Abstract
Axonal transport, the process by which membrane-bound organelles and soluble protein complexes are transported into and out of axons, ensures proper function of the neuron, including that of the synapse. As such, abnormalities in axonal transport could lead to neuronal pathology and disease. Similar to many neurodegenerative diseases, axonal transport is deficient in Alzheimer’s disease (AD), a neurodegenerative brain disorder that affects old-age humans and is characterized by the deterioration of cognitive function and progressive memory loss. It was proposed that the synaptic pathology and neuronal degeneration that develops in AD could be caused by an abnormal axonal transport, and that the mutated proteins that cause early-onset AD, as well as the genetic variants that confer predisposition to late-onset AD might somehow impede axonal transport. This paper analyzes the data that support or contradict this hypothesis. Together, they indicate that, although abnormalities in axonal transport are part of the disease, additional studies are required to clearly establish to what extent deficient axonal transport is the cause or the effect of the neuronal pathology in AD, and to identify mechanisms that lead to its perturbation.
Keywords: Alzheimer’s disease, amyloid-β peptide, amyloid-β precursor protein, apolipoprotein E4, axonal transport, JNK-interacting protein-1, kinesin, neurodegeneration, presenilin-1, sortilin-related receptor
Alzheimer’s disease (AD), the most prevalent neurodegenerative disorder causing mental failure in the elderly [1–3], is a multifactorial syndrome of as yet unknown etiology [4], predicted to affect 14 million individuals by 2050 [5]. In a small number of cases (~1%), AD starts early in life, before the age of 60–65 years; these forms of early-onset AD (EOAD, or familial AD) are caused by rare autosomal dominant mutations in three genes: APP, PS1 or PS2, all of which alter the metabolism of the amyloid-β (Aβ) precursor protein (APP) and the production of the amyloidogenic peptide, Aβ [6–9]. For most cases of AD – those termed late-onset AD (LOAD, or sporadic AD) – the first signs of disease appear at much older ages, and are thought to be caused by yet unknown conditions associated with aging, long-term exposure to detrimental environmental factors and specific genetic predisposition. The latter includes certain common genetic variants with low penetrance [10]. So far, only one clearly established (APOE4), and one potential (SORL1) susceptibility gene for LOAD have been identified [6,7, 11–13].
The clinical symptomatology of AD is complex, and is highlighted by the deterioration of cognitive function and progressive memory loss. Pathologically, AD has been associated with synaptic dysfunction and neuronal loss, as well as with the presence of neuritic plaques and neurofibrilary tangles in specific brain regions, both of which consist of protein aggregates. Neuritic plaques are extracellular deposits of material (i.e., proteins, proteoglycans and metal ions) that contain aggregated Aβ at their core, generated by the proteolytic processing of APP. Neurofibrillary tangles are intraneuronal, cytoplasmic lesions consisting of aggregated, hyperphosphorylated tau (a microtubule binding protein). The clinical symptomatology in AD is likely caused by synaptic dysfunction followed by neuronal loss [14,15]. However, what triggers this neuronal pathology, and its relation to the plaques and tangles, is still not clear.
Disruption of axonal transport is part of the pathogenic process in AD, but is the disruption the cause or the result of it?
An interesting idea that has recently gained support is that the synaptic pathology and neuronal degeneration in AD might be linked to abnormal axonal transport [16–18], the process by which membrane-bound organelles and soluble protein complexes are transported into and out of axons [19]. Transport of membranous cargo within axons, known as fast axonal transport, occurs along microtubule tracks and is powered by molecular motors: kinesins (for movement in the anterograde direction) and cytoplasmic dynein (for movement in the retrograde direction) [19]. Kinesin-1, consisting of two heavy chains, which contain the motor domains, and two light chains (KLCs), which bind to the cargo, is the major anterograde motor responsible for transport of many cargoes, including APP. The recruitment of the motors to the transport vesicles, and their release – usually occurring after completion of the transport – are regulated events that determine the rate of transport.
Axonal transport is likely to be abnormal in degenerating neurons of AD brains, judged from the axonal pathology detected in affected brain regions in humans and in mouse models of the disease (see [20–22] for examples; reviewed in [23–25]). However, whether the abnormality is the cause, a facilitating factor, or the result of the disease is unknown. In principle, a block or reduction in axonal transport could cause the depletion of mitochondria, peroxisomes and synaptic vesicle precursors from axons, leading to decreased energy production, increased oxidative stress and dysregulation of synaptic function. An impaired axonal transport could also cause the accumulation of material destined for the axon within the cell body, which could lead to perikaryal degeneration.
Another possibility is that small, more difficult to detect changes in axonal transport of specific proteins, rather than its global perturbation, initiate the neuronal pathology leading to AD. Such subtle deficiencies in the transport of a limited number of proteins may lead to their mislocalization, and loss (or gain) of function at specific intracellular sites. For example, a defective transport of APP, a protein highly relevant for AD, might perturb its proteolytic processing and increase the production of the amyloidogenic peptide, Aβ [26]. Similarly, an abnormal axonal transport of kinases (e.g., cyclin-dependent kinase 5 [Cdk5], glycogen synthase kinase 3β [GSK3β], casein kinase 2 [CK2], c-Jun N-terminal kinase [JNK]) could result in improper localization of the entire signaling cascades, and promote aberrant phosphorylation of proteins. Hyperphosphorylation of tau, followed by its aggregation into neurofibrillary tangles [27,28], or of APP, causing increased susceptibility to proteolytic cleavage by secretases [29], may be the result of such processes. Perturbation of the transport of a limited set of proteins has been occasionally reported for other neurodegenerative diseases. For example, a recent study demonstrated that the palette of signaling molecules transported by retrograde axonal transport is changed in neurons from a transgenic mouse model for familial amyotrophic lateral sclerosis [30].
A different view is that the AD-specific neuronal pathology is triggered by dysregulation of cellular processes that are not linked to axonal transport. For example, such conditions could prevent proper recycling of synaptic vesicles [31], or could interfere with normal mitochondrial function [32], both of which could affect the function of the synapse. These abnormalities may thus lead to axonal pathology that is independent of axonal transport. Eventually, this neuronal pathology could also perturb the axonal transport, in a subsequent phase, and the axonal transport deficiency may accelerate and accentuate the neuronal pathology. However, in this case, the alteration of transport is a consequence rather than the cause of the initial pathogenic event.
