Abstract
Obesity and weight gain in adulthood are associated with an increased risk of several cancers. Telomeres play a critical role in maintaining genomic integrity, and may be involved in carcinogenesis. Using data from 647 women aged 35–74 in the U.S. and Puerto Rico (2003-4), we examined the association between current and past anthropometric characteristics and telomere length in blood cells. In a multivariate linear regression model, higher current body mass index (BMI) and hip circumference were inversely associated with telomere length. Higher BMI in the 30s was associated with shorter telomere length among women aged 40 years or older (p for trend < 0.01). Weight gain since age 30s (p for trend = 0.07) and weight cycling (p for trend = 0.04) were also inversely associated with telomere length. When current BMI and BMI at ages 30–39 were considered together, the most marked decrease in telomere length was found for women who had overweight or obese BMI at both time points (mean T/S ratio=1.26, 95% CI: 1.21–1.30) compared with women who had normal BMI at both times (mean T/S ratio = 1.33, 95% CI: 1.30–1.36). These findings support the hypothesis that obesity may accelerate aging, and highlight the importance of maintaining a desirable weight in adulthood.
Introduction
Telomeres, non-coding double-stranded repeats (TTAGGG in humans) at the ends of chromosomes, become shortened with each cell division due to incomplete replication of lagging strand (1). This process is further accelerated by oxidative stress and inflammation (2, 3). While the findings are not completely consistent, short telomeres have been associated with an increased risk of insulin resistance (4, 5), cardiovascular disease (6, 7), and several cancers (8–11).
Obesity is implicated in many age-related disorders (12), and has been recognized as a state of increased oxidative stress (13) and inflammation (14). Shorter telomeres have been associated with increasing body mass index (2, 3), and more recently with increasing waist and hip circumferences in women (15).
The present study investigated the relationship between various measures of current body size and telomere length in 647 women aged 35–74 years. Changes in weight and obesity status since women were in their 30s were also examined in relation to telomere length among 608 women aged 40 years or older.
Materials and Methods
Study population
The NIEHS Sister Study (www.SisterStudy.org) is a prospective cohort study of environmental and genetic risk factors for breast cancer and other endpoints in approximately 50,000 women aged 35–74 who do not have breast cancer themselves but have a sister who was diagnosed with the disease. Details of the study design have been described elsewhere (16). The study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, NIH and the Copernicus Group IRB. Selection criteria were developed for another study (Parks et al., 2008, manuscript in press). Briefly, women who enrolled during the vanguard phase of the Sister Study, were included in the first annual health update follow-up mailing in June 2005, and were not known to have developed breast cancer at the time of follow-up were eligible for the study. Of 2,086 eligible women, 295 were excluded for one or more of the following reasons: Missing or inadequate blood or urine sample, urine sample that was not a first morning void, major dental procedure or surgery within the past week, current shift work, chemotherapy or radiation treatment for cancer, or missing value for race or smoking status. A further 18 women were excluded due to known breast cancer diagnosis prior to follow-up. Of 1,773 women left, we selected all women with high or very high perceived stress (score = 6–16 based on the 4-item version of perceived stress scale developed by Cohen (17)), nonwhite race, and current smoking, and a random sample of the remaining women, for a final weighted random sample of 740 women. Duplicate measurements were available for 647 women because 54 were run in pilot assays without adequate plate controls, 29 had failed PCR-reactions, and 10 samples were run only in a single plate. For methodological consistency among samples, only women with duplicate measurements were included in the present analysis. However, the distributions of telomere length were similar in those with and without duplicate measurements, and the relationship between obesity and telomere length did not change by further inclusion of women with a single measurement.
Anthropometric measurement
Current height, weight, and hip and waist circumferences were measured during a home visit and were used to derive current body mass index (BMI) and waist-to-hip ratio. BMI was categorized based on WHO definitions (12) as normal or underweight (BMI <25.0 kg/m2), overweight (BMI 25–29.9 kg/m2), or obese (BMI ≥30.0 kg/m2). Waist circumference was classified based on the American Diabetes Association criteria for abdominal obesity as normal (<80 cm), action level 1 (80–87.9 cm), or action level 2 (≥88 cm) (18). Other body size variables without known standard classification criteria were categorized into quartiles (for height and weight) or tertiles (for hip circumference and waist-to-hip ratio) based on distributions of the entire study population.
