Abstract
Aim
To explore the relationship between alteration in the expression of TWIST, highly conserved transcription factor from the basic helix-loop-helix family, and apoptosis of Hep-2 cells induced by chemotherapeutic agent paclitaxel.
Methods
Morphological changes of Hep-2 cells were observed by acridine orange cytochemistry staining. Viability of Hep-2 cells treated with various concentrations of paclitaxel was examined by cell proliferation assay. Apoptosis was examined by flow cytometry. The mRNA and protein expression of TWIST in response to paclitaxel at 24 hours, 48 hours, and 72 hours was examined by reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting, respectively.
Results
Typical morphological changes of apoptotic cells at 24 hours, 48 hours, or 72 hours after treatment with paclitaxel (10 × 10−9 mol/L) were observed. The cell survival rates significantly decreased in a concentration- and time-dependent manner (P = 0.001). Paclitaxel-induced apoptosis increased with culture time (22.6 ± 5.3% after 24 hours, 38.7 ± 7.9% after 48 hours, and 52.4 ± 14.3% after 72 hours; P = 0.002). Both mRNA and protein expression of TWIST was markedly decreased at both mRNA levels and protein levels, at 24 hours, 48 hours, and 72 hours in the paclitaxel-induced apoptosis of Hep-2 cells (P < 0.001).
Conclusion
TWIST, which has a significantly decreased expression in response to paclitaxel in Hep-2 cells, may play a pivotal role in paclitaxel-induced apoptosis of Hep-2 cells.
Laryngeal carcinoma is one of the most aggressive human malignancies, which causes substantial annual morbidity and mortality (1,2). Over the two past decades, the survival rate of the patients has not considerably improved, despite multimodal therapeutic strategies, especially chemotherapy (3,4). Of the chemotherapeutic agents, paclitaxel (Taxol), a natural product derived from the bark of Pacific Yew, has been clinically used alone or in combination with other drugs to treat human cancers. It has been shown that paclitaxel exerts effective inhibitory effect on certain types of human malignant tumors, including the carcinomas of the breast, ovary, lung, esophagus, and head and neck (5-7). Although in vitro studies have shown that paclitaxel-induced cell death occurs via the apoptotic pathway in several tumor cell lines (8), few studies have been conducted to examine how paclitaxel exerts its inhibitory effects on Hep-2 cells, the human laryngeal carcinoma cell line. Recently, Li et al (9) found that paclitaxel induced apoptosis in Hep-2 cell, which was related to the disturbance of cell cycle. However, the precise mechanism by which paclitaxel elicits apoptosis in Hep-2 cells is still unclear.
TWIST, which belongs to basic helix-loop-helix family, is a highly conserved transcription factor. Previous research has confirmed that overexpression of TWIST is found in several kinds of human cancers (10), which not only promotes the immigration and invasion of cancers cells, but also decreases the sensitivity to chemotherapy (11,12). Overexpression of TWIST may a key for tumor metastasis and drug resistance, but the precise mechanism is still unclear (11). As yet, there are no studies on the role of TWIST in paclitaxel-exerted effect on Hep-2 cells. The present study was designed to investigate the possible role of TWIST activity in paclitaxel-induced apoptosis in Hep-2 cells.
Materials and methods
Cell culture
The human laryngeal carcinoma cell line Hep-2 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The Dulbecco’s modified Eagle’s medium (DMEM) and serum was purchased from GIBCO (Carlsbad, CA, USA). TWIST antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Taxol was obtained from Bristol-Myers Squibb Company (New York, NY, USA). All other agents were purchased from Sigma (St. Louis, MO, USA). Hep-2 cells were cultured in DMEM containing 10% fetal calf serum, 100 U/mL penicillin, and 100 mg streptomycin at 37°C in a humidified atmosphere composed of 95% air and 5% CO2. Cells were regularly sub-cultured with 0.25% trypsin and seeded into a Petri dish, 24 or 96-well plate, for different experiments.
