitor, oseltamivir phosphate, is the most commonly used antiviral drug. However, a number of reports suggest the emergence of oseltamivir phosphate resistance in new seasonal influenza viruses and highly pathogenic avian influenza (H5N1) (for example, de Jong et al, 2005).
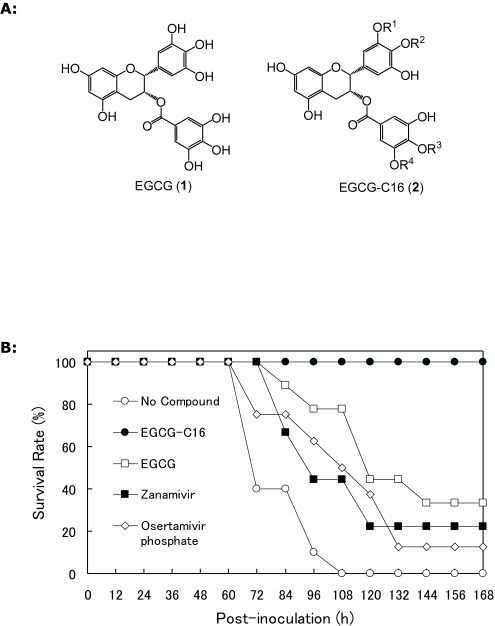
(–)-Epigallocatechin-3-O-gallate (EGCG: 1), a major component of green tea plant (Camellia sinensis), has been recognized to possess antiviral properties. Reports on the anti-influenza activity of 1 found that it inhibited virus adsorption (Nakayama et al, 1993), as well as acidification of endosomes and lysosomes (Imanishi et al, 2002). Such virus inhibition activity is different from other current NA or proton pump inhibitors, suggesting that 1 can be developed into a new class of antiviral compounds that are effective against current drug resistant influenza strains. However, 1 has not been used as an antiviral compound because of its poor lipid membrane permeability and low chemical stability. It was previously reported that the introduction of long alkyl chain groups to 1 improved its lipid membrane permeability (Tanaka et al, 1998), while protection of its hydroxyl groups with acyl groups increased its chemical stability under physiological conditions (Lam et al, 2004). Recently, we reported a method to synthesize EGCG-fatty acid monoesters using lipase-catalyzed transesterification and demonstrated that EGCG-fatty acids monoesters possessed improved influenza virus inhibitory effect against influenza A/PR8/34/(H1N1) in an alkyl length dependent manner (Mori et al, 2008). Here, we investigated the spectrum of influenza virus inhibition activity of EGCG (1) and EGCG-C-16 (2). As shown in Figures 1a, 2 is a mixture of four regio-isomers and the ratio of each regio-isomer 2a:2b:2c:2d is 38:35:7:20, respectively. Because the B-ring-modified esters (2a and 2b) and D-ring-modified esters (2c and 2d) showed exactly the same antiviral activities (data not shown), we used the mixture of four regio-isomers (2a-d) in the following assays.
Figure 1.
A. Chemical structure of EGCG (1) and EGCG-C16 (2). 2 is a mixture of four regio-isomers (2a-d). 2a: R2=R3= R4=H, R1= CO(CH)14CH3, 2b: R1=R3=R4=H, R2= CO(CH)14CH3, 2c: R1=R2=R4=H, R3= CO(CH)14CH3, 2d: R1=R2=R3=H, R4= CO(CH)14CH3. B. Avian influenza virus inhibition activity of 1 and 2 in chicken embryonated eggs. Influenza A/Duck/Hong Kong/342/78 (H5N2) was pretreated with or without 1.0μM compounds and then inoculated (50 pfu/egg) into the allantoic fluid of embryonated eggs for 7 days at 37°C. Open circle, no compound; Closed circle, 1.0μM EGCG-16 (2); Open square, 1.0μM EGCG (1); Closed square, 1.0μM Zanamivir; Open lozenge, 1.0μM Oseltamivir phosphate.
A series of human influenza viruses, an experimental strain (A/Puerto Rico/8/34/(H1N1)), vaccine strains (A/Beijing/262/95/(H1N1), A/Panama/2007/99/(H3N2), and B/Yamanashi/166/98/), drug-resistant strains (Yokohama/77/2008/(H1N1) OPR: oseltamivir phosphate-resistant (OPR), Yokohama/63/2007/(H1N1) AR: amantadine-resistant (AR), A/Yokohama/91/2008/(H1N1) OPR/AR: (OPR/AR) and avian pathogenic influenza (A/Duck/Hong Kong/342/78/(H5N2)), were directly incubated with 1 or 2 for 30 min prior to inoculation into MDCK cells. The cells were inoculated with virus, with or without the compounds, for 1hr, and the direct virus inhibitory effects were assessed by a plaque formation assay at 54hr post-incubation. The plaque inhibition activity was calculated relative to no compound. The cytotoxicity of compounds on MDCK cells were evaluated by the MTT cell proliferation assay. Briefly, the cells were incubated with 1 or 2 for 24hr for the MTT assay. The results of the plaque inhibition assay and MTT assay are expressed as mean ± standard error of three independent experiments.