In a computer assisted telephone interview, women aged 40 or older were asked the following question: “Thinking back to your 30s [when you were not pregnant, breastfeeding, or in the 6 months after pregnancy], what was your average weight?” The self-reported average weight at ages 30–39 (hereafter referred to as past weight) was used to assess changes in weight and obesity status during adulthood. Weight change since their 30s was calculated as the difference between current and past weights. Cutpoints for weight change categories were chosen in consideration of those used in previous literature on weight change (19). Change in obesity status from the 30s was classified based on BMI in the 30s (hereafter referred to as past BMI) and current BMI as follows: 1) having a normal BMI at both time points if both past and current BMI fall in the normal range; 2) gaining weight from normal past BMI to currently overweight BMI; 3) gaining weight from normal past BMI to currently obese BMI; 4) having an overweight or obese BMI at both time points if both past and current BMI fall in overweight or obese categories; and 5) losing weight from overweight past BMI to currently normal BMI, or from obese past BMI to currently normal or overweight BMI. Additionally, women were asked the following question about lifetime episodes of weight cycling: “How many times in your life have you lost 20 pounds (9 kilograms) or more, and then later gained all of the weight back? [Do not count weight changes related to pregnancy.]” The response to this question was categorized as never, once, 2–3 times, or 4 times or more.
Assessment of telomere length
DNA was extracted from whole blood stored at −80 °C. Telomere length was quantified using the real-time quantitative PCR assay previously described(20), with some modifications. Briefly, each sample was amplified for telomeric DNA (T) and a single-copy control gene (S) in 1ul aliquots containing 100–200ng template DNA. Based on a standard curve with serial dilutions, cycle threshold was transformed into nanograms of DNA. A total of 647 specimens were run in triplicate on duplicate plates. The ratio of telomere repeat copy number to single copy gene copy number (T/S ratio) was calculated from the average T and S of triplicate measurements in a single plate. Additionally, three controls with known T/S ratio (one each at high, medium, and low T/S ratio) were included in all assay batches to adjust for inter-batch variation. After the adjustment, the coefficient of variation of the adjusted replicates was 8.5%. Data are the average of the adjusted replicates of T/S ratio. The average T/S ratio is an indicator of telomere length; a lower T/S ratio reflects shorter telomere length.
Statistical analysis
Overall, relative telomere length was normally distributed (kurtosis = 3.1, skewness = 0.3). Therefore, we used a linear regression model to estimate mean relative telomere length and 95% confidence interval (95% CI) according to current and past anthropometric characteristics controlling for the following variables: race, smoking status [never, past or current], perceived level of stress [5 categories]), age in years (continuous), education level (≤high school, some college or associate degree, or college graduate or more), regular use of nonsteroidal anti-inflammatory drugs [NSAIDs] (yes/no), regular use of vitamin supplement (yes/no) and medical history of hypertension (yes/no) and cardiovascular disease (yes/no). Other covariates considered in multivariate models included menopausal status (pre- or post-menopause), dietary intakes of calories and total fat (quartiles based on distributions of the entire sample), physical activity in the past 12 months (total MET [metabolic equivalents]-hours per week from sports and exercise activities as well as activities of daily living and domestic chores, quartiles based on distributions of the entire sample) and diagnosis of diabetes mellitus (yes/no); however, adding these variables did not change the association between body size variables and telomere length. Menopausal status and regular use of NSAIDs and vitamin supplements were investigated as potential effect modifiers. Strong correlations existed between past and current body size variables with correlation coefficients of 0.74 (p<0.01) for current and past weights, and 0.67 (p<0.01) for current and past BMI. Therefore, past body size variables were evaluated with additional adjustment for current body size variables by fitting a simple linear regression model for current body size variables given past body size variables, and entering the residuals into the model. Tests for linear trend were performed by treating an ordered categorical variable as a continuous variable. Analyses were performed using Stata 10.0 (College Station, TX), and all statistical tests were two-sided, with an alpha level of 0.05.
Results
Selected characteristics of the study population by BMI are presented in Table 1. Mean ages were similar across three BMI categories. However, women in higher BMI categories tended to be physically inactive and have higher intakes of energy and fat compared to women with normal BMI. BMI was also positively associated with nonwhite race, regular use of NSAIDs, menopause and comorbidity but inversely associated with regular use of vitamin supplement.
Table 1.