Cell viability assessment
Methylthiazoletetrazolium (MTT) assay was employed to assess cell viability (13). MTT assay was based on the ability of the viable cells to reduce soluble yellow MTT to blue formazan crystals. In this assay, optical density (OD) values represented the absorption of formazan dissolved by dimethyl sulfoxide at 570 nm. Cells (1 × 104 per well) were seeded in a 96-well plate containing 100 μL culture medium. After 24 hours of incubation, the medium was removed and refilled with new serum-free medium containing different concentrations of paclitaxel for 24 hours, 48 hours, and 72 hours incubation. At the indicated time points, MTT was added to reach a final concentration of 0.5 mg/mL. After 4 hours of incubation at 37°C, the medium was removed and the precipitate in each well was dissolved in dimethylsulfoxide. The plates were incubated for another 20 minutes in the dark. The OD values were measured at 570 nm using an ELISA reader (Multiskan MK3, Thermo Labsystems, Vantaa, Finland). Cell relative viability was calculated according to the following formula:
Cell relative viability (%) = ODexperiment/ODcontrol × 100% (ODblank was used as base point)
Morphological observation
Morphological changes of Hep-2 cells in response to paclitaxel treatment were evaluated by staining cells with acridine orange. Cells (5 × 104/per well) were seeded in 24-well dishes containing 1 mL culture medium. After 24 hours of incubation, the medium was removed and refilled with new serum-free medium containing paclitaxel (10 × 10−9 mol/L) for 24 hours, 48 hours, and 72 hours incubation. The cells were washed twice by phosphate buffered solution, fixed by 95% alcohol for 10 minutes, and stained by 0.01% acridine orange for 5 minutes. The morphological changes were examined by fluorescence microscope. The cells with typical features of nuclear fragmentation and/or marked condensation of chromatin with reduced nuclear size were interpreted as apoptotic cells.
Flow cytometry analysis
Cell apoptotic progression was assessed by flow cytometry using Annexin V-FITC and propidium iodide (PI). Cells (5 × 104/ml) were seeded in 6-well dishes and were treated with paclitaxel for 24 hours, 48 hours, and 72 hours. After treatment, cells were trypsinized and washed with phosphate-buffered saline. After washing, cells (1 × 107/mL) were resuspended in binding buffer (200 μL) containing Annexin V-FITC (5μL, 20 μg/mL) and PI (10 μL, 20 μg/mL) for at least 15 minutes at room temperature, and then added binding buffer (300 μL) before analysis with a System II, version 3.0 (Beckman Coulter Company, Miami, FL, USA)
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and was further purified with RNeasy purification kit (Qiagen, Chatsworth, CA, USA). The reverse transcription reaction was performed using the ExScriptRT reagent kit (TaKaRa, Dalian, China) in a final volume of 20 μL containing 1 μg total RNA, 4 μL 5 × ExScript buffer, 1μL deoxynucleotide triphosphate mixture, 1 μL OligodT primer, 0.5 μL ExScript RTase, 0.5 μL RNase inhibitor, and RNase-free water to a volume of 20 μL. The reverse-transcription reaction was performed at 42°C for 15 minutes and terminated by heating at 95°C for 2 minutes. PCR was performed following the instruction of TaKaRa TaqTM under the following conditions: pre-degeneration at 95°C for 3 minutes, degeneration at 95°C for 60 seconds, renaturation at 58°C for 45 seconds, and elongation at 72°C for 60 seconds, for a total of 25 cycles. All experiments were conducted 3 times. The primers were as follows:
TWIST: 5′-GG AGTCCGCAGTCTTACGAG-3′;
5′-TCTGGAGGACCTGGTACAGG-3′.
β-actin:5′-CTCCTTAATGTCACGCACGATTT-3′,
5′-GTGGGGCGCCCCAGGCACCA -3′.
Protein extraction and Western blot analysis
The Hep-2 cells were harvested and lysed with a cold radioimmune precipitation buffer protein lysis buffer for 30 minutes on ice. The lysates were transferred to Eppendorf tubes and clarified by centrifugation at 12 000 × g for 15 minutes at 4°C. The supernatant was kept at -80°C for future use. The Bradford method was used to determine the protein concentration of the supernatant. Samples (60 μg of total protein each) were boiled at 95°C for 5 minutes and loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (5% stacking gel and 15% separating gel), followed by a separation at 80 V for about 2 hours and subsequent transfer to a polyvinylidene difluoride membrane. The membrane was blocked in 5% defatted milk for 1 hour at room temperature, incubated in the first antibodies (TWIST1:200, rabbit anti-human; actin, 1:2000, mouse anti-human), and diluted in 5% defatted milk/Tris-buffered saline-Tween (TBS-T) overnight at 4°C. After washing with TBS-T, the membrane was incubated with a secondary antibody against mouse or rabbit IgG accordingly and the signals were visualized using electrochemiluminescence). The actin and TWIST bands were visualized at apparent molecular weights of 43 kDa and 28 kDa, respectively. Relative OD value ratio was calculated with National Institutes of Health software Image J (http://rsbweb.nih.gov/ij/), by comparing it with actin from 3 experiments.
