Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) are neuroprotective in numerous models. Impairment of cerebrovascular reactivity (CR) contributes to ischemia/reperfusion (I/R)-induced neuronal damage. We tested whether PACAP and/or VIP preserve CR to I/R-sensitive dilator responses dependent on endothelial and/or neuronal function. Accordingly, changes in pial arteriolar diameters in response to hypercapnia (5–10% CO2 ventilation) or topical N-methyl-d-aspartate (NMDA, 10–4 M) were determined before and after I/R via intravital microscopy in anesthetized/ventilated piglets. Local pretreatment with non-vasoactive doses of PACAP (10–8 M) and VIP (10–9 M) prevented the attenuation of postischemic CR to hypercapnia; to 10% CO2, the CR values were 27±8% vs 92±5%* vs 88±13%* (vehicle vs PACAP38 vs VIP, CR expressed as a percentage of the response before I/R, mean±SEM, n=8–8, *p<0.05). PACAP, but not VIP, preserved CR to NMDA after I/R, with CR values of 31±10% vs 87±8%* vs 35±12% (vehicle vs PACAP38 vs VIP, n=6–6). Unlike PACAP, VIP-induced vasodilation has not yet been investigated in the piglet. We tested whether VIP-induced arteriolar dilation was sensitive to inhibitors of cyclooxygenase (COX)-1 (SC-560, 1 mg/kg), COX-2 (NS-398, 1 mg/kg), indomethacin (5 mg/kg), and nitric oxide synthase (L-NAME, 15 mg/kg). VIP (10–8–10–7–10–6 M, n=8) induced reproducible, dose-dependent vasodilation of 16±3%, 33±6%*, and 70±8%*. The response was unaffected by all drugs, except that the vasodilation to 10–8 M VIP was abolished by SC-560 and indomethacin. In conclusion, PACAP and VIP differentially preserve postischemic CR; independent of their vasodilatory effect.
Keywords: Piglet, Pial arteriole, Cranial window, NMDA, Hypercapnia
1. Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) are structurally related neuropeptides. PACAP has two naturally occurring isoforms; PACAP38, a basic, amidated 38-residue peptide, and PACAP27, the N-terminal portion consisting of 27 amino acids (Miyata et al. 1989; Miyata et al. 1990). PACAP possesses specific receptors (8 subtypes of PAC1), but also shares common receptors with VIP (VPAC1, VPAC2) (Harmar et al. 1998; Vaudry et al. 2000). Thus, despite the high sequence homology, these peptides are expected to activate multiple pathways resulting in distinct biological actions.
In the cerebral circulation of the newborn pig, PACAP27 and PACAP38 have been reported to induce prominent pial arteriolar dilation (Tong et al. 1993; Lenti et al. 2007). We found that PACAP38-induced vasodilation was abolished by a cyclooxygenase (COX)-1-inhibitor, while PACAP27-induced vasodilation was insensitive to either COX-1 or COX-2 blockade (Lenti et al. 2007). Ischemia/reperfusion (I/R), which frequently occurs in the perinatal period (Berger et al. 2002), has been shown to impair several cerebrovascular regulatory mechanisms of the neurovascular axis in piglets (Leffler et al. 1989a; Leffler et al. 1989b; Busija et al. 1996). Impaired cerebrovascular reactivity (CR) probably contributes to and aggravates neuronal cell death following hypoxic/ischemic brain injury. Previous studies identified N-methyl-d-aspartate (NMDA)- and CO2-induced vasodilatory responses as prototypes of such I/R-sensitive mechanisms. However, these stimuli dilate cerebral arterioles by completely different mechanisms. NMDA-induced pial arteriolar dilation is a neuronal-triggered multi-step process involving the activation of neuronal nitric oxide (NO) synthase (NOS) (Meng et al. 1995; Domoki et al. 2002), while CO2 induces vasodilation via an endothelium-dependent, indomethacin-sensitive process in the newborn pig (Leffler et al. 1993; Leffler et al. 1994b).
PACAP and VIP are considered as endogenous neuroprotective mediators that may be released from neural and vascular cells during I/R and could possibly reduce neuronal injury (Brenneman 2007; Stumm et al. 2007). Conceivably, these neuroprotective peptides may protect CR after I/R as well. Therefore, the major goal of our study was to determine whether PACAP27, PACAP38 and/or VIP preserves CR to NMDA and/or hypercapnia after I/R, and whether the protective effects of the neuropeptides are dependent on their vasoactivity. Since the pial arteriolar response to VIP has not yet been described in the piglet, we also characterized VIP-induced vasodilation using peptide antagonists (PACAP6-38 and PACAP6-27), COX- and NOS inhibitors.