Characteristics of study population by current body mass index1
| BMI (kg/m2) | |||
|---|---|---|---|
| <25 (n=260) |
25–29.9 (n=183) |
≥30 (n=201) |
|
| Mean (SD) | |||
| Age at enrollment (yr) | 53.1 (9.8) | 53.6 (9.4) | 54.0 (9.5) |
| Physical activity (met/hr/wk) | 13.8 (14.3) | 11.2 (17.0) | 7.3 (9.7) |
| Calories (kcal/d)3 | 1,450.3 (540.4) | 1,546.1 (580.0) | 1,603.7 (593.8) |
| Total fat (g/d)3 | 63.9 (29.4) | 70.4 (32.7) | 71.4 (31.9) |
| Number (%) | |||
| Non-white race2 | 31 (11.9) | 27 (14.8) | 50 (24.9) |
| High or very high perceived stress | 98 (37.7) | 66 (36.1) | 84 (41.8) |
| Education level | |||
| High school grad or less | 34 (13.1) | 36 (19.7) | 32 (15.9) |
| Some college/Associate degree | 96 (36.9) | 84 (45.9) | 81 (40.3) |
| College grad or more | 130 (50.0) | 63 (34.4) | 88 (43.8) |
| Smoking | |||
| Never smoker | 123 (47.3) | 89 (48.6) | 92 (45.8) |
| Past smoker | 73 (28.1) | 52 (28.4) | 61 (30.3) |
| Current smoker | 64 (24.6) | 42 (23.0) | 48 (23.9) |
| Postmenopause | 166 (63.8) | 127 (69.4) | 143 (71.1) |
| Regular use of NSAIDs | 113 (43.5) | 79 (43.2) | 122 (60.7) |
| Regular use of vitamin supplement3 | 180 (69.2) | 120 (65.6) | 129 (64.2) |
| Medical history | |||
| Hypertension | 34 (13.1) | 39 (21.3) | 85 (42.3) |
| Cardiovascular disease | 28 (10.8) | 29 (15.8) | 46 (22.9) |
| Diabetes mellitus | 2 (0.8) | 10 (5.5) | 23 (11.4) |
Out of 647, 3 subjects with missing values for BMI were not included
Includes blacks, Hispanic whites, and others
21 subjects (7 in BMI<25, 5 in BMI 25–29.9, and 9 in BMI ≥ 30 kg/m2) with missing dietary data, and 18 subjects (7 in BMI<25, 4 in BMI 25–29.9, and 7 in BMI ≥ 30 kg/m2) with missing vitamin supplement use
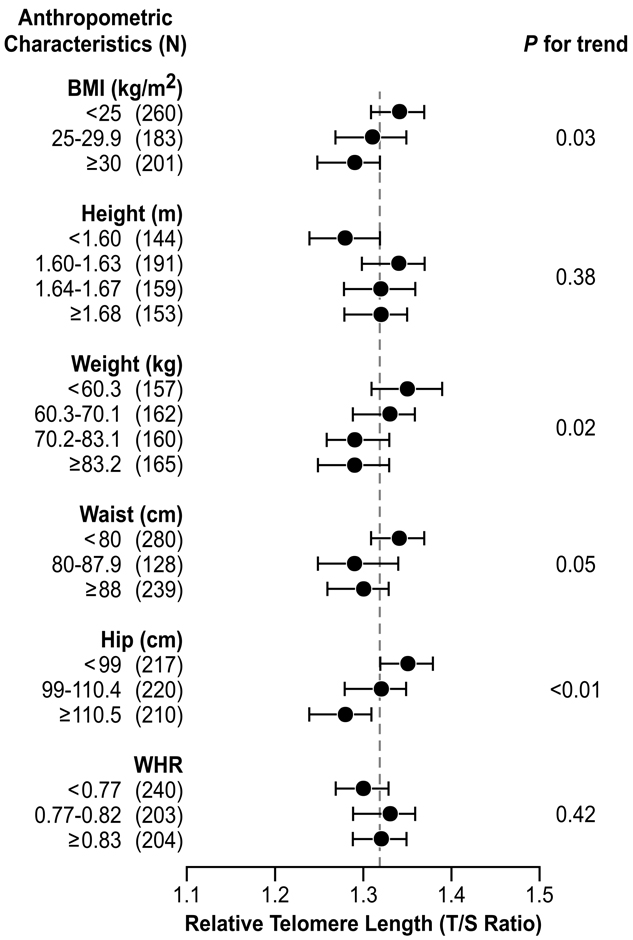
There was a linear decrease in mean relative telomere length with increasing BMI (p for trend =0.03) (Figure 1). The respective mean T/S ratios were 1.34 (95% CI: 1.31–1.37) for BMI <25 kg/m2, 1.31 (95% CI: 1.27–1.35) for BMI 25–29.9 kg/m2 and 1.29 (95% CI: 1.25–1.32) for BMI ≥30 kg/m2. Increase in weight was also associated with shorter mean relative telomere length, though the association did not appear to be strictly linear. Reduced telomere length was found with increasing hip and, to a lesser extent, waist circumferences, but no association was observed with waist-to-hip ratio. Women who were shorter than 1.6 meters had the shortest telomere length (mean relative T/S ratio = 1.28, 95% CI: 1.24–1.32) but telomere length did not increase with increasing height (p for trend = 0.38).
