Abstract
The ligand specificity of transforming growth factor beta (TGFβ) in vivo in mouse cardiac cushion epithelial-to-mesenchymal transition (EMT) is poorly understood. To elucidate the function of TGFβ in cushion EMT, we analyzed Tgfb1 −/−, Tgfb2−/− and Tgfb3−/− mice between embryonic day (E) 9.5 and E14.5 using both in vitro and in vivo approaches. Atrioventricular (AV) canal collagen gel assays at E9.5 indicated normal EMT in both Tgfb1−/− and Tgfb3−/− mice. However, analysis of Tgfb2−/− AV explants at E9.5 and E10.5 indicated that EMT, but not cushion cell proliferation, was initially delayed but later remained persistent. This was concordant with the observation that Tgfb2−/− embryos, and not Tgfb1−/− or Tgfb3−/− embryos, develop enlarged cushions at E14.5 with elevated levels of well validated indicators of EMT. Collectively, these data indicate that TGFβ2, and not TGFβ1 or TGFβ3, mediates cardiac cushion EMT by promoting both the initiation and cessation of EMT.
Keywords: Transforming growth factor beta, epithelial mesenchymal transformation, Valvulogenesis, Heart development
INTRODUCTION
Congenital heart disease affects approximately 5% of live births in the United States (Pierpont et al. 2007). About ¼ of these patients have structural anomalies of one or more heart valves (Supino et al. 2004). It is also thought that defects in valve formation can cause various forms of valvuloseptal defects (Bartram et al. 2001a). Valve formation involves EMTs that result in endocardial cushions in the atrioventricular (AV) and outflow tract (OFT) regions of the developing heart (Person et al. 2005;Barnett and Desgrosellier 2003).
In mouse cardiac development, EMT occurs between somite stage S21 and S29 (E9.5-E10.5) and leads to the formation of rudimentary cushions by S30–35 (E10.5-E11.0), and it significantly diminishes by E13 (Camenisch et al. 2002). There is considerable evidence suggesting that both the initiation and cessation of EMT involves signals from the endocardium, adjacent myocardium and cushion mesenchyme, and that they involve multiple growth factors and signaling pathways (Person et al. 2005). Trophic factors belonging to the TGFβ superfamily (i.e., TGFβs and BMPs) secreted from the AV or OFT myocardium are known to induce EMT by activating a subset of endocardial cells to break away from the endocardium, migrate into hyaluronan-rich cardiac jelly, and transform into cushion mesenchyme cells (Butcher and Markwald 2007;Barnett and Desgrosellier 2003;Armstrong and Bischoff 2004). This activated endocardium can be identified by its distinct cellular and subcellular changes (Markwald et al. 1975). EMT is a transient process and it is difficult to visualize in vivo during heart development, therefore, numerous investigators have used change in cellular (cell-cell contact, cell size) and molecular (reduced endothelial or increased mesenchymal gene expression) properties as a standard approach to assess EMT (Markwald et al. 1977;Timmerman et al. 2004;Paruchuri et al. 2006).
Several explant studies in avian and mammalian systems have suggested that TGFβ1, 2 and 3 play important roles in the regulation of cardiac EMT (Mercado-Pimentel and Runyan 2007). However, the ligand specificity of their roles in EMT is not clear. Following TGFβ ligand activation TGFβ usually interacts with a heteromeric complex of TGFβ type II (TGFβR2) and TGFβ type I (ALK5) receptors. This interaction is strengthened by type III coreceptor-like molecules, TGFβR3 or endoglin (Brown et al. 1999;Mercado-Pimentel et al. 2007). Canonical TGFβ signaling culminates in the activation of SMAD2 or 3 which then regulate transcription of target genes that regulate EMT (Lan et al. 2007;Massague and Gomis 2006;Bharathy et al. 2008).
Some clarity for TGFβ ligand specificity in EMT has been afforded by expression studies. In general, the Tgfb1, Tgfb2 and Tgfb3 are expressed in significantly non-overlapping fashion in mouse during EMT (Azhar et al. 2003;Millan et al. 1991;Dickson et al. 1993;Camenisch et al. 2002). Tgfb1 is specifically expressed in mouse, but not in chick AV endocardial cells during EMT (Molin et al. 2003;Potts et al. 1992). Intriguingly, chick AV endocardial cells express Tgfb3, which is not significantly expressed in mouse cushion endocardium during EMT (Boyer et al. 1999;Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002), suggesting differential utilization of TGFβ1 and TGFβ3 in EMT in chick and mouse. Tgfb2 is the most widely and intensely expressed in both mouse and chicken (Boyer et al. 1999;Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002). Tgfb2 is predominantly localized to both the cushion myocardium and endocardium in mouse and chick during EMT (Boyer et al. 1999;Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002). The endothelium-derived mesenchymal cells, which begin to populate the AV endocardial cushions as a consequence of EMT also express Tgfb2 (Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002). Overall, in mouse, Tgfb1 and Tgfb2 both express in AV endocardium, but Tgfb1 expression is stronger than Tgfb2, and only Tgfb2 is expressed intensely in cushion myocardium and invading mesenchymal cells; and there is no detectable expression of Tgfb3 during EMT. Post-EMT in mouse (E11.0 and after), Tgfb1 continues to be expressed in cushion endocardium; whereas, Tgfb2 is expressed predominately in cushion mesenchyme and myocardium, and Tgfb3 is expressed less intensely only in cushion mesenchyme (Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002). Collectively, the differential temporal and spatial gene expression pattern, as well as the differential intensity of expression of the three Tgfb ligand genes suggests non-overlapping roles in cushion EMT in mouse.
Even though there are differential expression patterns for TGFβ ligands in mouse heart development, no cardiac developmental defects have been detected in TGFβ1- or TGFβ3-deficient mice (Azhar et al. 2003;Shull et al. 1992;Kulkarni et al. 1993;Diebold et al. 1995;Proetzel et al. 1995;Kaartinen et al. 1995). Only Tgfb2−/− mice exhibit multiple congenital cardiovascular malformations, and they develop enlarged valves at E18.5 (Sanford et al. 1997;Bartram et al. 2001b). In vitro studies have indicated that while only TGFβ2 is required for EMT in mouse and man, addition of the other ligands can also induce EMT in chicken and sheep in embryonic and adult heart, respectively (Camenisch et al. 2002;Yang et al. 2008;Boyer et al. 1999;Potts and Runyan 1989;Nakajima et al. 1994). However, these in vitro findings are not consistent with the fact that EMT occurs in Tgfb1, Tgfb2 and Tgfb3 knockout mouse strains (Azhar et al. 2003). Thus, the role of TGFβ ligands in vivo in cardiac cushion EMT in mice warrants further investigation.
In the present study we have cultured AV canal explants from Tgfb1, Tgfb2 and Tgfb3 knockout mice in vitro on collagen gels and determined that TGFβ2 is the only ligand whose absence affects cardiac cushion EMT. The results have delineated two stages of EMT that are differentially affected by TGFβ2. EMT is delayed in E9.5 AV explants of TGFβ2-defcient mice, indicating that TGFβ2 promotes early-stage EMT. However, in TGFβ2-deficient E10.5 AV explants, EMT is increased, indicating that TGFβ2 is also required for promoting the downregulation of EMT after it has completed the cellularization of the cushion. BrdU labeling assays at E10.5 indicate that dysregulated EMT is not due to altered cushion cell proliferation. The persistence of EMT is concordant with the observations that Tgfb2−/− embryos, and not Tgfb1−/− or Tgfb3−/− embryos, develop enlarged cushions at E14.5 with elevated levels of validated endothelial or mesenchymal indicators of EMT. Collectively, these data indicate that TGFβ2 is the major TGFβ that mediates EMT by affecting both the initiation and cessation stages of EMT.