2. Results
2.1. Effects of PACAP/VIP pretreatment on pial arteriolar responsiveness to CO2 after I/R
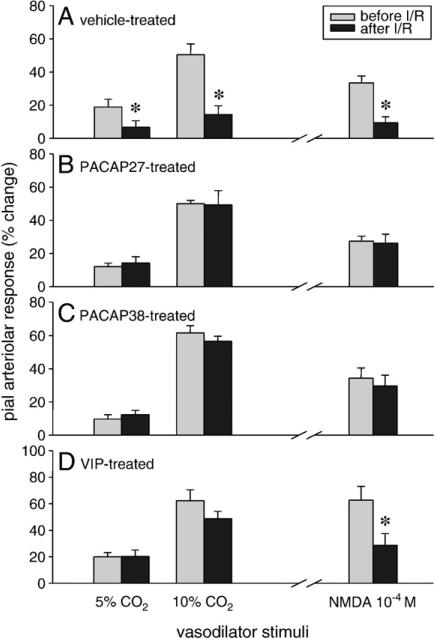
Graded hypercapnia (5–10% CO2 ventilation) resulted in large, dose-dependent, reversible increases in pial arteriolar diameters. In the vehicle-treated animals (group 1), I/R (10 min of ischemia and 1 h of reperfusion) severely attenuated the hypercapnia-induced arteriolar vasodilation (Fig. 1A, left). Incubation of the brain surface with PACAP27 (10–8 M), PACAP38 (10–8 M) or VIP (10–9 M) for 30 min did not affect the pial arterioles significantly (2±1%, 2±1%, and 3±1% maximal dilation in response to PACAP27, PACAP38, and VIP, respectively); however, they efficiently preserved CR to both levels of hypercapnia after I/R (groups 3, 5, and 7; Figs. 1B–D, left).
Fig. 1.
Effects of ischemia/reperfusion (I/R) on hypercapnia- or NMDA-induced pial arteriolar vasodilation. Arteriolar responses to 5–10% CO2 ventilation and topical NMDA were recorded before and after 10 min of global cerebral ischemia followed by 1 h of reperfusion. Hypercapnia elicited concentration-dependent pial arteriolar vasodilation, which was markedly attenuated after I/R in vehicle-treated piglets (A, left). PACAP isoforms and VIP preserved the vascular responsiveness to CO2 (B–D, left). Vasodilation in response to 10–4 M NMDA was also deteriorated after I/R (A, right). PACAP27 and PACAP38 (B–C, right), but not VIP (D, right), protected the vasodilator effect of NMDA. (Data are mean±SEM, n=6–8, *p<0.05.)
2.2. Effects of PACAP/VIP pretreatment on pial arteriolar responsiveness to NMDA after I/R
Topical application of NMDA (10–4 M) induced a marked vasodilation that was also sensitive to I/R (group 2, Fig. 1A, right). PACAP27 (10–8 M) or PACAP38 (10–8 M) pretreatment of the brain surface protected the NMDA-evoked pial arteriolar dilation after I/R, whereas incubation of the brain surface with 10–9 M VIP was not effective (groups 4, 6, and 8; Figs. 1B–D, right). Furthermore, a higher dose of VIP (10–8 M, group 8b) was still unable to preserve CR to 10–4 M NMDA reduced by I/R. CR values were 36±12% vs 51±6% (vehicle vs VIP, CR expressed as a percentage of the preischemic response). In group 8c, lower, 10–5 M and 5×10–5 M concentrations of NMDA also dilated the pial arterioles with 4.4±0.2% and 12.6±0.4%*, respectively (*p<0.05). I/R reduced these vascular reactions significantly, and moreover, a tendency towards vasoconstriction was observed after I/R (–2±3% and –3±4% changes in diameter to 10–5 M and 5×10–5 M NMDA), irrespectively of the VIP (10–8 M) treatment.
2.3. Effects of PACAP/VIP treatment on CO2- and NMDA-induced pial arteriolar dilation
Topical PACAP27 (10–8 M), PACAP38 (10–8 M) or VIP (10–9 M) treatment alone did not affect the vasodilation induced by either hypercapnia or NMDA. Vascular responses to 10% CO2 were 50±2% and 57±7% before and after PACAP27 (group 9), and 63±5% and 64±6% before and after PACAP38 incubation (group 11). Topical NMDA (10–4 M) evoked 36±7% and 33±5% vasodilation before and after PACAP27, and 37±5% and 43±7% vasodilation before and after PACAP38 application (groups 10 and 12). Responses to 10% CO2 were 60±9% and 56±11% (group 13), and to 10–4 M NMDA were 49±7% and 48±7% (group 14), before and after VIP treatment. Accordingly, the protective effects of these neuropeptides are not caused by direct facilitation of the vascular reactivity to hypercapnia or to NMDA.