How could the AD-specific conditions lead to abnormal axonal transport? Do the proteins implicated in the pathogenesis of AD impinge on intracellular transport? This scenario seems likely, since the genetics of AD suggest a link between the proteins associated with the disease and axonal transport. For example, APP and the presenilins – proteins that are mutated in EOAD – have been implicated in the regulation of axonal transport (see later). In addition, APOE4 and SORLA, proteins encoded by genes that confer susceptibility to LOAD, also appear to participate in the regulation of intracellular transport processes (see later). Given the multiple connections between axonal transport and the pathobiology of AD, the question whether a deficient axonal transport is the cause, a facilitating factor or the consequence of AD is essential for the prevention, early detection and, possibly, treatment of the disease. In the following sections, we will briefly analyze the evidence that links the proteins associated with increased risk for AD to abnormal axonal transport.
Is APP a cargo linker or only a cargo protein for kinesin-1?
Approximately one decade ago, a report demonstrating that APP can bind directly to the microtubule motor, kinesin-1 [33] prompted a heated debate aimed at determining how relevant for the in vivo situation this interaction is and, if true, how it might impact the field of AD (see [34,35] for examples).
Amyloid-β precursor protein is a type I glycoprotein with receptor-like structure [36], comprising a family of ubiquitously expressed, alternatively spliced and post-translationally processed polypeptides [37]; the neuronal isoform is APP695. Part of the newly synthesized APP follows the secretory pathway and is targeted, as full-length protein, into axons, by fast axonal transport [36]. A fraction of the synthesized, full-length APP reaches the plasma membrane and re-enters the cell via endocytic vesicles. Along the secretory and endocytic pathway, APP undergoes endoproteolytic cleavages by proteases, known as secretases [38,39], which generate three classes of biologically active peptides: the soluble N-terminal fragments (sAPPs); short middle segments, such as the amyloidogenic, 40–42-amino-acid long Aβ; and the carboxy-terminal fragments (CTFs) (Figure 1) [9]. Thus, the processing and transport of APP are coupled events that influence each other.
Figure 1. Processing of amyloid-β precursor protein via the β-secretase pathway.
The positions of the transmembrane domain, of Aβ (red), and of the cleavage sites are marked. The APP-derived proteolytic fragments, sAPPβ, Aβ, AICD, and the intermediary cleavage product, CTF-β, are also shown. The cleavage by γ-secretase at positions 636/638 generates Aβ40/42.
Aβ: Amyloidβ; APP: Amyloid-β precursor protein; AICD: APP intracellular domain; CTF-β: Carboxy-terminal fragment-β.
Two findings from the Goldstein laboratory revived the interest in the axonal transport of APP, in relation to AD. The first showed that APP might function as a receptor for kinesin-1 on transport vesicles, directly binding to the tetratricopeptide repeat (TPR) domain of the KLCs [33,40]. The second finding suggested that Aβ can be generated in transit, within the transport vesicles – thus, linking the transport of APP to AD [26]. Although these studies have since been challenged by several laboratories [35,41,42], they reignited the interest in investigating the intricate relationship between APP processing and transport, and the role a deficient axonal transport might play in the pathogenic process in AD. Let us take a closer look at the possibility that APP is a linker for kinesin-1 to cargo vesicles.
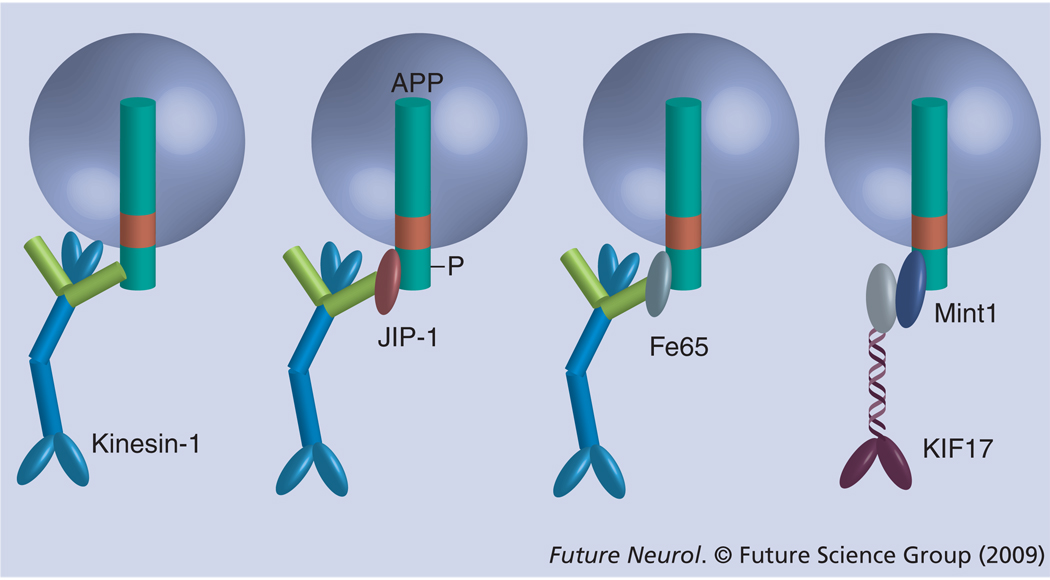
Numerous studies implicate kinesin-1 in the transport of APP into axons [43–47]. However, the notion that APP could have the function of recruiting kinesin-1 to cargo vesicles, as proposed by the Goldstein laboratory, confers to APP a major role in regulating the axonal transport. Although it was initially believed that APP anchors kinesin-1 to a significant fraction of axonally transported vesicles [33], the pool size of vesicles that recruit kinesin-1 via a direct interaction with APP may be small. In addition, more recent data show that the binding of kinesin-1 to APP may not be direct, but instead mediated by scaffolding proteins capable of binding simultaneously to APP and kinesin-1 [48–50]. The JNK-interacting protein-1 (JIP-1) is such a bridging protein that binds APP, via its phosphotyrosine-binding (PTB) domain [51–53], and the KLC subunits of kinesin-1, via a C-terminal motif [54,55]. Under endogenous conditions, JIP-1 mostly recruits kinesin-1 to a phosphorylated form of APP (pAPP; generated by phosphorylation of Thr668 of APP [56,57]), which constitutes a small fraction of the total cellular APP [50]. Fe65, another APP-binding scaffolding protein may fulfill a similar function, since it is capable of binding with high affinity to the KLCs of kinesin-1 [35] and APP [58]. Importantly, since Fe65 binds nonphosphorylated APP with an affinity approximately sevenfold higher compared with pAPP [59], it may primarily recruit kinesin-1 to nonphosphorylated APP (Figure 2).