EXPERIMENTAL PROCEDURES
Mouse strains and embryo collection
All procedures are approved by the Institutional Animal Care and Use the Committee at University of Arizona. Tgfb1−/− (129/CF-1 (50:50 mix) and BALB/c (N7)), Tgfb2−/− (129/Black-Swiss; 50:50 mix) and Tgfb3−/− (CF-1 (N7)) mice (Shull et al. 1992;Bommireddy et al. 2006;Sanford et al. 1997;Proetzel et al. 1995) were generated and genotyped as described.
AV canal collagen gel assays
Mouse AV canal collagen gel assays were performed as previously described (Camenisch et al. 2002). Because embryos develop at different rates under the same incubation conditions or within the same litter, somite number was utilized as an additional method for comparing wild-type and Tgfb2−/− mice. To assess early-stage EMT, AV canal collagen gel assays were performed on wild-type and Tgfb2−/− embryos at S21–29 stages (E9.5-E10.5) of gestation. All mesenchymal cells produced by each AV explant were counted (cells observed below the gel surface using Hoffman Differential Contrast microscopy). Mesenchymal cell numbers in AV explants were averaged from wild-type and Tgfb1−/−, Tgfb2−/− or Tgfb3−/− animals, and compared between the genotypes. To determine whether EMT is prolonged in Tgfb2−/− mice, AV explants undergoing late-stage EMT (S30–35 or E10.5-E11.0) were used. Briefly, the epicardial layer was disrupted by scraping the surface of the heart with a fine tungsten needle at the time the AV explants were harvested to remove or destroy this potential source of mesenchyme, and AV explants were then cultured for 1 day to allow the invasion of existing cushion mesenchymal cells into the gel matrix. These explants were then transferred to fresh collagen gels and cultured for an additional 48 hr. The newly formed mesenchymal cells (below the gel surface) on the transplanted gels were then identified based on their morphology by using Alexa Flour 488 phalloidin and counted as described (Camenisch et al. 2000). Although we removed or destroyed the epicardial cell layer at the time AV explants were harvested, we could not distinguish clearly between any possible remaining contaminant epicardial cells and endocardial cells. Confirmation of endothelial origin in AV explants on collagen gels, is not trivial because embryonic cardiac endothelia do not display many of the normal markers used in adult cells and those that are, including VE-Cad and P-CAM, are lost after EMT (Todd Camenisch, personal communication). As the endocardium and epicardium are the only possible sources of these cells, differences between the wild type and Tgfb2−/− mice reflect the unimpeded endothelial source and, perhaps, a few cells that may have escaped the epicardial disruption procedure. As both explant types were collected at the same time and multiple explants were measured in the comparison in these experiments, measured differences in mesenchymal cell formation are principally due to differences in EMT by the endocardium after loss of TGFβ2.
To determine the effects of the genotypes on cell proliferation in similar but separate experiments, 100 µM BrdU (BD Pharmingen) was added 2 hr prior to the termination of the experiment, and % BrdU cells was determined according to published literature (Mercado-Pimentel et al. 2007).
Histology and light microscopic morphometry
Hematoxylin and eosin staining was performed on serial tissue sections for routine histological examination and estimating total cushion cell count (Sanford et al. 1997). About 10 consecutive and corresponding tissue sections per animal were scored to determine relative total cushion cell count. Plastic blocks containing embryos were sectioned at 2 µm thick, and stained with toluidine blue for light microscopic morphometry to determine cellular size (µm) in cushion cells (Okunade et al. 2007). All sections were analyzed using bright-field optics with a Zeiss Axio Imager M1 microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY), and morphometric measurements on the captured images was done by AxioVision 4.6.3 imaging software.
BrdU incorporation assays
BrdU labeling was used for an in vivo quantification of cell proliferation at E10.5. Mouse embryos (E10.5) were labeled for 4 hr with 120 µg/gm body weight of BrdU labeling solution (Sigma), by means of an i.p. injection into the body cavity of timed-pregnant mothers. Consecutive serial sections of wild-type (N =3) and Tgfb2−/− (N = 3) embryos were evaluated by H-E for histology and immunostaining using anti-muscle actin (HHF-35, Dako) for cardiac morphology and anti-BrdU antibody (Cat # B-2531; Sigma) for cell proliferation (Abdelwahid et al. 2002). About 10 consecutive and corresponding tissue sections per animal were scored to determine relative % BrdU cushion cells.
Transmission electron microscopy
The cushion endothelial cell ultrastructure of control (N =4) and Tgfb2−/− (N =4) hearts (E14.5) was examined as described (Okunade et al. 2007). The junctional complexes (tight junctions, gap junctions and desmosomes) per micron of lateral cellular membrane were counted. In addition, a subjective assessment of the junctional complexes, basal membrane of the endocardial cell, and the heterochromatin was made from the micrographs. These morphometric measurements were done according to published literature (Okunade et al. 2007).
Quantitative real-time RT-PCR (Q-RT-PCR) procedures
Atrioventricular tissues from 6 mice were pooled per genotype and total RNA was isolated by RNeasy Mini kit (Cat # 74704: Qiagen, Valencia, CA). Three different pooled samples representing a total of 18 mice per genotype were assessed (each in triplicate) by Q-RT-PCR. Q-RT-PCR was performed according to the method of Rajan (Rajan et al. 2006) using an Opticon 2 real-time PCR machine (Biorad, Hercules, CA). The relative amount of target mRNA normalized to Gapdh was calculated according to the method described by Pfaffl (Pfaffl 2001). Specific primers that were used for the Q-RT-PCR amplification included (all sequences in 5’–3’ directions): VE-Cadherin forward, CCTGACTGGAACCAGCACGCT; VE-Cadherin reverse, GTGTGTCGTATGGGGGGCCAC; Pecam1 forward, GTCATGGGCCATGGTCGAGTA; Pecam1 reverse, CTCCTCGGCATCTTGCTGAA; Fibronectin forward, CCGGGCCTCAATCCAAA; Fibronectin reverse, GGAACGGCGTCCAAGAGAT; Mmp2 forward, ACAACTACCCCATGCCAATTG; Mmp2 reverse, CGCTCGTAGGCAGCACTGA; GalTase forward, TTTGACCGGATCGCACATAC; GalTase reverse, CCAACACCTTGTAGTAGGTAAGTGAGTTC; Gapdh forward, TGACCACAGTCCATGCCATC; Gapdh reverse, GACGGACACATTGGGGGTAG.
Immunohistochemistry
Immunohistochemistry (IHC) was done by using immunostaining kit (LSAB+ System-HRP (Cat #: K0690), according to the protocol of the manufacturer (DakoCytomation, CA). Antigen retrieval was performed in Target Retrieval Solution (Cat #: S1700; DakoCytomation, USA) for 15–20 minutes at 95°C. The following primary antibodies were used: muscle actin (Clone: HHF35, Cat # M0635; DakoCytomation) (1:50 dilution), VE-Cadherin (Cat # 144–11D4; Research Diagnostics, Inc) (1:100 dilution on acetone-fixed frozen sections), diphosphorylated ERK-1&2 (dp-ERK, clone MAPK-YT, Cat# M8159; Sigma, MO), Fibronectin (Cat# AB2033, Chemicon/Millipore, MA). For control staining, IgG isotype controls and/or preimmune serum (Pierce, IL) were used instead of the primary antibody.