2.4. Effects of different enzyme inhibitors on VIP-induced pial arteriolar vasodilation
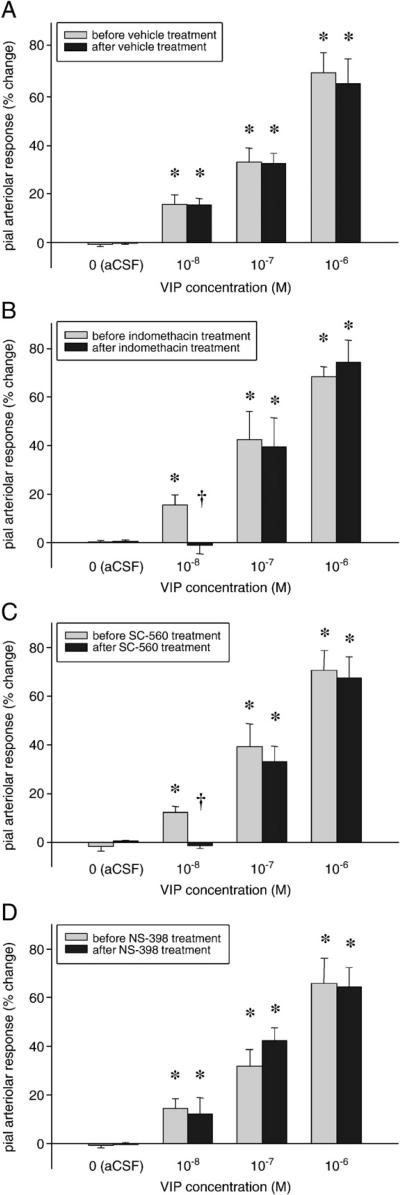
VIP induced dose-dependent, reproducible pial arteriolar vasodilation at 10–8–10–6 M concentrations (Fig. 2A). The VIP-induced vasodilation was partially indomethacin-sensitive (Fig. 2B), since administration (5 mg/kg iv) of this non-selective COX inhibitor significantly blocked the vasodilation evoked by the lowest (10–8 M) dose of VIP. Indomethacin was not effective when 10–7 or 10–6 M VIP was applied. Selective inhibition of COX-1 by SC-560 (1 mg/kg iv) produced a very similar outcome (Fig. 2C), but the COX-2 inhibitor NS-398 (1 mg/kg iv) failed to exert any inhibitory effect (Fig. 2D). Nitric oxide synthase (NOS) blockade had also no influence on the VIP-induced vasodilation; application of 10–8–10–6 M VIP onto the brain surface resulted in significant, 15±3–61±10% and 17±7–62±9% caliber changes before and after intravenous N-ω-nitro-l-arginine methyl ester (L-NAME, 15 mg/kg) administration.
Fig. 2.
Characterization of VIP-induced pial arteriolar vasodilation. VIP elicited significant, dose-dependent, reproducible increases in pial arteriolar diameters, while artificial cerebrospinal fluid (aCSF) did not evoke vasodilation (A). The non-selective cyclooxygenase (COX) inhibitor indomethacin abolished the pial arteriolar dilation in response to 10–8 M VIP, whereas it left the vasodilation to higher concentrations (10–7–10–6 M) of VIP essentially intact (B). The pial arteriolar response to VIP was differentially affected by selective COX inhibitors; the COX-1 inhibitor SC-560 abolished the arteriolar responses to 10–8 M VIP (C), whereas the COX-2 inhibitor NS-398 was ineffective (D). (Data are mean±SEM, n=6–8, *p<0.05: significantly higher than vasodilation to all smaller VIP concentrations, †p<0.05: significantly less than the corresponding value before treatment.)
2.5. Effects of PACAP receptor antagonists on VIP-induced pial arteriolar vasodilation
PACAP6-38 (10–5 M, group 20) pretreatment effectively diminished the VIP-induced vasodilation (62±10% and 33±6%* vasodilation in response to 10–6 M VIP, before and after PACAP6-38, *p<0.05); however, in group 21, PACAP6-27 (10–5 M) failed to exhibit any inhibitory effect on the VIP-related pial arteriolar dilation (61±12% and 66±11% changes in diameter before and after topical PACAP6-27 treatment).
2.6. Physiological variables
Graded (5 and 10%) hypercapnia significantly elevated the arterial pCO2 levels in all experimental groups where CR to hypercapnia was tested. The pCO2 changes were similar before and after I/R and between groups. For instance, in group 7, 5 and 10% CO2 elevated pCO2 from 34±2.5 to 52±2.1 and 79±5 mm Hg and from 37±2.7 to 56±2.7 and 84±5.9 mm Hg before and after I/R, respectively. The respective pO2 levels were 90±5.9–94±6.4–94±7.4 and 90±7.6–89±5.8–88±4.2 mm Hg in this group and were similar in all groups. Otherwise, body temperature, arterial pH, and blood gases were kept in the normal ranges and did not vary significantly among the different groups throughout the experiments. In group 1, for instance, the baseline values were as follows: core temperature= 37±0.2 °C, pH=7.43±0.03, pCO2=38±2 mm Hg, pO2=91±5 mm Hg. Similarly, the mean arterial blood pressure (MABP) values were always within the range characteristic for anesthetized newborn pigs and did not differ significantly between groups (e.g. 70±3 mm Hg and 69±3 mm Hg in groups 1 and 2, respectively). Furthermore, in each animal, care was taken to avoid any fluctuation in MABP during recordings so as to prevent effects of short-term changes in arterial pressure on vascular diameters. There were no significant differences between the baseline diameters in the groups; for example, in group 15 the values were 92±3 μm and 95±5 μm before the first and the second VIP application, respectively.
3. Discussion
The major findings of the present study: (1) PACAP27, PACAP38, and VIP prevent the attenuation of hypercapnia-induced vasodilation caused by I/R; (2) PACAP27 and PACAP38, but not VIP preserves NMDA-induced vasodilation; (3) VIP evokes concentration-dependent, reproducible pial arteriolar dilation that is independent of NOS activity, and essentially unaffected by COX inhibitors. Finally, (4) VIP-induced vasodilation is attenuated by PACAP6-38, but not by PACAP6-27.