Figure 2. Amyloid-β precursor protein recruits kinesin motors to cargo vesicles via adaptor proteins.
JIP-1, Fe65 and Mint1/X11α bind to APP via their phosphotyrosin-binding domains. JIP-1 and Fe65 also bind to the light chains of kinesin-1; Mint1/X11α binds to KIF17, a kinesin-2 motor with dendritic targeting. In the cellular context, JIP-1 binds preferentially to phosphorylated APP, while Fe65 binds to nonphosphorylated APP. The direct binding of kinesin-1 to APP, as shown to the left, could be less relevant for the in vivo situation.
APP: Amyloid-β precursor protein; JIP: JNK-interacting protein.
In addition to recruiting kinesin-1 via JIP-1 and, possibly, Fe65, APP may also recruit the kinesin-2 motor, KIF17, via the PTB domain-containing protein, Mint1/X11α, which also binds to KIF17 via a PDZ domain (Figure 2) [58,60]. Since KIF17 is primarily a dendritic motor [60], it is likely to transport APP species into dendrites. We note that significant amounts of APP are localized to dendrites. While in principle, APP could serve as an anchor for kinesins to cargo vesicles via the mechanisms described above, the extent to which this actually occurs in vivo, under native conditions, has only started to be evaluated. While under normal conditions, pAPP does bind kinesin-1 via JIP-1, and the two proteins are transported into neurites as a complex [50], it is possible that a significant fraction of non-phosphorylated APP are only cargo proteins for kinesins and not cargo linker proteins.
A slowed down axonal transport may increase production of amyloid-β, but does this mechanism operate in AD?
Since kinesin motors may bind – mostly indirectly – to APP, APP gains an important role not only for its own transport, but also for the transport of a number of membrane and soluble proteins that are sorted with APP in the transport vesicle. The premature cleavage of APP, resulting in the generation of Aβ inside the transport vesicle and shedding of the cytoplasmic C-terminal fragment, could theoretically lead to the release of the motor, and terminate the transport of many axonal proteins. In this scenario, the deficiency in axonal transport is a consequence of the premature cleavage of APP. A reversed situation, where an initial slowing down of axonal transport leads to APP cleavage and abnormal generation of Aβ can also be envisioned. This scenario is supported by earlier studies from the Goldstein laboratory, which suggest that the APP processing machinery (i.e., the secretases) is packaged into the same transport vesicle that carries APP [26,33]. Thus, an impeded transport could lead to premature proteolysis of APP and generation of cleavage products – including Aβ – in places where they may not be properly cleared (Figure 3A) [26]. According to this model, a retarded axonal transport is the initial event that leads to the neuronal pathology in AD, including the abnormal generation and aggregation of Aβ. The finding that the disruption of kinesin-1-driven transport in transgenic mice leads to increased axonal pathology, production of Aβ and amyloid deposition is consistent with the model described previously [22]. However, other studies failed to confirm key features of this model, such as the interaction of APP with kinesin-1 [35], and the cotransport of APP with BACE1, the major β-secretase [41]. Moreover, recent studies indicate that a significant fraction of APP is cleaved very early in the secretory pathway, prior to sorting into transport vesicles. Subsequently, these APP-derived fragments are sorted separately, and transported independently to various destinations within the neuron [61]. As a consequence, the axonal transport of APP becomes a vague concept, referring to transport of a large number of APP cleavage products, phosphorylated or not, in addition to full-length APP. Importantly, the cleavage of APP by secretases may or may not result in the release of the cleaved APP intracellular domain (AICD) – to which presumably the kinesin motors bind – from the cargo vesicle into the cytoplasm, as previously thought [61,62]. Thus, the processing of APP within the transport vesicle, if it occurs, would not necessarily release the kinesin, and halt transport. Although attractive, a mechanism where a slowed down axonal transport leads both to the abnormal generation of Aβ and to further inhibition of transport, owing to cleavage of APP and release of the kinesin motor, may not operate to a large extent in AD.
Figure 3. Hypothetical models for the release of kinesin-1 from transport vesicles, leading to disruption of the fast axonal transport in Alzheimer’s disease.
(A) A slowed down axonal transport at old age could allow premature processing of APP and release of kinesin-1 from the transport vesicle, leading to a halt in transport (crossed arrow) [26]. The cessation of transport further facilitates cleavage of APP molecules by cotransported secretases (not depicted), thus amplifying the loop where the deficiencies in axonal transport and processing of APP potentiate each other. Although this model is no longer regarded as a major mechanism operating in Alzheimer’s disease (AD), the basic principle for the disruption of the axonal transport by premature release of the motor from the transport vesicle remains valid. (B) The regulation of axonal transport by phosphorylation of the light chains of kinesin-1 by casein kinase 2 (CK2) [69] or glycogen synthase kinase 3β (GSK3β) [77,78]. The phosphorylated kinesin-1 is released from the vesicle, causing a halt in transport (crossed arrow). The signaling cascades leading to phosphorylation could be triggered by the AD-linked increased production of Aβ oligomers, or by a mutated presenilin-1 (PS1swe) present in some cases of early-onset AD. It should be noted that, (A) kinesin-1 binds to APP or (B) to another receptor protein. Other cargo proteins are shown.
Aβ: Amyloid-β; APP: Amyloid-β precursor protein.
Does amyloid-β block axonal transport in AD?
Amyloid-β is clearly associated with the pathogenic process in AD. As a result, numerous studies have asked whether Aβ could be a cause of the axonal transport abnormalities that accompany AD. There are many reasons to suspect that Aβ is detrimental to axonal transport. Thus, Aβ is highly toxic for neurons in cell culture and in animal models of disease. When generated in excess, Aβ (a hydrophobic peptide with high propensity for protein–protein interactions) is likely to affect a multitude of neuronal processes, including axonal transport. While APP-induced axonopathies might not necessarily be inflicted by a toxic effect of Aβ [63,64], several studies indicate that Aβ can lead to inhibition of axonal transport via a variety of mechanisms, depending on the oligomeric state of Aβ (i.e., monomeric, oligomeric or fibrillar) and the site of its action (extracellular or intracellular). We note that, although in AD Aβ accumulates primarily in extracellular plaques that contain high levels of fibrillar Aβ, the current thinking is that soluble Aβ oligomers – acting either from the extracellular space or from the inside of the cell – are the real culprits in AD [14,65].