Statistical analysis
Data were presented as mean ± standard deviation. One-way analysis of variance and least significance difference was applied to analyze the data. P values lower than 0.05 (two-tailed) were considered significant. Statistical calculations were performed using SPSS software package, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Effects of paclitaxel on cell viability in Hep-2 cells
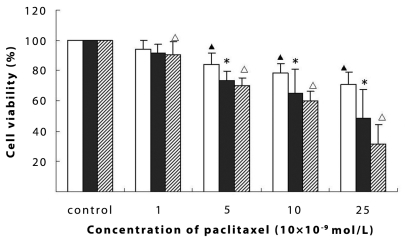
As evidenced by MTT assay, paclitaxel treatment resulted in the reduction of cell viability of Hep-2 cells in a concentration- and time-dependent (Figure 1).
Figure 1.
Effects of paclitaxel on cell viability of Hep-2 cells. The survival rates of Hep-2 cells decreased in concentration- and time-dependent manner. All experiments were performed in triplicate. Data are expressed as means ± standard deviation percent of the control. Significant differences vs control: asterisk – P = 0.001, closed triangle – P = 0.002, open triangle – P<0.001); open bars – 24 hours, closed bars – 48 hours, diagonally striped bars – 72 hours.
Effects of paclitaxel on morphological changes in Hep-2 cells
Morphological changes of Hep-2 cells were first examined by acridine orange cytochemistry staining at 24 hours, 48 hours, or 72 hours after treatment with paclitaxel (10 × 10−9 mol/L) (Figure 2). Cells without paclitaxel treatment showed polygonal shape and cells treated with paclitaxel became rounded and had cytoplasmic contraction and chromatin condensation. Apoptotic bodies, the main morphological characteristic of apoptosis were present.
Figure 2.
Morphological changes in Hep-2 cells treated with Paclitaxel (10 × 10−9mol/L) using acridine orange fluorescence microscopy ( × 200). (A) control; (B) 24 hours; (C) 48 hours; (D) 72 hours.
Flow cytometry analysis
Flow cytometric analysis (Figure 3 and Table 1) showed that the apoptotic rates at 24 hours, 48 hours, or 72 hours after the administration of paclitaxel (10 × 10−9 mol/L) were 22.6 ± 5.3%, 38.7 ± 7.9%, and 52.4 ± 14.3%, respectively, while the control values were 9.9 ± 5.8% (P = 0.002).
Figure 3.
Flow cytometric analysis of apoptotic cells of Hep-2 cells induced by paclitaxel after staining with Annexin V(AN)-FITC/propidium iodide (PI). Histogram for AN/PI bivariate analysis. x-axis represents the density of PI, y-axis represents the density of Annexin V-FITC. D1, upper-left quadrant, contains AN+/PI-, early apoptotic cells; D2, upper-right quadrant, contains AN+/PI+, secondary necrotic cells; D3, lower-left quadrant, contains AN-/PI-, viable, non-apoptotic cells; D4, lower-right quadrant, contains AN-/PI+, damaged viable cells. (A) control; (B) 24 hours; (C) 48 hours; (D) 72 hours.
Table 1.
Flow cytometric analysis of apoptotic cells of Hep-2 cells induced by paclitaxel after staining with annexin V(AN)-FITC/propidium iodide (PI)*
| Percent of cells (%) |
||||
|---|---|---|---|---|
| Quadrants | A | B | C | D |
| D1 |
9.85 ± 5.83 |
22.6 ± 5.30† |
38.7 ± 7.90† |
52.4 ± 14.27† |
| D2 |
4.16 ± 5.05 |
6.01 ± 5.14 |
4.57 ± 5.66 |
4.93 ± 5.18 |
| D3 |
80.30 ± 9.50 |
67.53 ± 6.01 |
55.83 ± 5.81 |
40.3 ± 5.06 |
| D4 | 4.83 ± 5.27 | 3.22 ± 5.00 | 3.07 ± 5.22 | 4.93 ± 5.79 |
*Raw data for each group derived from the Figure 3. Statistical analysis was performed to assess the difference in apoptosis in quadrant D1 between the treated and control group. Data were presented as mean ± standard deviation. All experiments were conducted three times.