Immunoblot analysis
A RAS pull-down assay was used for measuring activated RAS. Protein lysates from individual hearts of E14.5 wild-type (N = 3) and Tgfb2−/− (N =3) mice were made. The RAS Activation Assay Kit (Millipore, cat# 17–218) was used to determine the activated RAS and total RAS (before the pull-down) according to the manufacturer’s guidelines. Western blot procedures were used as described (Prasad et al. 2005). Densitometry was done by using the program ONE-DScan (Scanalytics, VA, USA).
Statistical analysis
Microsoft Excel was used for managing the data. Findings are reported as means ± S.E of the mean, and Student’s t-test (SigmaPlot, Systat Software, Inc., CA) was used for comparing groups. p-values were calculated, and a p<0.05 was considered significant.
RESULTS
TGFβ2 is the major TGFβ involved in cushion EMT
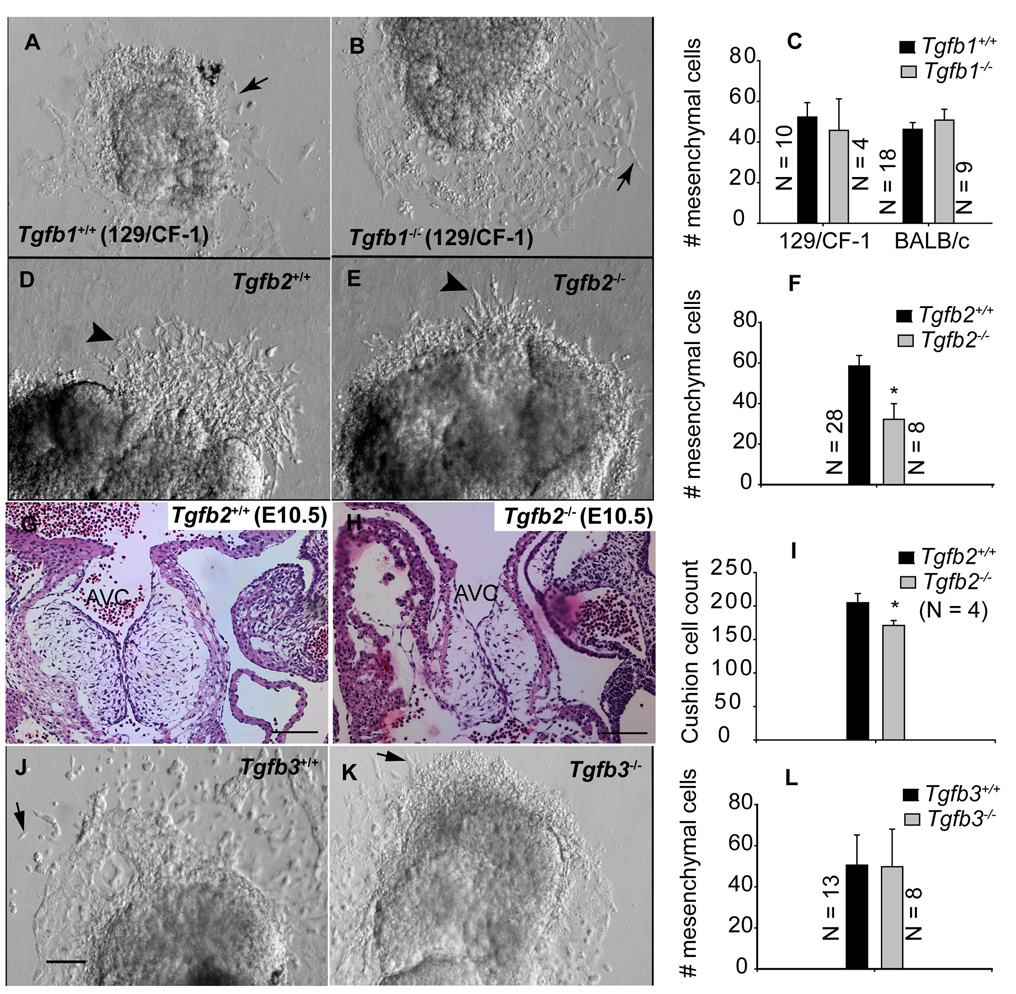
Tgfb1, Tgfb2 and Tgfb3 knockout mice at S21–29 (~E9.5) were subjected to AV canal collagen assays to elucidate ligand-specific functions of TGFβ in cushion EMT. The results indicated that the number of mesenchymal cells which invaded the collagen gels were not significantly different between wild-type and Tgfb1−/−mice (Fig. 1A–C). Analysis of AV explants of Tgfb2−/− mice ex vivo on collagen gels revealed a significant reduction in the number of invading mesenchymal cells (Fig. 1D–F), suggesting that TGFβ2 is required for full and undelayed early-stage EMT. Determination of relative cushion cell count in H-E stained serial sections of E10.5 cushions of wild-type and Tgfb2−/− mice indicated a reduced number of mesenchymal cells (Fig. 1G–I). The AV canal collagen gel assays of Tgfb3−/− mice indicated that the number of transformed mesenchymal cells was not significantly different between wild-type and Tgfb3−/− mice (Fig. 1J–L). Overall, these results indicate that TGFβ2, and not TGFβ1 or TGFβ3, is the major TGFβ ligand that promotes early stage EMT both in vitro and in vivo.
Fig.1. Effect of gene targeted disruption of Tgfb1, Tgfb2 and Tgfb3 on cushion EMT at an early stage.
Representative images of in vitro AV canal collagen gel assays (A,B,D,E,J,K) of Tgfb1+/+ (A) and Tgfb1−/− (B), Tgfb2+/+ (D) and Tgfb2−/− (E), and Tgfb3+/+ (J) and Tgfb3−/− (K) embryos at S21–29 (E9.5). Images were taken after 24 hr of explant culturing. Note that the number of mesenchymal cells present in the collagen gels cannot be determined with precision from the micrograph as many of the mesenchymal cells in our explant cultures were found beneath endothelial cells, and therefore they were not captured in the micrographs. C,F,L: Quantification of the number of mesenchymal cells (mean±s.e.m) below the gel surface (e.g., arrows (A,B,J,K) or arrowheads (A,B)). The number of mesenchymal cells is not significantly changed in Tgfb1−/− mice (C) (P=0.6640 for 129/CF-1, P= 0.4573 for BALB/c) or Tgfb3−/− mice (L) (P= 0.9737), but it is significantly reduced in Tgfb2−/− mice (F) (*P=0.0203). G-I: H-E stained images of showing AV cushions of E10.5 wild-type (G) and Tgfb2−/− mice (H) and the relative total cushion cell count in these embryos (I). The total cushion count is significantly reduced in Tgfb2−/− cushions (*P= 0.0201). Images are representative of 4 wild-type/mutant embryo pairs. Scale bar in (J ) applies to (A-B, D-E, J-K) = 100 µm; (G,H) = 100µm. AVC, AV cushions.