PACAP and VIP are widely distributed throughout the central and peripheral nervous system, and they have been identified in nerve endings innervating pial and intraparenchymal vessels of the cerebral cortex (Fahrenkrug et al. 2000). These perivascular nerve fibers are of either parasympathetic, trigeminal sensory or cortical neuronal origin (Edvinsson and Krause 2002). Our results indicate that PACAP and VIP are markedly different in their respective protective and vasodilator actions also indicating that these neuropeptides may activate only partially overlapping mechanisms.
In porcine cerebral vessels, hypercapnia evokes vasodilation not only in vivo (Leffler et al. 1989a), but also in isolated, denervated vessels (Kokubun et al. 2009) suggesting that neuronal/glial factors are not essential for the hypercapnia-induced vasodilation. In the piglet, hypercapnia-induced vasodilation requires intact endothelium (Leffler et al. 1994b); more specifically, the endothelium appears to serve as a source of prostanoids for the vascular smooth muscle to permit the relaxation (Leffler et al. 1994a). Prostanoid synthesis increases in newborn pig brain microvascular endothelial cells in response to hypercapnia, but high CO2 level does not increase prostanoid production by cerebral microvascular smooth muscle or glial cells (Hsu et al. 1993). Hypercapnia-induced vasodilation is vulnerable to I/R; however, supplementation of arachidonic acid restores this vasodilation and hypercapnia-related increases in the cerebrospinal fluid 6-keto-prostaglandinF1α levels (Leffler et al. 1992). Based on these findings, I/R seems to reduce hypercapnia-induced dilation of pial arterioles through endothelial damage in piglets. Therefore, the present data indicate decreased/shortened postischemic endothelial dysfunction by PACAP or VIP pretreatment, as suggested by the preserved hypercapnia-induced vasodilation. We are not aware of any studies in which similar protective effects of PACAP and VIP have been demonstrated on the cerebrovascular endothelium. Our findings are in agreement with the findings of Lange et al., who demonstrated both the synthesis of VIP and the expression of VIP receptor associated protein in microvascular endothelial cells of pial vessels in piglets (Lange et al. 1999), allowing a direct protective effect of both VIP and PACAP. The function of endothelial VIP production/effects is unclear, but an autocrine growth factor role involved in postnatal endothelial cell differentiation has been suggested. The exact mechanism of endothelial protection by these neuropeptides is unclear and its exploration demands further experiments. Although most data suggest the principal involvement of endothelium, the role of other cell types cannot be excluded, since neuronal/glial components also contribute to hypercapnia-induced cerebrovascular dilation in other experimental models (Wang et al. 1999; Xu et al. 2004).
Our present study clearly demonstrates that PACAP27 and PACAP38, but not VIP preserves CR to NMDA after I/R. The mechanisms of NMDA-induced pial arteriolar dilation and the attenuation of this response after hypoxic/ischemic stress in piglets has been recently reviewed (Busija et al. 2007). Briefly, the activation of neuronal NMDA receptors leads to the subsequent activation of a specific population of neuronal NOS positive neurons via local neuronal connections (Faraci and Breese 1993; Bari et al. 1996b). The released NO then diffuses to and acts on the vascular smooth muscle, resulting in dilation of the pial arterioles (Meng et al. 1995; Domoki et al. 2002). The response is unaffected by damage to the vascular endothelium (Domoki et al. 2002), but have been shown to be vulnerable to even short periods of hypoxic stress (Bari et al. 1996a; Busija et al. 1996). In contrast, the pial arteriolar response to NO itself is unaffected by I/R (Busija et al. 1996). All available evidence strongly suggests the causative role of reactive oxygen species (ROS) in the attenuation of NMDA-induced vasodilation after I/R. In piglets, topical application of ROS scavengers preserves cerebral arteriolar dilator responses to NMDA after I/R (Bari et al. 1996a). The primary site of ROS action appears to be at the level of the NMDA receptor (Choi et al. 2000; Guerguerian et al. 2002). Alternatively, the functional coupling between NMDA receptor and nNOS expressing neuronal populations may be disrupted after I/R.
Although PACAP and VIP display neuroprotective properties against a wide range of pathological conditions, PACAP is generally more potent than VIP and its function has been more widely investigated (Tamas et al. 2002). Vasodilatory, antioxidant, anti-apoptotic, neurotrophic, and anti-inflammatory effects have been promoted as the putative mechanisms of neuroprotection in various experimental models. Our present results suggest that preservation of CR to NMDA after I/R is independent of the increase in cerebral blood flow mediated by vasodilation to PACAP. In fact, PACAP effectively preserved NMDA-induced vasodilation in a non-vasoactive dose, whereas VIP was ineffective in an equimolar, vasoactive dose. However, the reported antioxidant property of PACAP can be an important factor in the preservation of the NMDA receptor function, especially that an analogous antioxidant capacity of VIP is absent (Reglodi et al. 2004). The PACAP-induced initiation of anti-apoptotic and anti-inflammatory mechanisms may also lead to increased general viability of the neurons. More specifically, PAC1 receptor stimulation leads to activation of Bcl-2/inhibition of Bad resulting in enhanced mitochondrial integrity/decreased release of apoptotic cytochrome c. Conceivably, preservation of mitochondrial function fastens the restoration of cellular ATP levels and also reduces postischemic ROS production. PACAP binding sites in the rat cerebral cortex are ten times more numerous as compared to VIP; this difference may also explain the higher general neuroprotective potency of PACAP (Masuo et al. 1991). Alternatively, PACAP isoforms may act on PAC1 receptors expressed on a specific population of cortical neurons critically involved in NMDA-induced vasodilation. Furthermore, the differences observed between PACAP and VIP can be due to different signal transduction mechanisms coupled to PACAP and/or VIP-stimulated receptors in these cells.