The effect of extracellular Aβ on axonal transport was investigated in mice in vivo [66], and in neuronal cultures [67]; in all cases, axonal transport of vesicular cargo was severely impaired. The impairment of transport was acute, suggesting that it occurs through activation of specific signaling pathways. Interestingly, the trafficking of mitochondria was particularly sensitive to treatment with Aβ, and the signaling pathway leading to the blockade of mitochondrial motility was mediated by GSK3β [68]. Whether this signaling alters the microtubules, the motor itself, or the attachment of the motor to the cargo or to the microtubules, remains to be established. We note that the inhibition of transport appears to be more pronounced for fibrillar compared with monomeric Aβ, although there is no consensus on this among the different studies.
The effect of intracellular Aβ on axonal transport was studied in isolated squid axoplasm [69] – an in situ system that shows robust vesicular transport [70]. Perfusion of the axoplasm with soluble Aβ oligomers, but not monomeric or fibrillar Aβ, disrupted the fast axonal transport via a pathway that led to the phosphorylation of KLCs by casein kinase 2, and release of kinesin-1 from the transport vesicles (Figure 3B) [69]. We note that the perfusion of squid axoplasm with Aβ, as performed in this study, does not reflect the expected topology of Aβ, which normally should be confined within membrane compartments rather than being free in the cytoplasm. Even if Aβ is taken up from the extracellular space via endocytosis, it should still remain encapsulated inside the endocytic vesicle. However, occasionally, intraneuronal Aβ was detected in the cytoplasm [71], where it could have escaped by translocation across the encapsulating membrane, or through leak-age from a damaged membrane compartment. While these remain real possibilities (since Aβ is a hydrophobic peptide and could, in principle, cross or translocate through membranes), they need to be confirmed experimentally.
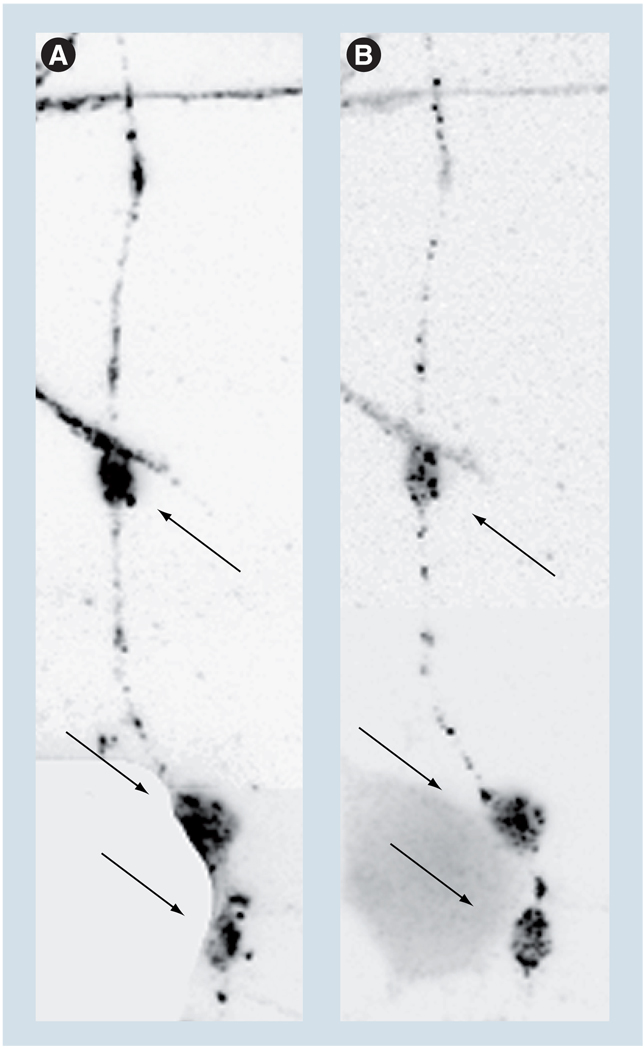
Unlike the soluble cytoplasmic Aβ, the intraneuronal accumulations of Aβ, present occasionally within membrane compartments in the neurites of cultured neurons, do not globally perturb the axonal transport [72]. However, transport and distribution of mitochondria within the neurites that contain such accumulations are perturbed (Figure 4), and the neurites themselves appear dystrophic [73]. These and other results indicate that transport of mitochondria is particularly sensitive to AD-related insults and may thus be a major cause of the detected axonopathy.
Figure 4. Clusters of mitochondria colocalize with amyloid-β deposits.
Clusters of mitochondria colocalize with amyloid-β (Aβ) deposits (arrows) within the neurite of a CAD cell (a CNS-derived neuronal cell line [117]). Neurites containing Aβ deposits also contain fewer mitochondria [73]. (A) The mitochondria and (B) Aβ accumulations were detected with an antibody to lipoic acid and antibody 6E10 (Signet, Dedham, MA, USA), respectively. The inverted, fluorescence microscopy images were adjusted for contrast and brightness, to facilitate the evaluation of colocalization.
The studies previously summarized clearly show that Aβ has a detrimental effect on axonal transport, acting via a multitude of pathways. However, whether this is a mechanism for impairment of axonal transport in AD is still unknown.
Presenilin-1 regulates vesicle transport, but its enzymatic activity may not be required
The idea that APP processing and generation of Aβ could regulate axonal transport prompted the re-investigation of presenilins – proteins encoded by genes mutated in EOAD, which participate in APP processing – as possible regulators of axonal transport. Presenilin (PS)-1 had been previously shown to play a role in intracellular trafficking of various proteins (see [74–76] for examples). Several recent studies have indeed demonstrated that PS1 regulates axonal transport, and that EOAD-linked mutations in PS1 lead to the impairment of fast axonal transport [77,78]. The exact mechanisms by which PS1 controls axonal transport are not fully understood, but may not involve the proteolytic activity of PS1. It was proposed that EOAD-linked PS1 mutants might somehow activate GSK3β, leading to increased phosphorylation of kinesin-1 and its release from the cargo vesicles (Figure 3B) [77,78]. The PS1 mutants would thus trigger axonal pathology by acting directly on axonal transport. However, this may be an oversimplification, since a recent study demonstrated that EOAD-linked PS1 mutants may actually alleviate axonal transport defects caused by EOAD-linked APP mutants [64].