Tgfb2−/− mice, and not Tgfb1−/− or Tgfb3−/− mice, develop enlarged cushions at E14.5
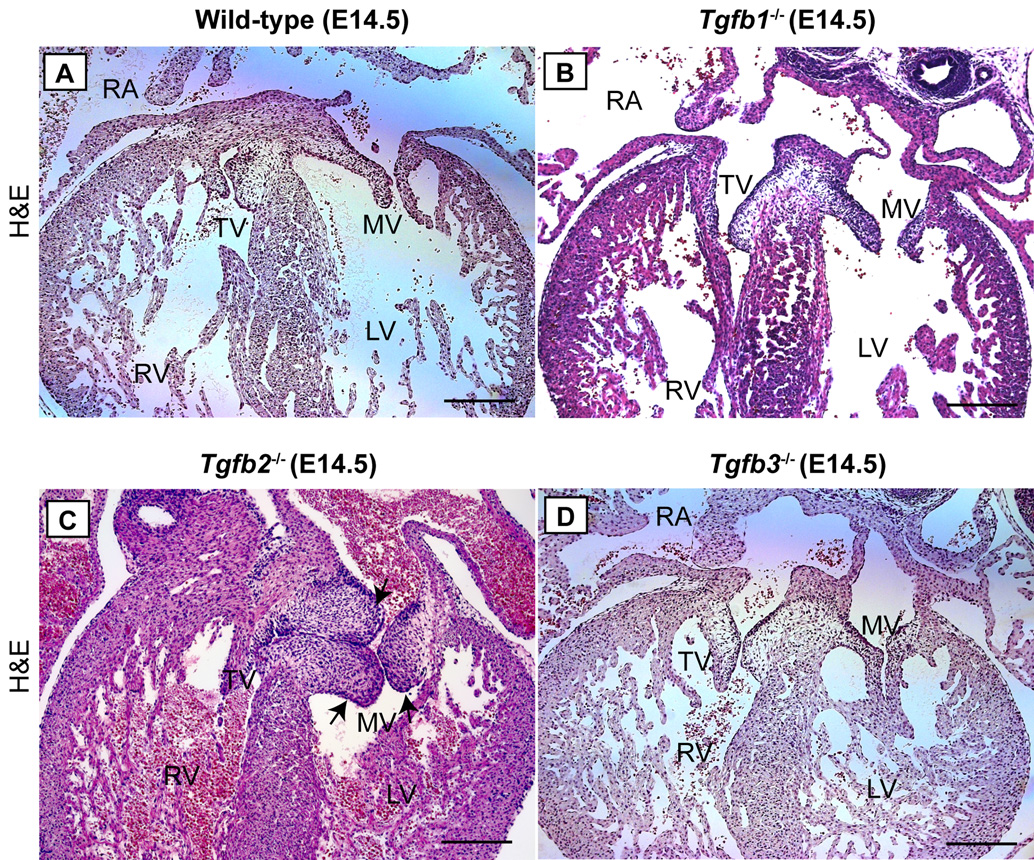
Histological examination of H-E stained serial sections of E14.5 Tgfb knockout mice was performed to determine the TGFβ-ligand specific requirement in cushion formation. The results showed that Tgfb1−/− or Tgfb3−/− mice developed normal cushions (Fig. 2A,B,D). These data are consistent with the normal cushion EMT found in vitro on collagen gel explants from these mutant mice (Fig. 1A–C,J–L). In contrast, the histological analysis of Tgfb2−/− mice indicated multiple cardiac malformations as reported by us previously (Bartram et al. 2001a) and enlarged AV cushions at E14.5 (Fig. 2A,C). Average relative count of the total cushion cells (mean ± s.e.m) was also significantly increased in these mutant hearts (Wild type = 369.67 ± 34.8537, Tgfb2−/− = 570.00 ± 23.4592. N = 3, P = 8.8508e-3). Although these data validate our previous findings that Tgfb2−/− mice have enlarged cushions at E18.5 (Bartram et al. 2001a), they indicate for the first time that the hypercellular nature of cushions in these mice is established by mid-gestation and suggest that prolonged EMT or cell proliferation is involved in the cushion defects in Tgfb2−/− mice. Together, these results indicate an important role for TGFβ2, and not TGFβ1 or TGFβ3, in cushion formation during heart development.
Fig.2. Cushion morphology in Tgfb1−/−, Tgfb2−/− and Tgfb3−/− mice at E14.5.
A-D: Representative of serially sectioned H-E stained images of wild-type (A), Tgfb1−/− (B), Tgfb2−/− (C) and Tgfb3−/− (D). Only Tgfb2−/− mice develop dysplastic or enlarged cushions (C, arrows) (see Results section for a quantification of total cushion cells). Notably, this mutant heart also has overriding tricuspid valves. Scale bar: 200 µm (A–D). TV, tricuspid valves; MV, mitral valves; RA, right atrium; RV, right ventricle; LV, left ventricle.
TGFβ2 promotes downregulation of EMT after the cardiac cushions are formed
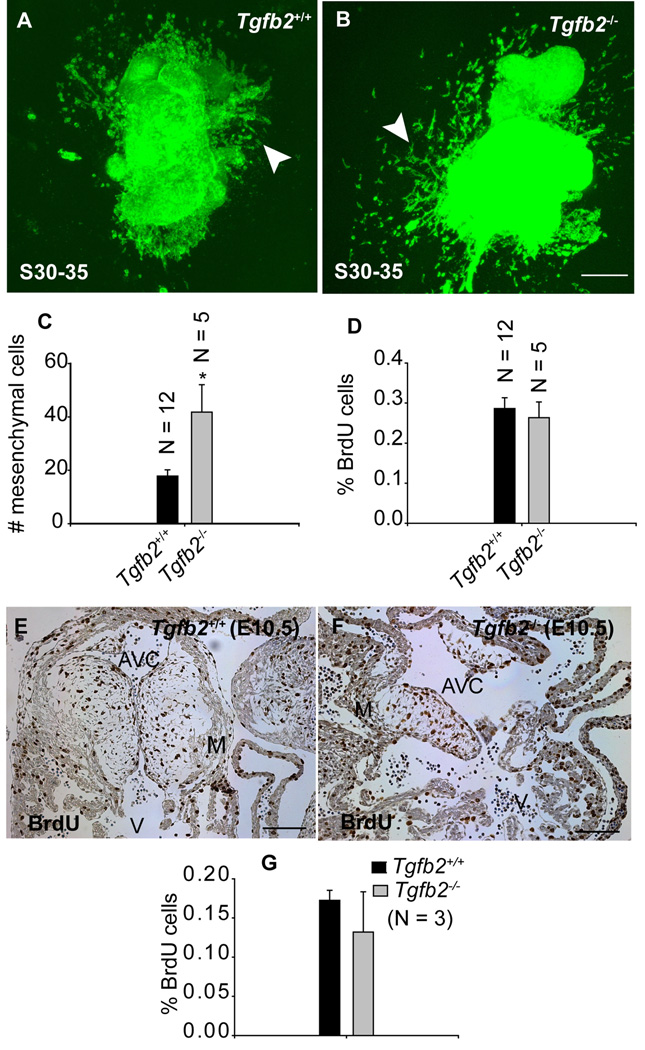
To determine whether TGFβ2 plays a role in regulating EMT after cushions are formed, cushion explants undergoing late-stage EMT (S30–35 or E10.5) in wild-type and Tgfb2−/− mice were cultured for 1 day on collagen gels to allow the invasion of initially formed mesenchyme. Care was taken to disrupt the epicardial cell layer before the explants were cultured. The AV explants were then transplanted to fresh collagen gels for an additional 48 hr. The number of newly formed mesenchymal cells was then counted. The results indicated that the EMT that is diminished in wild-type mice (compare Fig. 1D,F with Fig. 3A,C) did not abate in Tgfb2−/− mice, and there was a significant increase in the number of mesenchymal cells freshly invading the gels from mutant tissue (compare Fig. 1D,E,F with Fig. 3A,B,C). Since epicardium is disrupted in these explants leaving the endocardial cells the only remaining source of mesenchymal cells in the transplanted gels (see Experimental Procedures for details), these results suggest that endocardial EMT was prolonged in Tgfb2−/− mice. BrdU labeling of the serially transplanted AV explants on collagen gels was performed in order to determine whether these increased numbers of transformed mesenchymal cells in mutants were due to increased cell proliferation. The results indicated no significant change in % BrdU cells between wild-type and Tgfb2−/− mice (Fig. 3D), suggesting that cell proliferation was not responsible for the increased number of mesenchymal cells seen in AV canal collagen gel assays in Tgfb2−/−mice. BrdU labeling assays in vivo at E10.5 also indicated normal cushion cell proliferation in cardiac cushions in the absence of TGFβ2 (Fig. 3E,F,G). Collectively, these results demonstrate that after cushions are formed, TGFβ2 plays an essential role in bringing late-stage EMT to a conclusion.