Vasodilator neuropeptides such as PACAP and VIP have substantial roles in cerebrovascular control mechanisms (Edvinsson and Krause 2002). In our previous study indomethacin and the selective COX-1 inhibitor SC-560 abolished PACAP38-induced vasodilation to all concentrations used (10–8–10–6 M); however, the vascular action of PACAP27 was completely independent of COX activity (Lenti et al. 2007). Our present results show that vasodilation to 10–8 M VIP resembles the vasodilator effect of PACAP38 characterized by indomethacin- and SC-560-sensitivity. In contrast, at 10–7–10–6 M VIP concentrations the response is unaffected by COX-1 inhibition similar to PACAP27. Since NOS inhibition has also no effect on VIP-induced vasodilation, VIP probably exerts a robust direct effect on vascular smooth muscle cells at these doses. Similarly, VIP dilates the isolated porcine ophthalmic artery via COX-mediated mechanisms only at lower (10–10–10–9 M) VIP concentrations (Vincent 1992). VIP-induced pial arteriolar vasodilation is also indomethacin-sensitive in cats (Wei et al. 1980), whereas involvement of NOS activity in VIP-induced vasodilation was also reported in other, adult animal models (Gaw et al. 1991; Grant et al. 2006). The presumed activation of COX-1 by VIP and PACAP38 is unique, since COX-2 is known to be the predominant COX isoform in the newborn central nervous system (Peri et al. 1995; Parfenova et al. 1997), and SC-560 did not affect the vasodilation in response to any other COX-dependent stimuli studied (Domoki et al. 2005b).
The VIP/PACAP receptor antagonist PACAP fragments (PACAP6-38 and PACAP6-27) inhibited both PACAP27- and PACAP38-induced vasodilation in our previous study (Lenti et al. 2007). PACAP6-38, unlike PACAP6-27, is often reported to be a selective blocker of the PAC1 receptor group (Edvinsson and Krause 2002); however, in the present study, only PACAP6-38 but not PACAP6-27 attenuates VIP-induced vasodilation. This finding is in accordance with studies identifying PACAP6-38 as a nonspecific VIP/PACAP antagonist (Harmar et al. 1998). Possibly, PACAP6-38 simply is a more potent inhibitor than PACAP6-27 on the VIP/PACAP receptors involved in VIP-induced vasodilation. Unfortunately, we are unaware of the existence of any widely accepted, subtype-selective VIP/PACAP receptor blockers that would be required to unveil the functional relevance of each receptor subtype in cerebrovascular and neuroprotective effects of these neuropeptides (Edvinsson and Krause 2002).
In conclusion, PACAP27, PACAP38, and VIP all protect postischemic vascular reactivity to CO2, an ischemia-sensitive indicator of endothelial function in the newborn pig. However, PACAP isoforms but not VIP preserves the NMDA-induced neuron-dependent vasodilation. Although PACAP27, PACAP38, and VIP all induce dose-dependent pial arteriolar dilation by activating partially overlapping mechanisms, the protective effect of these neuropeptides is independent of their vasoactivity. This neurovascular protection probably supports the restoration of adequate perfusion of the brain tissue after I/R likely enhancing the direct neuroprotective effects of VIP and PACAP.
4. Experimental procedures
4.1. Animals
Newborn piglets of either sex (1–2 days old, body weight 1–3 kg, n=138) were used. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Szeged.
Anesthesia was initiated with thiopental sodium (40 mg/kg ip.; Biochemie, Vienna, Austria) and a bolus injection of α-chloralose (40 mg/kg iv.; Sigma, St. Louis, MO, USA). Additional doses of α-chloralose (3–7 mg/kg/h iv.) were given to maintain a constant level of anesthesia. A catheter was inserted into the right femoral artery to monitor blood pressure and to sample blood for determination of blood gas tensions and pH. Fluid and drugs were administered through a second catheter placed in the right femoral vein. The animals were intubated via tracheotomy and mechanically ventilated with room air. The ventilation rate (~30 breaths/min) and tidal volume (~20 ml) were adjusted to maintain arterial blood gas values and pH in the physiological range. A water-circulating heating pad was used to maintain the body temperature at ~37°C. Core temperature was monitored with a rectal probe. The animals were equipped with a closed cranial window as previously described (Domoki et al. 2005b).