†Significant difference in apoptotic rate in group B, C, or D, each in comparison with group A (P = 0.002).
Alteration in expression of TWIST mRNA in Hep-2 cells
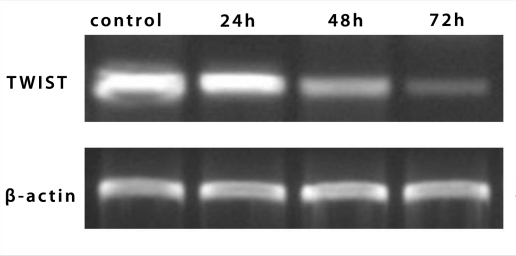
To study role of TWIST in response to paclitaxel treatment, TWIST expression at RNA level was first examined by semiquantitative RT-PCR. The expression of TWIST in Hep-2 was decreased at 24 hours, 48 hours, or 72 hours after treatment with paclitaxel (Figure 4).
Figure 4.
The alteration in expression of TWIST mRNA in Hep-2 cells at different times, as assessed by reverse transcription-polymerase chain reaction analysis.
Alteration in expression of TWIST protein in Hep-2 cells
The expression of TWIST protein was further examined by Western blotting (Figure 5). The data from Western blotting experiment showed that the expression in the control group was 3.21 ± 0.05 optical density units, compared with 2.68 ± 0.06 at 24 hours, 1.69 ± 0.03 at 48 hours, and 0.95 ± 0.03 at 72 hours in treated groups (P < 0.001). The alteration in protein paralleled the changes in mRNA levels.
Figure 5.
The alteration in expression of TWIST protein in Hep-2 cells. (A) The alteration in expression levels of TWIST protein in different times treated with paclitaxel by Western blotting analysis. (B) The optical densities value of TWIST protein in each lane using National Institutes of Health software Image J system were demonstrated in different times treated with paclitaxel. Each data point in the figure represents the mean ± standard deviation of 3 separate experiments. All experiments were conducted 3 times. Asterisk represents statistically significant difference vs control (P < 0.001).
Discussion
The present study demonstrated that paclitaxel decreased cell survival rates in a concentration- and time-dependent manner as evidenced by MTT assay, indicating that paclitaxel can block cell growth of Hep-2 cells. Hep-2 cells treated with paclitaxel also showed cellular rounding, cytoplasmic contraction, chromatin condensation, and apoptotic body formation. These morphological changes are in accordance with typical characteristics of cells experiencing apoptosis (14). Moreover, the apoptotic rates of cells exposed to paclitaxel were increased, further confirming the occurrence of apoptosis rather than necrosis. Our data are similar to a previous report, which showed that paclitaxel induced apoptosis in other solid tumor cell in in vitro studies (15).
Wang et al (16) reported that up-regulation of TWIST was responsible for development of acquired resistance to paclitaxel in HNE1-T3, a nasopharyngeal carcinoma cell line. Subsequent studies also demonstrated that the TWIST-mediated paclitaxel resistance may be regulated through its positive involvement in the Akt pathway (17). Although paclitaxel-induced cell death is thought to trigger apoptotic pathway in certain types of carcinoma, the exact mechanisms responsible for paclitaxel-induced cell death in head and neck cancers are not well understood. To address this issue, we focused on investigating whether TWIST was a key determinant in paclitaxel-induced Hep-2 cell death. In this study, it was for the first time clearly demonstrated that the both mRNA and protein expression of TWIST decreased in the paclitaxel-induced apoptosis of Hep-2 cells. This suggested that TWIST might be involved in paclitaxel-induced apoptosis of Hep-2 cells. It has been reported that high expression of TWIST can lead to increase in chemoresistance to paclitaxel (18,19); conversely, inactivation of TWIST in the nasopharyngeal carcinoma cells through small RNA interference led to increase in sensitivity to paclitaxel (17). Our findings, together with these reports, reaffirm the positive role of TWIST in the development and progression of human cancer. At present, two key apoptotic pathways are known, ie, the mitochondrial pathway and the death receptor pathway, both of which lead to activation of caspase cascades though a series of interactions. This poses the question whether TWIST is involved in these two classical pathways, which remains to be answered in our future work.