Fig.3. Effect of gene targeted disruption of Tgfb2 on cushion EMT and cell proliferation after the rudimentary cushions are formed at E10.5.
A-B: Representative confocal images of alexa-fluor488-phalloidin stained S30–35 (E10.5) AV canal explants of Tgfb2+/+ (A) and Tgfb2−/− (B) embryos after 60 hr of culturing. The AV explants in which the epicardial cell layer is removed or destroyed at the time of harvesting are cultured for 12 hr to let the pre-existing cushion mesenchymal cells migrate out of the AV explants and then transferred to fresh collagen gels for an additional 48 hr. (C) The number of mesenchymal cells (mean±s.e.m) below the gel surface (arrowhead, a-b). Notably, as the endocardium and epicardium are the only possible sources of mesenchyme in this experiment, differences between the wild type and Tgfb2−/− mice reflect the unimpeded endothelial source and, perhaps, a few cells that may have escaped the epicardial disruption procedure (see Experimental Procedures for details). (D) % of BrdU-incorporated mesenchymal cells (mean±s.e.m) in vitro on collagen gels. The number of mesenchymal cells is significantly increased in Tgfb2−/− mice (C) (*P=0.0032) but there is no difference in % of BrdU cells between wild-type and mutants (D). e-g: Anti-BrdU-stained sections of E10.5 AV cushions of wild-type (E) and Tgfb2−/− (F) mice and the % of BrdU-incorporating cushion cells (G) (brown color staining in E-F). Cell proliferation is comparable between wild-type and mutant cushions at E10.5 (P=0.9416). Images (E,F) are representative of three wild-type/mutant embryo pairs. Scale bar: 100 µm (A,B,E,F).
Enhanced cushion endothelial cell-cell separation during heart development in Tgfb2−/− mice
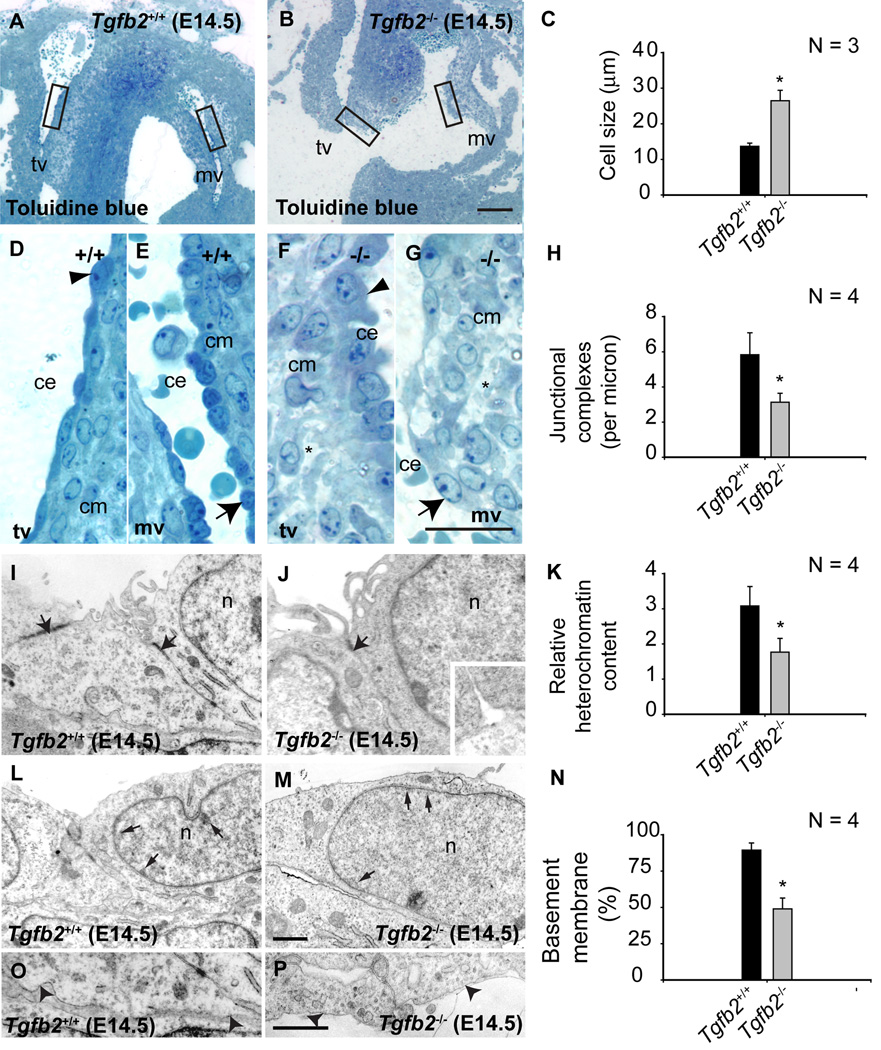
Histological and morphometric examination of toluidine-blue-stained thin sections at E14.5 revealed enlarged cushion endothelial cells in Tgfb2−/− mice (Fig. 4A–G). Transmission electron microscopy and the morphometric analysis of E14.5 endocardial cushions showed that junctional complexes between cushion endothelial cells (Fig. 4H,I,J), their nuclear heterochromatin content (Fig. 4K,L,M), and their basement membrane (Fig. 4O,P,Q) were significantly reduced. Since endothelial cell-cell contacts are re-established after E10.5 during the reduction in EMT that occurs in normal heart development (Timmerman et al. 2004), these data indicate that in the absence of TGFβ2 there are significant morphological and sub-cellular changes consistent with enhanced cushion endothelial cell-cell separation and endothelial activation, suggesting prolonged EMT in vivo during cardiac development.
Fig.4. Cellular and ultrastructural changes in cushion endothelial cells in Tgfb2 −/− mice at E14.5.