We also investigated the virus inhibition activity of 1 and 2 on avian influenza A/Duck/Hong Kong/342/78 (H5N2) virus in ovo using 11-day-old chicken embryonated eggs (n=12) inoculated with compound-treated or untreated viruses.
As shown in Table 1, both 1 and 2 showed broad virus inhibitory effects on MDCK cells. The EC50 values of 2 on these viruses were between 10 to 61nM, which were approximately 7.1 to 44-fold lower than those of 1. The CC50 value of 2 was 82μM, which was only 3.1-fold lower than that of 1 (255 μM). Thus, the SI value of 2 was improved 2.2 to 14-fold compared to 1.
Table 1.
Direct virus inhibitory effect of 1 and 2 based on the plaque formation assay
| Direct virus inhibitory effecta | SIb | |||
|---|---|---|---|---|
| Virus | EC50c (μM) | CC50d / EC50 | ||
| 1 | 2 | 1 | 2 | |
| A/Puerto Rico8/34 (H1N1) | 0.391±0.056 | 0.020±0.007 | 653 | 4062 |
| A/Beijing/262/95 (H1N1) | 0.436±0.081 | 0.061±0.008 | 585 | 1331 |
| A/Yokohama/77/2008 (H1N1) OPRe | 0.400±0.042 | 0.017±0.008 | 638 | 4779 |
| A/Yokohama/63/2008 (H1N1) ARf | 0.540±0.042 | 0.027±0.008 | 472 | 3009 |
| A/Yokohama/91/2008 (H1N1) OPR/AR | 0.597±0.015 | 0.036±0.012 | 427 | 2256 |
| A/Panama/2007/99 (H3N2) | 0.173±0.006 | 0.010±0.004 | 1476 | 8125 |
| A/Duck/Hong Kong/342/78 (H5N2) | 0.657±0.022 | 0.015±0.006 | 388 | 5416 |
| B/Yamanashi/166/98 | 0.490±0.053 | 0.024±0.008 | 521 | 3385 |
Direct virus inhibitory effect: Virus treated with 1 or 2 for 30min at 37 ºC prior to the inoculation and then inoculated for 2 days at 37ºC. MDCK cells, MIO: 2.5 x 10−4 pfu/cell.
SI (selectivity index) is the ratio of CC50 and EG50.
EC50 represents the concentration of compound required to reduce plaque number by 50% relative to the control well without test compound.
CC50 represents the concentration of compound required to reduce cell viability by 50% relative to the control well without test compound. The CC50 for 1 and 2 were 255.7 ± 6.0 and 81.2 ± 12.5μM, respectively.
OPR: oseltamivir phosphate-resistant virus
AR: amantadine-resistant virus
With respect to the avian influenza virus inhibition assay in ovo, virus treated with 1, zanamivir, or oseltamivir phosphate showed a moderate viral inhibitory effect (Figures 1b). However, 2 almost completely inhibited the infection (Figures. 1b), and the efficacy was retained even at 0.1μM (data not shown).
In summary, 2 inhibited human and avian influenza A and B viruses, including drug-resistant viruses. 2 was found to be more effective than neuraminidase inhibitors, and strongly inhibited the infection of avian influenza (H5N2) virus in chicken embryonated eggs. This unique viral inhibitory action has the potential to be utilized to effectively control a broad spectrum of influenza viruses.
ACKNOWLEDGEMENTS
This study was supported by the Industrial Technology Research Grant Program in 2006 from New Energy and Industrial Technology Development Organization (NEDO) of Japan.
COMPETING INTERESTS
None declared.
REFERENCES
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- Imanishi N, Tuji Y, Katada Y, et al. Additional inhibitory effect of tea extract of the growth of influenza A and B viruses in MDCK cells. Microbiol Immunol. 2002;46:491–494. doi: 10.1111/j.1348-0421.2002.tb02724.x. [DOI] [PubMed] [Google Scholar]
- Lam WH, Kazi A, Kuhn DJ, et al. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (–)-epigallocatechin gallate [(–)-EGCG] Bioorg Med Chem. 2004;12:5587–5593. doi: 10.1016/j.bmc.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Mori S, Miyake S, Kobe T, et al. Enhanced anti-influenza A virus activity of (–)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effects of alkyl chain length. Bioorg Med Chem Lett. 2008;18:4249–4252. doi: 10.1016/j.bmcl.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Suzuki K, Toda M, et al. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kusano R, Kouno I. Synthesis and antioxidant activity of novel amphipathic derivatives of tea polyphenol. Bioorg Med Chem Lett. 1998;8:1801–1806. doi: 10.1016/s0960-894x(98)00311-4. [DOI] [PubMed] [Google Scholar]