Following surgery, the closed window was filled with artificial cerebrospinal fluid (aCSF) which was similar to the endogenous CSF (aCSF composition: KCl 220, MgCl2 132, CaCl2 221, NaCl 7710, urea 402, dextrose 665, and NaHCO3 2066, in mg/l), warmed to 37 °C and equilibrated with a gas mixture containing 6% O2, 6.5% CO2 and 87.5% N2 to obtain pH 7.33, pCO2=46 mm Hg and pO2=43 mm Hg. Pial arterial vessels were observed with an operating microscope (Wild, Switzerland) equipped with a video camera (Sanyo digital color CCD camera, Japan), and a video monitor (Panasonic, Japan). Vascular diameters were measured with a video microscaler. In each experiment, a ~100-μm-diameter (range 76–114 μm) pial arteriole was selected. We chose this vessel size because this is the level of the first-order pial arterioles and the primary site of vascular resistance. After a stable baseline diameter was reached, the window was flushed with aCSF as control. Topical stimuli were applied to the pial surface through one of the injectable ports of the cranial window. The pial arterioles were exposed to each vasodilator stimulus for 5 min, while arteriolar diameters were measured continuously. After completion of a stimulus, the cranial window was flushed with aCSF and the vessel diameter was allowed to return to the baseline level.
To induce global cerebral ischemia, a 3-mm hole was made with an electric drill in the left frontal cranium rostral to the cranial window and the dura was exposed. A hollow brass bolt was inserted and secured in place with dental acrylic. Cerebral ischemia was induced by the infusion of aCSF so as to raise the intracranial pressure above the arterial pressure. Ischemia was verified by the cessation of blood flow in the vessels observed through the cranial window. Venous blood was withdrawn as necessary to keep MABP near the normal values. At the end of the ischemic period, the infusion tube was clamped and the intracranial pressure returned to the preischemic levels. The withdrawn and heparinized blood was reinfused (Domoki et al. 2005a).
At the end of the experiments, the animals were euthanized with an iv. injection of saturated KCl solution.
4.2. Drugs
VIP (Sigma Chemical Co.), PACAP38, PACAP27, PACAP6-27, and PACAP6-38 (Department of Medical Chemistry, University of Szeged, Hungary) were prepared as stock solutions in saline (10–4 M) and, before use, were diluted with aCSF (10–9–10–5 M). Indomethacin (Merck & Co, Whitehouse Station, NJ) and L-NAME (Sigma Chemical Co.) were dissolved in saline (30 mg/ml and 10 mg/ml, respectively). NS-398 (Sigma Chemical Co.) and SC-560 (Sigma Chemical Co.) were dissolved in dimethyl sulfoxide (DMSO, 5 mg/ml) and further diluted with saline to 1 ml. NMDA (Sigma Chemical Co.) was dissolved in aCSF. Selection of enzyme inhibitor doses was based on previous results in this experimental model (Bari et al. 1996b; Domoki et al. 2005b; Lenti et al. 2007).
4.3. Protocol
In the first series of experiments, the effect of global cerebral I/R (10 min of ischemia and 1 h of reperfusion) was tested on CO2- and NMDA-induced vasodilation, with or without PACAP or VIP treatment. Prior to the induction of ischemia, the brain surface was incubated for 30 min with vehicle (aCSF, groups 1–2, n=6–6), 10–8 M PACAP27 (groups 3–4, n=6–6), 10–8 M PACAP38 (groups 5–6, n=6–6), or 10–9 M VIP (groups 7–8, n=8–6). Pial arteriolar caliber changes to 5 and 10% CO2 ventilation (groups 1, 3, 5, and 7), or to topical, 10–4 M NMDA (groups 2, 4, 6, and 8) were measured before the pretreatments and after 1 h of reperfusion. We performed additional experiments on 4–4 animals in groups 8b and 8c to test whether a higher (10–8 M) dose of VIP can protect NMDA-induced vasodilation after I/R. In group 8b we used the NMDA at 10–4 M concentration similar to the original experiments, while in group 8c we tested if the vascular response to a lower, 10–5 M and 5×10–5 M concentration of NMDA can be protected by VIP (10–8 M) after I/R. The effects of the same PACAP27, PACAP38 or VIP pretreatments in the absence of I/R were also tested on the vasodilation evoked by hypercapnia (groups 9, 11, and 13, n=6–5–6) or by NMDA (groups 10, 12, and 14, n=5–7–5), respectively.
In the second series of experiments, the brain surface was exposed to VIP before and after the intravenous administration of different enzyme inhibitors or topical application of different PACAP fragments. VIP application (10–8–10–6 M cumulatively) was repeated 20 min after the intravenous administration of vehicle (group 15, saline in 5, and diluted DMSO in 3 animals), indomethacin (group 16, n=7, 5 mg/kg), SC-560 (group 17, n=6, 1 mg/kg), NS-398 (group 18, n=7, 1 mg/kg) or L-NAME (group 19, n=8, 15 mg/kg). Pial arteriolar dilation to 10–6 M VIP was determined before and after 20 min topical application of PACAP6-38 (group 20, n=6, 10–5 M) or PACAP6-27 (group 21, n=7, 10–5 M).
4.4. Statistical analysis
The pial artery diameter data (absolute diameters or maximal percentage changes from the baseline values) were analyzed by one-way repeated measures ANOVA. For post hoc analysis, Tukey's test was performed where appropriate. Values of p<0.05 were considered significant. Data are reported as mean±SEM.