A-G: Toluidine blue staining of wild-type (A) and Tgfb2−/− (B) AV cushions. Boxes in (A) and (B) are magnified in (D,E) and (F,G), respectively. Asterisks (F,G) indicate the abnormal organization and composition of cardiac jelly in mutants. Cushion endothelial cells are enlarged in mutants (arrowheads (D,F) and arrows (E,G)). C: Morphometric comparison showing significantly increased cushion endothelial cell size (mean±s.e.m) in Tgfb2−/− embryos (*P= 0.0138). I-Q: Ultrastructural and morphometric comparison of wild-type and Tgfb2−/− mice by transmission electron microscopy. Electron micrographs of representative E14.5 Tgfb2+/+ (I,L,P) and Tgfb2−/− (J,M,Q) mice, illustrating decreased endothelial junctional complexes (arrows, I,J), heterochromatin organization (arrows, L,M), and basement membrane (arrowheads, P,Q). Morphometric comparison (mean±s.e.m) of junctional complexes (H), relative heterochromatin content (K) and % of basement membrane (O) confirms these findings. *P=0.0320 (junctional complexes), 0.0306 (heterochromatin content), 8.8927e-5 (basement membrane). Images (A,B,D-G) and (I,J,L,M,P,Q) are representative of four wild-type/mutant embryo pairs. Scale: 100 µm (a,b), 25 µm (D,E,F,G), 1 µm (scale in Q applies to I,J,L,M,P). mv, mitral valves; tv, tricuspid valves; ce, cushion endothelium; cm, cushion mesenchyme; n, nucleus.
Reduced expression of endothelial markers of EMT in Tgfb2−/− mice during heart development
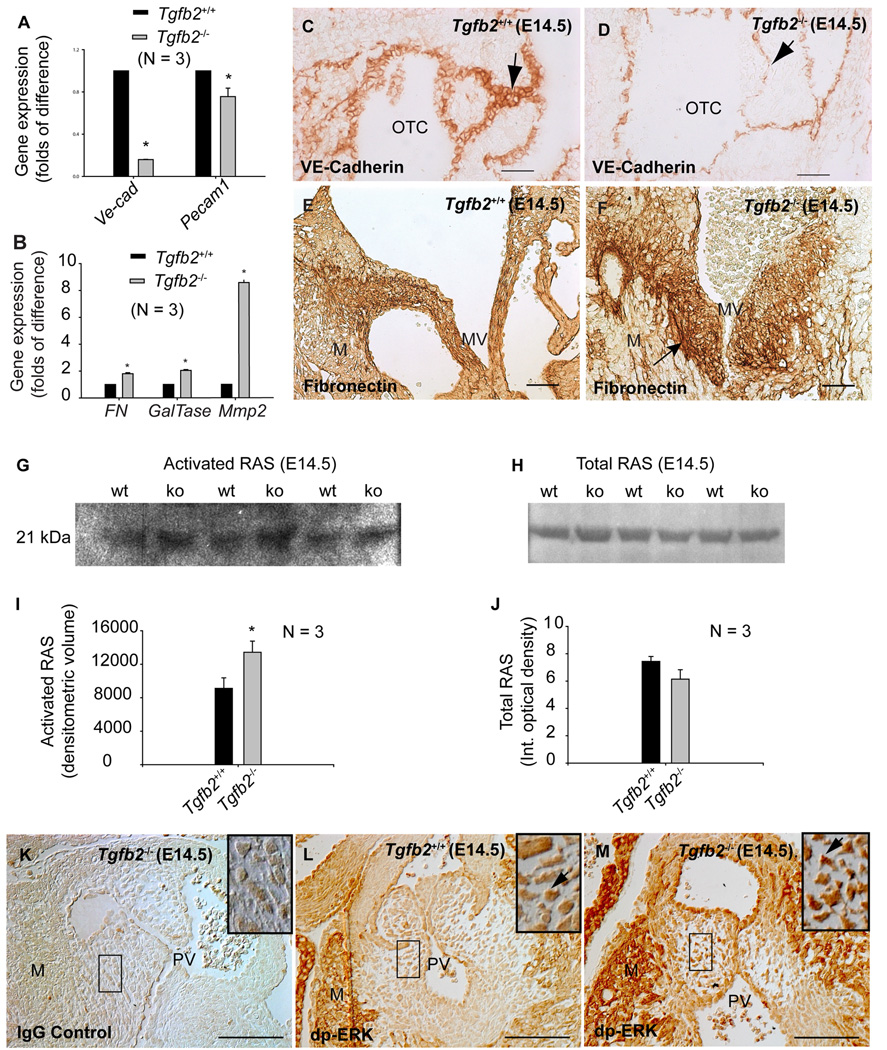
Gene expression analysis of the validated endothelial EMT markers VE-Cadherin (VE-Cad) (Timmerman et al. 2004;Mercado-Pimentel et al. 2007) and PECAM1 (Pecam1) (Enciso et al. 2003;Paruchuri et al. 2006) was undertaken to assess EMT in vivo in wild-type and Tgfb2−/− mice at E14.5. Q-RT-PCR analysis of AV explants indicated significant reductions of VE-Cad and Pecam1 (Fig. 5A). Consistent with prolonged EMT, there were more cushion endothelial cells in E14.5 mutants (mean±s.e.m: 228.33±14.52, P=0.0203) than in wild-type (mean±s.e.m: 159.00±11.59) as determined by cushion cell counting of H-E stained serial sections; yet there was less VE-Cadherin staining (Fig. 5C,D) as well as less VE-Cad expression (Fig. 5A). Therefore, since every cushion endothelial cell is stained positive with VE-Cadherin (Fig. 5C,D), there must be less VE-Cad expression in each cushion endothelial cell in mutants. Since EMT is not diminished after E10.5 in Tgfb2−/− mice (compare Fig. 1F with Fig. 3C), these data collectively indicate that in the absence of TGFβ2 there is a prolonged EMT in vivo during heart development.
Fig.5. Cushion EMT marker expression in Tgfb2−/− mice at E14.5.
A-B: Q-RT-PCR (mean±s.e.m.) showing significantly decreased VE-Cad (*P=7.0000e-10) and Pecam1 (*P=0.0392) (A), and increased Fibronectin (*P = 0.0016), GalTase (*P=2.6790e-4) and Mmp2 (*P=6.4998e-6) (B) in AV explant tissue of Tgfb2−/− mice. Each wild-type value is normalized to 1.0. C-D: Anti-VE-Cadherin staining of wild-type (C) and Tgfb2−/− (D) mice shows that VE-Cadherin is reduced in mutants (arrows, C-D). E-F: Anti-FN staining of wild-type (E) and Tgfb2−/− (F) mice confirms the increased FN in AV cushions of Tgfb2−/− mice (arrow, F). Note that Tgfb2−/− cushions in (F) are naturally enlarged. G,H: Representative western blots showing increased activated RAS (G) and unaltered total RAS (H) in Tgfb2−/− hearts. The blots are a representative of at least 3 wild-type/mutant embryo pairs. I,J: Densitometric quantification (mean±s.e.m) confirms increased activated RAS in mutants (I) (*P=0.0394) without any significant change in the total RAS (J) (P=0.1710). K-M: Tgfb2−/− cushions stained with IgG (K) and anti-dp-ERK (L) are compared with anti-dp-ERK stained cushions of Tgfb2+/+ mice (M). Areas under the box in (K–M) are magnified (insets). Tgfb2−/− cushions have increased dp-ERK (arrows, L-M). Images (C-D, E-F, K-M) are representative of at least three wild-type/mutant embryo pairs. Scale bar: 50 µm (C-D, E-F, K-M). OTC, outflow tract cushion; MV, mitral valves; M, myocardium; PV, pulmonary valves.