Opposing effects of tau on kinesin-1-based transport
Although no mutations in MAPT (the gene encoding tau) were found in AD, tau is clearly linked to the pathology of AD [7]. The binding of tau to microtubules leads to their stabilization [79], and should thus enhance the transport of cargo along microtubules. In principle, this positive effect on axonal transport could be counterbalanced by the inhibition of kinesin-1-driven transport [80], likely due to blocking of kinesin-1 binding to microtubules by tau [81–83]. Thus, the net effect of increased tau levels, presumably occurring in AD [84], on axonal transport may not be very detrimental. For the same reason, a reduced affinity for microtubules of hyperphosphorylated tau [85] – also observed in AD [86–88] – may not necessarily perturb axonal transport through the diminished binding of phosphorylated tau to microtubules (causing thus decreased microtubule stability), but rather via other mechanisms. However, tau overexpression and abnormal phosphorylation likely contribute to the formation of disease-related intraneuronal structures, such as the neuropile threads and neurofibrillary tangles, which themselves may physically damage the microtubules and obstruct transport within the neurites [89,90]. Moreover, the extent by which the opposing effects of tau on axonal transport may balance each other out depends on the concentration of tau and on its affinity for microtubules (regulated, in part, through phosphorylation of tau). This could explain the apparently contradictory results obtained using different experimental systems, with regard to the effect of tau on axonal transport. Indeed, while overexpression of tau in Drosophila motor neurons [91,92] or mammalian neurons [80] was shown to interfere with organelle transport (including transport of mitochondria), other studies, using the squid axon system or transgenic mice, found that axonal transport is unaffected by increased concentrations of tau [93,94].
The effect of tau on axonal transport in AD may be difficult to assess in experimental systems that do not reproduce the full palette of disease-related changes, such as the presence of Aβ. In this respect, one study demonstrated that tau, instead of stabilizing microtubules, facilitates their disassembly, initiated by the addition of extracellular, prefibrillar Aβ [95]. Thus, tau may have opposing effects on the stability of microtubules, depending on specific extracellular and intracellular conditions. Interestingly, aberrant activation of Cdk5 in transgenic mice leads to a typical AD brain pathology, including the development of neurofibrillary tangles (aggregated and hyperphosphorylated tau), enhanced amyloidogenic processing of APP, intraneuronal accumulation of Aβ, and neuronal degeneration and loss [96,97]. Although the neuronal degeneration observed in these mice is compatible with axonal transport defects, it is difficult to determine if this is a cause or the result of the AD-like pathology. However, a recent study demonstrated that the selective disruption of kinesin-1-driven transport in a transgenic mouse triggers a cascade of events that leads to tau hyperphosphorylation and accumulation [98]. Whether such a mechanism may operate in AD is difficult to predict, since no tau pathology develops in most mouse models of AD, in spite of extensive axonal pathology. We note that axonal transport defects are seen in familial forms of frontotemporal dementia with parkinsonism linked to chromosome 17, as well as other tauopathies, which are caused by mutations in the MAPT gene, and show extensive tau pathology, similar to AD [24,99,100]. Future studies are needed to fully elucidate how tau might regulate axonal transport in vivo, and how this regulatory role might be compromised in AD.
APOE4 allele: a major risk factor for LOAD that may cause disruption of axonal transport
ApoE, the major apolipoprotein in the brain, with an essential role in cholesterol uptake and transport, is encoded by the APOE gene, which exists as a polymorphic locus represented by three alleles, ε2–ε4. Of these, the ε4 allele confers high risk for LOAD. The mechanism by which APOE4 predisposes to AD is not fully understood, but may include modulation of APP metabolism and Aβ aggregation, regulation of brain lipid metabolism and synaptic function, as well as an effect on axonal transport [23,101]. In support of the latter, it was found that transgenic expression of human APOE4 causes significant tau hyperphosphorylation, axonal pathology and disruption of axonal transport, in brain and spinal cord neurons [102,103]. The disruption of axonal transport may be caused by a direct effect on the cytoskeleton (possibly by the phosphorylated tau), or on the interaction of kinesin-1 with the cargo. Indeed, the APOE4 receptor, APOER2, is known to bind to JIP-1 [104], and this interaction may compromise the role of JIP-1 as anchor of kinesin-1 to the cargo vesicle (Figure 2).
Sortilin-related receptor, SORLA, a possible risk factor for LOAD, regulates sorting & intracellular trafficking pathways
Systematic meta-analyses of AD genetic association studies have identified additional loci that confer AD risk, but the risk is modest and inconsistent among different studies [6,7]. Of these, the sortilin-related receptor, SORLA, with a role in sorting and intracellular trafficking pathways [105], was shown to interact with APOE and APP, and to affect Aβ generation [106–108], and thus, it could be, via these proteins, indirectly involved in the regulation of axonal transport. However, the association of SORL1 (the gene encoding SORLA) with LOAD is still debated [7].
The chicken or the egg dilemma of AD: which comes first, axonal transport deficiency or neuronal pathology?