Acknowledgments
The authors thank Valéria Tóth-Szűki for her excellent technical assistance. This study was supported by grants from the National Institutes of Health (HL30260, HL65380, HL77731), Hungarian Health Science Board (ETT 194042006) and from the National Scientific Research Fund of Hungary (OTKA, K68976, K63401, IN69967). Ferenc Domoki was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Abbreviations
- PACAP
pituitary adenylate cyclase activating polypeptide
- VIP
vasoactive intestinal peptide
- NMDA
N-methyl-d-aspartate
- COX
cyclooxygenase
- NOS
nitric oxide synthase
- NO
nitric oxide
- L-NAME
N-ω-nitro-l-arginine methyl ester
- DMSO
dimethyl sulfoxide
- aCSF
artificial cerebrospinal fluid
- ROS
reactive oxygen species
- CR
cerebrovascular reactivity
- I/R
ischemia/reperfusion
- MABP
mean arterial blood pressure
- ANOVA
analysis of variance
- SEM
standard error of the mean
REFERENCES
- Bari F, Errico RA, Louis TM, Busija DW. Differential effects of short-term hypoxia and hypercapnia on N-methyl-d-aspartate-induced cerebral vasodilatation in piglets. Stroke. 1996a;27:1634–1639. doi: 10.1161/01.str.27.9.1634. discussion 1639–1640. [DOI] [PubMed] [Google Scholar]
- Bari F, Errico RA, Louis TM, Busija DW. Interaction between ATP-sensitive K+ channels and nitric oxide on pial arterioles in piglets. J. Cereb. Blood Flow Metab. 1996b;16:1158–1164. doi: 10.1097/00004647-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Berger R, Garnier Y, Jensen A. Perinatal brain damage: underlying mechanisms and neuroprotective strategies. J. Soc. Gynecol. Investig. 2002;9:319–328. [PubMed] [Google Scholar]
- Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–1726. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Busija DW, Meng W, Bari F, McGough PS, Errico RA, Tobin JR, Louis TM. Effects of ischemia on cerebrovascular responses to N-methyl-d-aspartate in piglets. Am. J. Physiol. 1996;270:H1225–H1230. doi: 10.1152/ajpheart.1996.270.4.H1225. [DOI] [PubMed] [Google Scholar]
- Busija DW, Bari F, Domoki F, Louis T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007;56:89–100. doi: 10.1016/j.brainresrev.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- Domoki F, Perciaccante JV, Shimizu K, Puskar M, Busija DW, Bari F. N-methyl-d-aspartate-induced vasodilation is mediated by endothelium-independent nitric oxide release in piglets. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1404–H1409. doi: 10.1152/ajpheart.00523.2001. [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Nagy K, Farkas E, Busija DW, Bari F. Diazoxide preserves hypercapnia-induced arteriolar vasodilation after global cerebral ischemia in piglets. Am. J. Physiol. Heart Circ. Physiol. 2005a;289:H368–H373. doi: 10.1152/ajpheart.00887.2004. [DOI] [PubMed] [Google Scholar]
- Domoki F, Nagy K, Temesvari P, Bari F. Selective inhibitors differentially affect cyclooxygenase-dependent pial arteriolar responses in newborn pigs. Pediatr. Res. 2005b;57:853–857. doi: 10.1203/01.PDR.0000161415.62776.0A. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Krause DN. Cerebral Blood Flow and Metabolism. Lippincott Williams and Wilkins; Philadelphia, USA: 2002. [Google Scholar]
- Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: relation to PACAP and VIP containing nerves. J. Cereb. Blood Flow Metab. 2000;20:1205–1214. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR. Nitric oxide mediates vasodilatation in response to activation of N-methyl-d-aspartate receptors in brain. Circ. Res. 1993;72:476–480. doi: 10.1161/01.res.72.2.476. [DOI] [PubMed] [Google Scholar]
- Gaw AJ, Aberdeen J, Humphrey PP, Wadsworth RM, Burnstock G. Relaxation of sheep cerebral arteries by vasoactive intestinal polypeptide and neurogenic stimulation: inhibition by l-NG-monomethyl arginine in endothelium-denuded vessels. Br. J. Pharmacol. 1991;102:567–572. doi: 10.1111/j.1476-5381.1991.tb12213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Lutz EM, McPhaden AR, Wadsworth RM. Location and function of VPAC1, VPAC2 and NPR-C receptors in VIP-induced vasodilation of porcine basilar arteries. J. Cereb. Blood Flow Metab. 2006;26:58–67. doi: 10.1038/sj.jcbfm.9600163. [DOI] [PubMed] [Google Scholar]
- Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-d-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res. Mol. Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Hsu P, Shibata M, Leffler CW. Prostanoid synthesis in response to high CO2 in newborn pig brain microvascular endothelial cells. Am. J. Physiol. 1993;264:H1485–H1492. doi: 10.1152/ajpheart.1993.264.5.H1485. [DOI] [PubMed] [Google Scholar]
- Kokubun S, Fukuda S, Shimoji K, Sakamoto H, Gamou S, Ogura M, Yunokawa S, Morita S. Differential responses of porcine anterior spinal and middle cerebral arteries to carbon dioxide and pH. Crit. Care Med. 2009;37:987–992. doi: 10.1097/CCM.0b013e3181961330. [DOI] [PubMed] [Google Scholar]