Mesenchymal markers of EMT are elevated in Tgfb2−/− mice during heart development
Increased expression of Fibronectin (Fn) (Ahmed and Nawshad 2007), β-1,4-galactosyltransferase (GalTase) (Boyer et al. 1999) and matrix-remodeling enzyme (Mmp2) (Enciso et al. 2003) are commonly used mesenchymal indicators for monitoring an ongoing EMT process. Gene expression analysis by Q-RT-PCR showed that expression of Fn, GalTase and Mmp2 in AV explants of E14.5 Tgfb2−/− mice was increased from basal levels (Fig. 5B). IHC analysis showed higher levels of Fibronectin in cushions of E14.5 Tgfb2−/− mice, corroborating the Q-RT-PCR results (Fig. 5E,F). These data indicate prolonged EMT in mutants during heart development which contributes to their enlarged cushions.
Enhanced in vivo expression of activated RAS and dp-ERK indicates prolonged cushion EMT in Tgfb2−/− mice during heart development
Enhanced levels of both activated RAS and dp-ERK have been shown to result in enhanced cardiac cushion EMT both in vitro (EMT) (Lakkis and Epstein 1998) and in vivo (Gitler et al. 2003). RAS pull-down assays were used to determine in vivo levels of activated in AV explants of E14.5 Tgfb2−/− mice. The results indicated that the levels of activated RAS were significantly increased in mutants (Fig. 5G,I) without any significant alteration in the total RAS between wild-type and Tgfb2−/− mice (Fig. 5H,J). IHC analysis indicated that levels of dp-ERK, a downstream mediator of activated RAS, were also increased in cushions of E14.5 Tgfb2−/− mice (Fig. 5K–M). These data along with the data indicating a prolonged EMT in Tgfb2−/− mice in vitro on collagen gels (Fig. 3A–D) are concordant with the prolonged EMT seen in vivo during heart development in TGFβ2-deficient mice. Collectively, these data demonstrate that in the absence of TGFβ2 there is a prolonged EMT that leads to dysmorphic, thickened valves during heart development.
DISCUSSION
This study used ligand-specific Tgfb knockout mice to provide the first clear evidence that TGFβ2 is the major TGFβ ligand that mediates cushion EMT both ex vivo and in vivo during cushion formation in heart development in mouse. Previous studies utilizing TGFβ blocking antibodies on wild-type AV explants in collagen gel culture assays have suggested a positive role for TGFβ2 in EMT in both chicken and mouse AV explants (Boyer et al. 1999;Camenisch et al. 2002), but this seemed inconsistent with the original study showing that EMT occurs in Tgfb2−/− mice (Sanford et al. 1997). The study presented here resolves these seeming inconsistencies by demonstrating that TGFβ2 promotes early-stage EMT both in vitro and in vivo, and later promotes the downregulation of EMT both in vitro and in vivo. Hence, at E10.5 and in the absence of TGFβ2, cushion cell counts are reduced, but without any more of a difference in the mitotic index than is found in normal cushions, indicating attenuated EMT with no change in growth. At later stages (E14.5) the situation has reversed. In the absence of TGFβ2, cushions become enlarged and endothelial cell-cell separation and activation in vivo persists. These findings are consistent with the AV canal collagen gel assays of endocardial Tgfbr2 conditional knockout mice indicating poor EMT at an early stage, yet EMT occurs in vivo in these mice (Jiao et al. 2006).
There has been significant confusion about the role of TGFβ ligands in mediating EMT in cardiac development or adult cardiac valves in mammals (Mercado-Pimentel and Runyan 2007;Yang et al. 2008). Functional studies in chicken have indicated that both TGFβ2 and TGFβ3 can induce cushion EMT in AV canal assays (Boyer et al. 1999), whereas only TGFβ2 is required for EMT on collagen gels in mouse (Camenisch et al. 2002). All three TGFβ ligands can induce EMT in adult valve endothelial cell cultures, indicating in vitro redundant function (Yang et al. 2008). The present study has sorted out the confusion about the ligand-specific role of TGFβ in cushion EMT, not adult cardiac valve EMT, by unambiguously establishing a specific role for TGFβ2 in mediating EMT during heart development.
This study demonstrates that TGFβ1 and TGFβ3 are not required for cushion formation. These findings are concordant with normal heart development in Tgfb1−/− mice (Azhar et al. 2003) and in Tgfb3−/− mice ((Proetzel et al. 1995;Kaartinen et al. 1995) and Pexieder, personal communication), and this was the case in more than one genetic background for both knockout strains of mice (Shull et al. 1992;Kulkarni et al. 1993;Diebold et al. 1995). The conclusion that TGFβ3 plays an insignificant role in cushion EMT in mouse also corroborates well with previous studies showing undetectable expression of Tgfb3 during EMT in mouse hearts (Molin et al. 2003;Azhar et al. 2003;Camenisch et al. 2002). Two lines of evidence support the findings that TGFβ2 promotes both the initiation and cessation stages of EMT in mouse. Firstly, unique high level expression of Tgfb2 in AV myocardium during EMT is consistent with the requirement for promoting the initiation of EMT. Secondly, dynamic changes in Tgfb2 expression encompassing AV endocardial and invading cushion mesenchymal cells during EMT to predominantly cushion mesenchyme post-EMT are concordant with TGFβ2 playing a role in downregulation of EMT after the cushions are formed (Azhar et al. 2003;Molin et al. 2003;Camenisch et al. 2002).
Several lines of evidence suggest that ligands other than the prototypic TGFβs, such as BMP2, may be responsible for transformation in Tgfb2−/− AV explants. Bmp2 is expressed in similar fashion to Tgfb2 in AV cushions during cushion formation in mouse, and absence of BMP2, which results in downregulation of Tgfb2, leads to complete shut down of cushion EMT in mouse AV explant cultures (Ma et al. 2005;Rivera-Feliciano and Tabin 2006). Furthermore, Tgfb2 is decreased in the absence of ALK3 (Gaussin et al. 2005). BMP2 is known to function synergistically with Tgfb3 in the regulation of endocardial cushion morphogenesis (Yamagishi et al. 1999), and therefore it is possible that BMP2 may induce EMT by activating an endocardial TGFβ autocrine loop. In addition, it is known that TGFβR3 can bind with equal affinity to both TGFβ2 and BMP2 and can lead to cushion EMT (Kirkbride et al. 2008). TGFβ ligands can induce both TGFβ-specific and BMP-specific SMADs (Bharathy et al. 2008), and endothelial-specific deletion of Alk2, a BMP type 1 receptor, leads to the downregulation of both TGFβ- and BMP-specific SMADs (Wang et al. 2005). Recent findings have indicated that Alk5 Tie2-Cre-deficient embryos failed to undergo EMT both in vitro and in vivo (Sridurongrit et al. 2008;Mercado-Pimentel et al. 2007); whereas, Tgfbr2 Tie2-Cre-deficient embryos undergo EMT in vivo but not in vitro (Jiao et al. 2006), further suggesting the possibility that BMP2 and/or ligands other than the prototypic TGFβs (e.g., BMP2) can signal differentially through TGFβ receptors during valvulogenesis. The present study suggests that TGFβ2 mediates, in part, both the process of initiation as well as cessation of EMT. Perhaps, BMP2 plays a major role by inducing TGFβ2 to assist in initiation of EMT, and then to promote cessation of BMP2-induced EMT. What is not yet clear is whether TGFβ2 and BMP2 share some common receptor(s) and SMAD(s) in cushion formation in vivo. Future studies will sort out this possible crosstalk and its role in in vivo cushion formation.