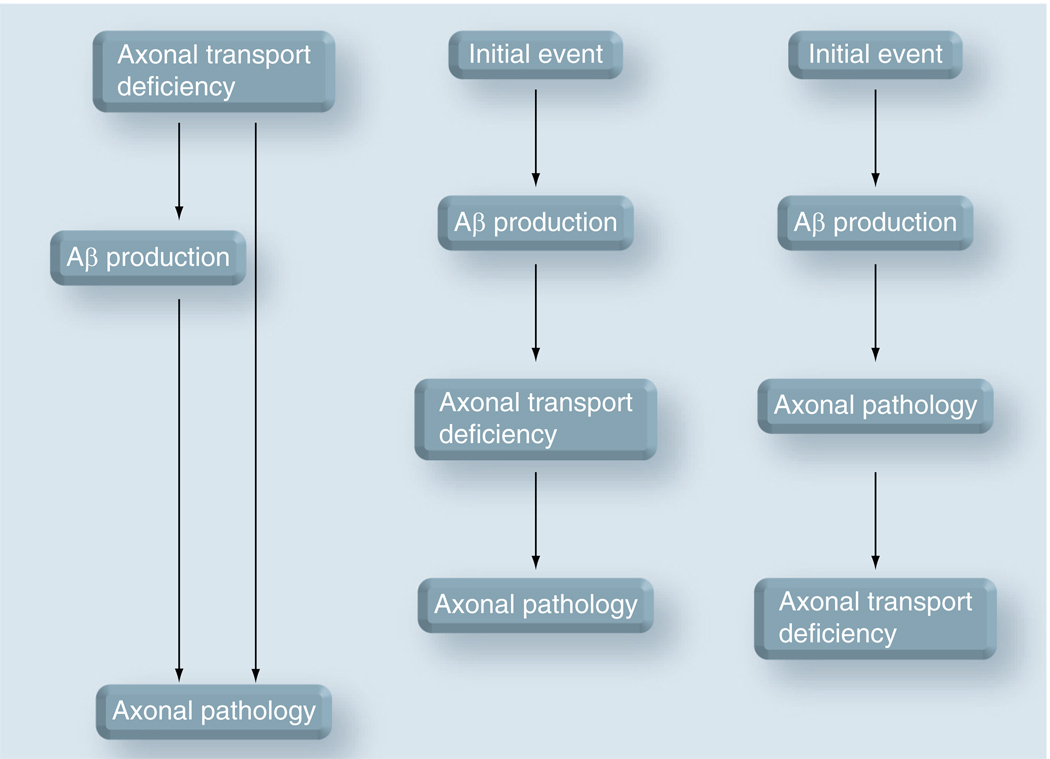
There is no doubt that an abnormal axonal transport is part of the pathogenic process in AD, as well as the evolution of the disease. However, it is still not clear whether a deficient axonal transport constitutes the very initial event that triggers the neuronal pathology in AD (Figure 5). As previously described, there is considerable evidence suggesting that the EOAD-linked genes, APP and PS1, encode proteins with a role in the regulation of axonal transport. There is also some evidence that the mutations or allelic variations in the genes that predispose for LOAD may adversely affect axonal transport. However, there is no definitive evidence demonstrating that the disruption of axonal transport through these AD-linked proteins is the cause of the axonal pathology and synaptic failure that are characteristic for the disease. The proteins that confer risk to AD, although having an effect on axonal transport, could also have other effects on processes that are not related to axonal transport. For example, they could target the activity of mitochondria, the generation of toxic reactive oxygen species or the composition of intracellular membranes. In this case, the abnormalities of axonal transport could be only a secondary factor, which does not itself trigger, but contributes to the disease process. Finally, it is possible that deficiencies in axonal transport, caused by factors that normally appear in old age, initiate the cascade of events that lead to neuronal pathology. For example, environmental stress (e.g., oxidative and osmotic) is known to perturb axonal transport via specific signaling [109]. This cascade may involve proteins that are linked to AD, such as APP and the presenilins.
Figure 5. Relationship between abnormal axonal transport and axonal pathology in Alzheimer’s disease.
(A) Deficiencies in axonal transport (caused by factors that normally appear at old age or by mutated proteins with a role in axonal transport) inflict axonal pathology by depriving the axon of essential factors, and/or by causing an abnormal/increased production of toxic amyloid-β (Aβ). In this case, the axonal transport deficiency is the cause of the axonal pathology. (B) An initial event, not related to axonal transport, induces abnormal/increased production of toxic Aβ, which blocks axonal transport and leads to axonal pathology. Although here, the axonal transport is not itself the initial cause, it is an essential part of the mechanism that leads to axonal pathology. (C) Axonal pathology is caused by a toxic effect of Aβ on the synaptic function, independently of axonal transport (see [31] for example), triggering neuronal death by a dying back mechanism [118], when axonal transport is also perturbed. In this case, the deficiencies in axonal transport are a result of the neuronal pathology of Alzheimer’s disease. The mechanisms described above are focused on Aβ. Other scenarios, where the abnormalities in axonal transport are a cause, a facilitating factor or a result of the neuronal pathology can be envisioned.
Aβ: Amyloid-β.
Since axonal transport both depends on, and affects many intracellular processes, it will be difficult to clearly establish the sequence of events that lead to its perturbation. According to a model of initiation of the neuronal pathology proposed by Kamal et al. [26], the abnormal axonal transport is both causative and consequential of the pathology (Figure 3A). In this case, the slowed down transport of the APP-carrying vesicle would lead to the premature cleavage of APP and the generation of Aβ, which in turn will further block axonal transport (due to the concomitant release of the kinesin motor). Vice versa, a premature cleavage of APP and generation of Aβ would lead to slowed down or blocked transport, which in turn, will facilitate further cleavage of APP. Although more recent studies no longer support this mechanism, the alternative models that were recently advanced also envision an abnormal axonal transport that is caused by the release of the kinesin motor from the cargo vesicle (Figure 3B). Whether this is triggered by a signaling downstream of intracellular Aβ, or from mutant presenilin, or by yet another mechanism, is a question of mechanistic details that needs to be solved by future studies.
Future perspective
The dilemma of whether an abnormal axonal transport is a cause or a result of the AD neuronal pathology will not be easily solved. This will require very sensitive assays to detect subtle defects in axonal transport and APP processing. In addition, these parameters should be accurately measured in vivo, at least in animal models of AD. Methods to image and noninvasively quantify axonal transport and axonal degeneration are already being developed [20,110–113]. For now, it appears that the deficiencies in axonal transport represent important components of AD, as they very likely appear relevant for a variety of other neurodegenerative diseases [24,114]. It is certain that, owing to the importance of axonal transport for the physiology of the neuron, any dysfunctionality in axonal transport will lead to some form of disease.