- Lange D, Funa K, Ishisaki A, Bauer R, Wollina U. Autocrine endothelial regulation in brain stem vessels of newborn piglets. Histol. Histopathol. 1999;14:821–825. doi: 10.14670/HH-14.821. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Beasley DG, Busija DW. Cerebral ischemia alters cerebral microvascular reactivity in newborn pigs. Am. J. Physiol. 1989a;257:H266–H271. doi: 10.1152/ajpheart.1989.257.1.H266. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Busija DW, Armstead WM, Mirro R, Beasley DG. Ischemia alters cerebral vascular responses to hypercapnia and acetylcholine in piglets. Pediatr. Res. 1989b;25:180–183. doi: 10.1203/00006450-198902000-00020. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Mirro R, Armstead WM, Shibata M. Topical arachidonic acid restores pial arteriolar dilation to hypercapnia of postischemic newborn pig brain. Am. J. Physiol. 1992;263:H746–H751. doi: 10.1152/ajpheart.1992.263.3.H746. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Mirro R, Shibata M, Parfenova H, Armstead WM, Zuckerman S. Effects of indomethacin on cerebral vasodilator responses to arachidonic acid and hypercapnia in newborn pigs. Pediatr. Res. 1993;33:609–614. doi: 10.1203/00006450-199306000-00016. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Mirro R, Pharris LJ, Shibata M. Permissive role of prostacyclin in cerebral vasodilation to hypercapnia in newborn pigs. Am. J. Physiol. 1994a;267:H285–H291. doi: 10.1152/ajpheart.1994.267.1.H285. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Mirro R, Shanklin DR, Armstead WM, Shibata M. Light/dye microvascular injury selectively eliminates hypercapnia-induced pial arteriolar dilation in newborn pigs. Am. J. Physiol. 1994b;266:H623–H630. doi: 10.1152/ajpheart.1994.266.2.H623. [DOI] [PubMed] [Google Scholar]
- Lenti L, Domoki F, Kis D, Hegyi O, Toth GK, Busija DW, Bari F. Pituitary adenylate cyclase-activating polypeptide induces pial arteriolar vasodilation through cyclooxygenase-dependent and independent mechanisms in newborn pigs. Brain Res. 2007;1165:81–88. doi: 10.1016/j.brainres.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Nagai Y, Suno M, Tsuda M, Fujino M. Autoradiographic distribution of pituitary adenylate cyclase activating polypeptide (PACAP) binding sites in the rat brain. Neurosci. Lett. 1991;126:103–106. doi: 10.1016/0304-3940(91)90529-3. [DOI] [PubMed] [Google Scholar]
- Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-d-aspartate receptors. Stroke. 1995;26:857–862. doi: 10.1161/01.str.26.5.857. discussion 863. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Eidson TH, Leffler CW. Upregulation of COX-2 in cerebral microvascular endothelial cells by smooth muscle cell signals. Am. J. Physiol. 1997;273:C277–C288. doi: 10.1152/ajpcell.1997.273.1.C277. [DOI] [PubMed] [Google Scholar]
- Peri KG, Hardy P, Li DY, Varma DR, Chemtob S. Prostaglandin G/H synthase-2 is a major contributor of brain prostaglandins in the newborn. J. Biol. Chem. 1995;270:24615–24620. doi: 10.1074/jbc.270.41.24615. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Fabian Z, Tamas A, Lubics A, Szeberenyi J, Alexy T, Toth K, Marton Z, Borsiczky B, Roth E, Szalontay L, Lengvari I. Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation, and production of reactive oxygen radicals. Regul. Pept. 2004;123:51–59. doi: 10.1016/j.regpep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J. Neurochem. 2007;103:1666–1681. doi: 10.1111/j.1471-4159.2007.04895.x. [DOI] [PubMed] [Google Scholar]
- Tamas A, Reglodi D, Szanto Z, Borsiczky B, Nemeth J, Lengvari I. Comparative neuroprotective effects of preischemic PACAP and VIP administration in permanent occlusion of the middle cerebral artery in rats. Neuro. Endocrinol. Lett. 2002;23:249–254. [PubMed] [Google Scholar]
- Tong S, Parfenova H, Shibata M, Zuckerman S, Armstead WM, Leffler CW. Pituitary adenylate cyclase-activating polypeptide dilates cerebral arterioles of newborn pigs. Proc. Soc. Exp. Biol. Med. 1993;203:343–347. doi: 10.3181/00379727-203-43609. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vincent MB. Cyclooxygenase inhibitors modify the relaxant effect of vasoactive intestinal polypeptide and substance P in isolated porcine ophthalmic artery. Cephalalgia. 1992;12:15–19. doi: 10.1046/j.1468-2982.1992.1201015.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bryowsky J, Minshall RD, Pelligrino DA. Possible obligatory functions of cyclic nucleotides in hypercapnia-induced cerebral vasodilation in adult rats. Am. J. Physiol. 1999;276:H480–H487. doi: 10.1152/ajpheart.1999.276.2.H480. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Said SI. Mechanism of action of vasoactive intestinal polypeptide on cerebral arterioles. Am. J. Physiol. 1980;239:H765–H768. doi: 10.1152/ajpheart.1980.239.6.H765. [DOI] [PubMed] [Google Scholar]
- Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of the glia limitans on pial arteriolar relaxation in the rat. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H331–H339. doi: 10.1152/ajpheart.00831.2003. [DOI] [PubMed] [Google Scholar]