We have provided the first in vivo evidence that TGFβ2 is required for normal VE-Cadherin levels during cushion formation. VE-Cadherin establishes endothelial cell-cell contacts, it induces TGFβ signaling by associating with TGFβ receptor complex, and it suppresses dp-ERK (Rudini et al. 2008;Grazia et al. 2003). It is also well documented that higher levels of activated RAS or dp-ERK can cause increased cushion EMT (Lakkis and Epstein 1998;Gitler et al. 2003). We have shown that Tgfb2−/− mice have reduced VE-Cadherin and persistently high levels of activated RAS and dp-ERK, all of which are consistent with the increased endothelial cell-cell separation, endothelial activation and EMT found in Tgfb2−/− cushions.
In summary, TGFβ1 and TGFβ3 do not play essential roles in cushion EMT and cushion formation in the mouse. TGFβ2 is the major TGFβ ligand that mediates cushion EMT by enhancing both the initiation and cessation stages of EMT during heart development without affecting cell proliferation differently than in normal heart development. Since dysregulated TGFβ signaling is associated both syndromic and non-syndromic heart valve diseases, understanding the ligand specificity in the pathogenesis of these valvular diseases could lead new therapies.
ACKNOWLEDGEMENTS
We thank Moying Yin, Kui Xu, Jade Rusche and Vikram Prasad for the technical support.This work was supported by funds from the National Institutes of Health Grant R01 HD26471 and BIO5 Institute (to Dr. Doetschman), R01 HD82851 (to Dr. Runyan), and Arizona Biomedical Research Commission (ABRC #0901) and The Steven M. Gootter Foundation (to Dr. Azhar).
REFERENCES
- Abdelwahid E, Pelliniemi LJ, Jokinen E. Cell death and differentiation in the development of the endocardial cushion of the embryonic heart. Microsc Res Tech. 2002;58:395–403. doi: 10.1002/jemt.10159. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Nawshad A. Complexity in interpretation of embryonic epithelial-mesenchymal transition in response to transforming growth factor-beta signaling. Cells Tissues Organs. 2007;185:131–145. doi: 10.1159/000101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Schultz JE, Grupp I, Dorn GW, Meneton P, Molin DG, Gittenberger-de Groot AC, Doetschman T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14:391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JV, Desgrosellier JS. Early events in valvulogenesis: a signaling perspective. Birth Defects Res C Embryo Today. 2003;69:58–72. doi: 10.1002/bdrc.10006. [DOI] [PubMed] [Google Scholar]
- Bartram U, Bartelings MM, Kramer HH, Gittenberger-de Groot AC. Congenital polyvalvular disease: a review. Pediatr Cardiol. 2001a;22:93–101. doi: 10.1007/s002460010169. [DOI] [PubMed] [Google Scholar]
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de GA. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in Tgfb2 knockout mice. Circulation. 2001b;103:2745–2752. doi: 10.1161/01.cir.103.22.2745. [DOI] [PubMed] [Google Scholar]
- Bharathy S, Xie W, Yingling JM, Reiss M. Cancer-associated transforming growth factor beta type II receptor gene mutant causes activation of bone morphogenic protein-Smads and invasive phenotype. Cancer Res. 2008;68:1656–1666. doi: 10.1158/0008-5472.CAN-07-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommireddy R, Pathak LJ, Martin J, Ormsby I, Engle SJ, Boivin GP, Babcock GF, Eriksson AU, Singh RR, Doetschman T. Self-antigen recognition by TGFbeta1-deficient T cells causes their activation and systemic inflammation. Lab Invest. 2006;86:1008–1019. doi: 10.1038/labinvest.3700460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and Distinct TGFbeta Ligand Requirements during Mouse and Avian Endocardial Cushion Morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro AJ, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MC, Slager HG, Duffie E, Mummery CL, Akhurst RJ. RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993;117:625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci U S A. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol. 2003;160:605–615. doi: 10.1083/jcb.200209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33:75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazia LM, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Langworthy M, Batts L, Brown CB, Moses HL, Baldwin HS. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development. 2006;133:4585–4593. doi: 10.1242/dev.02597. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J Biol Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkis MM, Epstein JA. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development. 1998;125:4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- Lan Y, Liu B, Yao H, Li F, Weng T, Yang G, Li W, Cheng X, Mao N, Yang X. Essential role of endothelial Smad4 in vascular remodeling and integrity. Mol Cell Biol. 2007;27:7683–7692. doi: 10.1128/MCB.00577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. American Journal of Anatomy. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Smith WN. Sturctural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42:160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Hubbard AD, Runyan RB. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2007;304:420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, Akhurst RJ. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111:131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Molin DG, Bartram U, Van der HK, Van Iperen L, Speer CP, Hierck BP, Poelmann RE, Gittenberger-de-Groot AC. Expression patterns of Tgfbeta1–3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev Dyn. 2003;227:431–444. doi: 10.1002/dvdy.10314. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Krug EL, Markwald RR. Myocardial regulation of transforming growth factor-beta expression by outflow tract endothelium in the early embryonic chick heart. Dev Biol. 1994;165:615–626. doi: 10.1006/dbio.1994.1280. [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the Atp2c1 secretory pathway Ca(2+)-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007;282:26517–26527. doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. 287–335. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Basson CT, Benson DW, Jr., Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989;134:392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Potts JD, Vincent EB, Runyan RB, Weeks DL. Sense and antisense TGF beta 3 mRNA levels correlate with cardiac valve induction. Dev Dyn. 1992;193:340–345. doi: 10.1002/aja.1001930407. [DOI] [PubMed] [Google Scholar]
- Prasad V, Boivin GP, Miller ML, Liu LH, Erwin CR, Warner BW, Shull GE. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res. 2005;65:8655–8661. doi: 10.1158/0008-5472.CAN-05-0026. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Williams SS, Jagatheesan G, Ahmed RP, Fuller-Bicer G, Schwartz A, Aronow BJ, Wieczorek DF. Microarray analysis of gene expression during early stages of mild and severe cardiac hypertrophy. Physiol Genomics. 2006;27:309–317. doi: 10.1152/physiolgenomics.00072.2006. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garre M, Liebner S, Letarte M, ten Dijke P, Dejana E. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de GA, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non- overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev Biol. 2008;322:208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supino PG, Borer JS, Yin A, Dillingham E, McClymont W. The epidemiology of valvular heart diseases: the problem is growing. Adv Cardiol. 2004;41:9–15. doi: 10.1159/000079779. 9–15. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Miyazono K, Nakamura H. Bone morphogenetic protein-2 acts synergistically with transforming growth factor-beta3 during endothelial-mesenchymal transformation in the developing chick heart. J Cell Physiol. 1999;180:35–45. doi: 10.1002/(SICI)1097-4652(199907)180:1<35::AID-JCP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]