Figure 1.
Adjusted mean of relative telomere length (T/S ratio) according to anthropometric characteristics among 647 women aged 35–74 years in the Sister Study, 2003–2004.
Mean values (●) were adjusted for age, race, smoking status, perceived level of stress, regular use of NSAIDs, regular use of vitamin supplement, diagnosis of hypertension, and history of cardiovascular diseases. Bars represent 95% confidence intervals. A reference line ( ) indicates the overall mean of relative T/S ratio in the study sample.
) indicates the overall mean of relative T/S ratio in the study sample.
The association between BMI and telomere length was similar among pre- and post-menopausal women. NSAIDs and vitamin supplements, which are known to have antioxidant properties (21, 22), were also examined as a potential effect modifier of the association between BMI and telomere length; however, there was little evidence for effect modification by these factors (data not shown).
After adjustment for current BMI, past BMI was inversely associated with relative telomere length (Figure 2). The mean T/S ratios were 1.33 (95% CI: 1.30–1.36), 1.29 (95% CI: 1.26–1.33) and 1.26 (95% CI: 1.21–1.30) for past BMI of <22.5 kg/m2, 22.5–24.9 kg/m2, and ≥25kg/m2, respectively. Weight gain since the 30s (p for trend = 0.07) and frequent weight cycling (p for trend = 0.03) were also associated with shorter telomere length. Further assessment of change in obesity status confirmed shorter telomere length associated with adult weight change but the most marked reduction in telomere length was found among women who had overweight or obese BMI at both time points with mean T/S ratio 1.26 (95% CI: 1.21–1.30). Those who lost weight compared to in their 30s also had short telomeres, but they were too few to be reliably examined.
Figure 2.
Adjusted mean of relative telomere length (T/S ratio) according to anthropometric history variables among 608 women aged 40 years or older in the Sister Study, 2003–2004.
Mean values (●) were adjusted for age, race, smoking status, perceived level of stress, regular use of NSAIDs, regular use of vitamin supplement, diagnosis of hypertension, history of cardiovascular diseases, and residuals of current weight or BMI. Bars represent 95% confidence intervals. A reference line ( ) indicates the overall mean of relative T/S ratio among all women aged 40 or older.
) indicates the overall mean of relative T/S ratio among all women aged 40 or older.
Discussion
Consistent with previous studies particularly among women (2, 3, 15), we found an inverse association between current BMI and telomere length. Shorter telomeres were also associated with higher hip, and, to a lesser extent, waist circumferences. Additionally, higher BMI at ages 30–39, adult weight gain and frequent weight cycling were inversely associated with telomere length.
Women with higher BMI at baseline tend to experience more frequent intentional weight loss and weight cycling than those with lower BMI; therefore, it is not easy to sort out whether the reduction in telomere length is due to higher BMI at baseline, weight change, or both. However, our finding that women who maintained an overweight or obese BMI since their 30s had shorter telomeres than those who became overweight or obese since their 30s suggests that duration of obesity may be more important than weight change per se.
One previous study has reported an inverse association between telomere length and insulin resistance only in premenopausal women (5). Estrogens (23) as well as vitamins7 and NSAIDs6 are known to have antioxidant properties. In our exploratory analyses, however, the relationship between current BMI and telomere length was not modified by these factors.
Oxidative stress and inflammation have been suggested as an underlying mechanism for the association between obesity and short telomeres (3). Accumulating adiposity increases oxidative stress and causes deregulation of inflammatory cytokines (13). Oxidative stress is a direct and indirect source of single strand breaks in DNA(23). Compared to genomic DNA, the G-rich telomeric sequence is an ideal target for acute oxidative damage, and telomeric DNA is relatively less capable of DNA repair, allowing acceleration of telomere loss during cell cycle and subsequent replicative senescence (23).
Limitations of the present study should be acknowledged. First, we had insufficient data to comment on the influence of weight loss on telomere length: only a few women were obese in their 30s and thus, very few had lost 5 kg or more. Additionally, weight at ages 30–39 was recalled in our study. Although a strong correlation between recalled and previously measured weight has been reported, accuracy of self-reported past body weight appears to be influenced by race and current body weight (24). Lastly, there was a one-time measurement of telomere length in this cross-sectional study. While evidence suggests that oxidative stress, possibly promoted by obesity, accelerates telomere shortening (23), we could not directly evaluate whether shorter telomeres in obese individuals relative to the non-obese are attributed to rapid telomere attrition rate, or some other reasons such as genetic differences (25).