From a clinical point of view, it will become necessary to identify ways to enhance axonal transport for preventive and therapeutical reasons. Implicitly, this requires the identification of the molecular species that trigger the abnormal transport, and of the proteins involved in axonal transport, whose functions are affected by old age or by the disease process. The interventions should also aim to stabilize the axonal microtubule network. This could be carried out by increasing expression of microtubule-associated proteins, capable to reduce the microtubule dynamics without interfering with kinesin motility, or by promoting post-translational modifications of tubulin that increase microtubule stability, such as acetylation and detyrosination (see [115,116] for examples). Since these modifications are the result of enzymatic reactions, the responsible enzymes could be targeted with specific drugs, pending the solving problems of delivery and interference with other intracellular processes. Eventually, correcting deficiencies of axonal transport associated with AD will also allow for the clear establishment of whether these deficiencies are a cause or consequence of the neuronal pathology. If the neuronal pathology is corrected by the intervention, then the abnormal axonal transport is most probably the cause or a facilitating factor of the pathology, but not a consequence of it.
Executive summary
Is abnormal axonal transport a common mechanism of disease in early-onset & late-onset Alzheimer’s disease?
Several lines of evidence suggest an affirmative answer to this question, and implicate proteins relevant to the disease process in the regulation of axonal transport.
The axonopathy observed in post-mortem Alzheimer’s disease (AD) brains and in mouse models of AD suggests impeded transport. Quantitatively, decreased rates of transport are detected in mouse models of AD.
Increased expression of Amyloid β precursor protein (APP) in transgenic mice causes abnormal transport of select proteins.
Amyloid-β, applied either intracellularly or extracellularly, disrupts axonal transport.
Mice expressing early-onset AD-specific presenilin 1 variant show impaired axonal transport.
Presenilin 1, a key enzyme in APP processing, can regulate axonal transport via mechanisms that target the binding of kinesin-1 to the cargo vesicles.
Tau can regulate kinesin-driven transport, but the mechanisms could involve more than regulating the stability of microtubules and the accessibility of kinesin-1 to microtubules.
Alteration of axonal transport in a KLC1-null mouse leads to tau hyperphosphorylation.
Aberrant activation of Cdk5 in a transgenic mouse featuring AD-related amyloid and tau pathology leads to axonal pathology consistent with transport deficiencies.
Mice expressing APOE4, which in humans predisposes for late-onset AD, display pathology consistent with disruption of axonal transport.
Conclusion
Overall, these observations are consistent with the notion that axonal transport slows down in old age, and suggest that an abnormal axonal transport could contribute to AD.
Nevertheless, the AD-related conditions could cause axonal pathology via mechanisms not involving axonal transport, but at the same time, could indirectly affect axonal transport.
Thus, the alterations in axonal transport could be both the cause and the consequence of AD-specific neuronal pathology.
Future perspective
Careful studies investigating the appearance and development of neuronal pathology in correlation with live imaging of axonal transport in animal models of AD are required. Since no animal models fully reproduce the pathology of the human disease, noninvasive methods to monitor the axonal transport in human brain neurons in situ are required.
Identifying the means to correct transport deficiencies by enhancing axonal transport will soon become a priority for AD therapeutics.
Footnotes
Financial & competing interests disclosure
Supported by NIH Grant GM068596 and funds from University of Medicine and Dentistry of New Jersey. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Contributor Information
Virgil Muresan, University of Medicine & Dentistry of New Jersey, New Jersey Medical School, Department of Pharmacology & Physiology, 185 South Orange Avenue, MSB, I-683 Newark, NJ 07103, USA, Tel.: +1 973 972 2392, Fax: +1 973 972 7950, muresazo@umdnj.edu.
Zoia Muresan, University of Medicine & Dentistry of New Jersey, New Jersey Medical School, Department of Pharmacology & Physiology, 185 South Orange Avenue, MSB, I-665. Newark, NJ 07103, USA, Tel.: +1 973 972 4385, Fax: +1 973 972 7950, muresavi@umdnj.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Sisodia SS. Alzheimer’s disease: perspectives for the new millennium. J. Clin. Invest. 1999;104:1169–1170. doi: 10.1172/JCI8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 5.Hedera P, Turner RS. Inherited dementias. Neurol. Clin. 2002;20:779–808. doi: 10.1016/s0733-8619(01)00020-2. vii. [DOI] [PubMed] [Google Scholar]
- 6.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 7.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 8.Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 9.Sisodia SS, St George-Hyslop PH. γ-Secretase, Notch, Aβ and Alzheimer's disease: where do the presenilins fit in? Nat. Rev. Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 10.Tanzi RE. A genetic dichotomy model for the inheritance of Alzheimer’s disease and common age-related disorders. J. Clin. Invest. 1999;104:1175–1179. doi: 10.1172/JCI8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 12.Farrer LA, Cupples LA, Haines JL, et al. APOE and Alzheimer Disease Meta Analysis Consortium. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 13.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 14.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 16.Frolkis VV, Tanin SA, Gorban YN. Age-related changes in axonal transport. Exp. Gerontol. 1997;32:441–450. doi: 10.1016/s0531-5565(96)00168-4. [DOI] [PubMed] [Google Scholar]
- 17.Minoshima S, Cross D. In vivo imaging of axonal transport using MRI: aging and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging. 2008;35 Suppl. 1:S89–S92. doi: 10.1007/s00259-007-0707-8. [DOI] [PubMed] [Google Scholar]
- 18.Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of Alzheimer’s disease. Neuroimage. 2007;35:1401–1408. doi: 10.1016/j.neuroimage.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muresan V. One axon, many kinesins: what’s the logic? J. Neurocytol. 2000;29:799–818. doi: 10.1023/a:1010943424272. [DOI] [PubMed] [Google Scholar]
- 20. Adalbert R, Nogradi A, Babetto E, et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009;132:402–416. doi: 10.1093/brain/awn312. ▪ Example of a careful and rigorous analysis of axonal pathology, using a combination of in situ imaging, electron microscopy and immunohistochemistry techniques.
- 21.Boutajangout A, Authelet M, Blanchard V, et al. Characterisation of cytoskeletal abnormalities in mice transgenic for wild-type human tau and familial Alzheimer’s disease mutants of APP and presenilin-1. Neurobiol. Dis. 2004;15:47–60. doi: 10.1016/j.nbd.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22. Stokin GB, Lillo C, Falzone TL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. ▪▪ Demonstrates that axonal defects consistent with impaired axonal transport are detected in the early stages of Alzheimer’s disease in humans, and in mouse models of Alzheimer’s disease, where they precede known disease-related pathology. The study also shows that the experimental impairment of axonal transport in transgenic mice causes axonal defects, increased amyloid-β (Aβ) levels and amyloid deposition, proving that axonal transport deficiencies can trigger the development of lesions typical for Alzheimer’s disease.