In conclusion, the present study demonstrated that transcription factor TWIST participated in paclitaxel-induced apoptosis of Hep-2 cells. The precise role of TWIST underlying the action of paclitaxel needs to be further explored. The intensive investigation of TWIST might not only contribute to obtaining a new insight into the mechanism of chemoresistance, but offers a new target for the treatment of tumor.
Acknowledgment
This work was supported in part by Department of Science & Technology of Shandong province (03BS020).
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 3.Friboulet L, Pioche-Durieu C, Rodriguez S, Valent A, Souquere S, Ripoche H, et al. Recurrent overexpression Of C-Iap2 in Ebv-associated nasopharyngeal carcinomas: critical role in resistance to toll-like receptor 3-mediated apoptosis. Neoplasia. 2008;10:1183–94. doi: 10.1593/neo.08590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 5.Tishler RB, Busse PM, Norris CM, Jr, Rossi R, Poulin M, Thornhill L, et al. An initial experience using concurrent paclitaxel and radiation in the treatment of head and neck malignancies. Int J Radiat Oncol Biol Phys. 1999;43:1001–8. doi: 10.1016/S0360-3016(98)00533-1. [DOI] [PubMed] [Google Scholar]
- 6.Haddad R, Tishler RB, Norris CM, Mahadevan A, Busse P, Wirth L, et al. Docetaxel, cisplatin, 5-fluorouracil (TPF)-based induction chemotherapy for head and neck cancer and the case for sequential, combined-modality treatment. Oncologist. 2003;8:35–44. doi: 10.1634/theoncologist.8-1-35. [DOI] [PubMed] [Google Scholar]
- 7.Haraf DJ, Rosen FR, Stenson K, Argiris A, Mittal BB, Witt ME, et al. Induction chemotherapy followed by concomitant TFHX chemoradiotherapy with reduced dose radiation in advanced head and neck cancer. Clin Cancer Res. 2003;9:5936–43. [PubMed] [Google Scholar]
- 8.Thomadaki H, Talieri M, Scorilas A. Treatment of MCF-7 cells with taxol and etoposide induces distinct alterations in the expression of apoptosis-related genes BCL2, BCL2L12, BAX, CASPASE-9 and FAS. Biol Chem. 2006;387:1081–6. doi: 10.1515/BC.2006.133. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Jiang AC, Dong P, Wan Y, Yu ZW. The characteristics of Hep-2 cell with multiple drug resistance induced by Taxol. Otolaryngol Head Neck Surg. 2007;137:659–64. doi: 10.1016/j.otohns.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–60. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 12.Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–83. [PubMed] [Google Scholar]
- 13.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–7. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 14.Gan Y, Wientjes MG, Schuller DE, Au JL. Pharmacodynamics of taxol in human head and neck tumors. Cancer Res. 1996;56:2086–93. [PubMed] [Google Scholar]
- 15.Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–28. doi: 10.1002/1097-0142(20000601)88:11<2619::AID-CNCR26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Ling MT, Guan XY, Tsao SW, Cheung HW, Lee DT, et al. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene. 2004;23:474–82. doi: 10.1038/sj.onc.1207128. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Wang Q, Ling MT, Wong YC, Leung SC, Wang X. Anti-apoptotic role of TWIST and its association with Akt pathway in mediating taxol resistance in nasopharyngeal carcinoma cells. Int J Cancer. 2007;120:1891–8. doi: 10.1002/ijc.22489. [DOI] [PubMed] [Google Scholar]
- 18.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 19.Syrigos KN, Karachalios D, Karapanagiotou EM, Nutting CM, Manolopoulos L, Harrington KJ. Head and neck cancer in the elderly: an overview on the treatment modalities. Cancer Treat Rev. 2009;35:237–45. doi: 10.1016/j.ctrv.2008.11.002. [DOI] [PubMed] [Google Scholar]