In summary, the present study showed reduced telomere length associated with higher BMI at ages 30–39 and weight gain in adulthood, and current BMI. These findings support the hypothesis that obesity accelerates aging (3) and provide further evidence of the advantages of maintaining a healthy weight. Further investigations are warranted to characterize the role of obesity-driven aging in the development of degenerative diseases and cancer.
Acknowledgment
The authors especially thank Ms. Teresa Stepanek in Dr. Cawthon’s laboratory for assistance in measuring telomere lengths and thank Dr. Stephanie London for her thoughtful review of this manuscript.
Funding support: This research was funded by the Intramural Research Program of the NIH, National institute of Environmental Health Sciences (Z01 ES04400509) with additional support from the National Center on Minority Health and Health Disparities, and Department of Defense Breast Cancer Research Concept Award (BC045286).
Footnotes
Contributors: SK, CP and DS conceptualized the study. RC did laboratory analysis. SK analyzed and interpreted the data, and drafted the manuscript. DS, LD and JT contributed to the interpretation of the data and provided editorial and scientific review. CP and DS obtained funding. All authors contributed to discussion of the findings and revision of the manuscript.
Conflict of interest: RC has submitted a patent application for the method of telomere length measurement by quantitative PCR. The lab that measured telomere lengths was blind with regard to all identifying and clinical information associated with the DNA samples. No other actual or potential conflict of interest is declared.
Contributor Information
Sangmi Kim, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599.
Christine G Parks, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599.
Lisa A DeRoo, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599.
Honglei Chen, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599.
Jack A. Taylor, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599
Richard M. Cawthon, Department of Human Genetics, University of Utah, Salt Lake City, UT 84112
Dale P Sandler, Epidemiology Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27599.
References
- 1.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr.Mol.Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 2.Gardner JP, et al. Rise in Insulin Resistance Is Associated With Escalated Telomere Attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 3.Valdes AM, et al. Obesity, cigarette smoking, and telomere length in women. The Lancet. 366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 4.Demissie S, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 5.Aviv A, et al. Menopause Modifies the Association of Leukocyte Telomere Length with Insulin Resistance and Inflammation. Journal of Clinical Endocrinology Metabolism. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 6.Brouilette SW, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet. 369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick AL, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, et al. Telomere Dysfunction: A Potential Cancer Predisposition Factor. JNCI Journal of the National Cancer Institute. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 9.Barwell J, et al. Is telomere length in peripheral blood lymphocytes correlated with cancer susceptibility or radiosensitivity? Br.J Cancer. 2007;97:1696–1700. doi: 10.1038/sj.bjc.6604085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen J, et al. Short Telomere Length and Breast Cancer Risk: A Study in Sister Sets. Cancer Research. 2007;67:5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- 11.Svenson U, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Research. 2008;68:3618–3623. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- 12.WHO Consultation on Obesity. Geneva: World Health Organization. WHO Technical Report Series (TRS); Obesity: Preventing and managing the Global Epidemic. 894 6-3-1997. [PubMed]
- 13.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 15.Nordfjall K, et al. Telomere Length Is Associated With Obesity Parameters but With a Gender Difference. Obesity (Silver.Spring) 2008 doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg CR, et al. Using Risk-based Sampling to Enrich Cohorts for Endpoints, Genes, and Exposures. American Journal of Epidemiology. 2007;166:447–455. doi: 10.1093/aje/kwm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc.Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Ardern CI, et al. Development of health-related waist circumference thresholds within BMI categories. Obes.Res. 2004;12:1094–1103. doi: 10.1038/oby.2004.137. [DOI] [PubMed] [Google Scholar]
- 19.Eng SM, et al. Body size changes in relation to postmenopausal breast cancer among women on Long Island, New York. Am J Epidemiol. 2005;162:229–237. doi: 10.1093/aje/kwi195. [DOI] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podhaisky HP, et al. Aspirin protects endothelial cells from oxidative stress--possible synergism with vitamin E. FEBS Lett. 1997;417:349–351. doi: 10.1016/s0014-5793(97)01307-0. [DOI] [PubMed] [Google Scholar]
- 22.Mayne ST. Antioxidant Nutrients and Chronic Disease: Use of Biomarkers of Exposure and Oxidative Stress Status in Epidemiologic Research. Journal of Nutrition. 2003;133:933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 23.von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 24.Perry GS, et al. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, et al. Offspring's Leukocyte Telomere Length, Paternal Age, and Telomere Elongation in Sperm. PLoS.Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]