- 23.Adalbert R, Gilley J, Coleman MP. Aβ, tau and ApoE4 in Alzheimer's disease: the axonal connection. Trends Mol. Med. 2007;13:135–142. doi: 10.1016/j.molmed.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, Zhang B, Lee VM, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. (Berl.) 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- 25.Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu. Rev. Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- 26.Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LSB. Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP. Nature. 2001;414:643–648. doi: 10.1038/414643a. [DOI] [PubMed] [Google Scholar]
- 27.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo ES, Shin RW, Billingsley ML, et al. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Kao SC, Lemere CA, et al. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlson E, Jeong GB, Ross JL, et al. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J. Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno H, Yu E, Pigino G, et al. Synaptic transmission block by presynaptic injection of oligomeric amyloid β. Proc. Natl Acad. Sci. USA. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 33.Kamal A, Stokin GB, Yang Z, Xia C, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein L, Almenar A, Kamal A, Stokin G. Letter to the Editor. J. Neurosci. 2005;25 [Google Scholar]
- 35.Lazarov O, Morfini GA, Lee EB, et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J. Neurosci. 2005;25:2386–2395. doi: 10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 37.Selkoe DJ. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer's disease. Annu. Rev. Cell Biol. 1994;10:373–403. doi: 10.1146/annurev.cb.10.110194.002105. [DOI] [PubMed] [Google Scholar]
- 38.Sisodia SS. β-amyloid precursor protein cleavage by a membrane-bound protease. Proc. Natl Acad. Sci. USA. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisodia SS, Annaert W, Kim SH, De Strooper B. γ-secretase: never more enigmatic. Trends Neurosci. 2001;24:S2–S6. doi: 10.1016/s0166-2236(00)01987-1. [DOI] [PubMed] [Google Scholar]
- 40.Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 41.Goldsbury C, Mocanu MM, Thies E, et al. Inhibition of APP trafficking by tau protein does not increase the generation of amyloid-β peptides. Traffic. 2006;7:873–888. doi: 10.1111/j.1600-0854.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 42.Sisodia SS. Biomedicine. A cargo receptor mystery APParently solved? Science. 2002;295:805–807. doi: 10.1126/science.1069661. [DOI] [PubMed] [Google Scholar]
- 43.Amaratunga A, Leeman SE, Kosik KS, Fine RE. Inhibition of kinesin synthesis in vivo inhibits the rapid transport of representative proteins for three transport vesicle classes into the axon. J. Neurochem. 1995;64:2374–2376. doi: 10.1046/j.1471-4159.1995.64052374.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J. Cell Biol. 1992;117:595–606. doi: 10.1083/jcb.117.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira A, Caceres A, Kosik KS. Intraneuronal compartments of the amyloid precursor protein. J. Neurosci. 1993;13:3112–3123. doi: 10.1523/JNEUROSCI.13-07-03112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki T, Selkoe DJ, Koo EH. Trafficking of cell surface β-amyloid precursor protein: retrograde and transcytotic transport in cultured neurons. J. Cell Biol. 1995;129:431–442. doi: 10.1083/jcb.129.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inomata H, Nakamura Y, Hayakawa A, et al. A scaffold protein JIP-1β enhances amyloid precursor protein phosphorylation by JNK and its association with kinesin light chain 1. J. Biol. Chem. 2003;278:22946–22955. doi: 10.1074/jbc.M212160200. ▪ Demonstrates that JNK-interacting protein-1 enhances the interaction of amyloid precursor protein (APP) with the light chains of kinesin-1, thus providing the proof-of-principle that JIP-1 could mediate the interaction of APP with kinesin-1.
- 49. Matsuda S, Matsuda Y, D’Adamio L. Amyloid-β protein precursor (AβPP), but not AβPP-like protein 2, is bridged to the kinesin light chain by the scaffold protein JNK-interacting protein 1. J. Biol. Chem. 2003;278:38601–38606. doi: 10.1074/jbc.M304379200. ▪ Demonstrates that the interaction of APP with the light chains of kinesin-1 is not direct, but is mediated by JIP-1.
- 50. Muresan Z, Muresan V. Coordinated transport of phosphorylated amyloid-β precursor protein and c-Jun NH2-terminal kinase-interacting protein-1. J. Cell Biol. 2005;171:615–625. doi: 10.1083/jcb.200502043. ▪ Demonstrates that transport of APP into neurites occurs via several pathways, which use distinct vesicle populations that recruit the kinesin-1 motor by different mechanisms. The study also shows that, under normal conditions, only the phosphorylated APP recruits kinesin-1 via JIP-1, to be transported to neurite terminals.
- 51.Matsuda S, Yasukawa T, Homma Y, et al. c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer’s amyloid precursor protein with JNK. J. Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheinfeld MH, Roncarati R, Vito P, Lopez PA, Abdallah M, D’Adamio L. Jun NH2-terminal kinase (JNK) interacting protein 1 (JIP1) binds the cytoplasmic domain of the Alzheimer’s β-amyloid precursor protein (APP) J. Biol. Chem. 2002;277:3767–3775. doi: 10.1074/jbc.M108357200. [DOI] [PubMed] [Google Scholar]
- 53.Taru H, Iijima K, Hase M, Kirino Y, Yagi Y, Suzuki T. Interaction of Alzheimer’s β-amyloid precursor family proteins with scaffold proteins of the JNK signaling cascade. J. Biol. Chem. 2002;277:20070–20078. doi: 10.1074/jbc.M108372200. [DOI] [PubMed] [Google Scholar]
- 54.Verhey KJ, Meyer D, Deehan R, et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitmarsh AJ, Kuan CY, Kennedy NJ, et al. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 2001;15:2421–2432. doi: 10.1101/gad.922801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muresan Z, Muresan V. c-Jun NH2-terminal kinase-interacting protein-3 facilitates phosphorylation and controls localization of amyloid-β precursor protein. J. Neurosci. 2005;25:3741–3751. doi: 10.1523/JNEUROSCI.0152-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki T, Oishi M, Marshak DR, Czernik AJ, Nairn AC, Greengard P. Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J. 1994;13:1114–1122. doi: 10.1002/j.1460-2075.1994.tb06360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borg JP, Ooi J, Levy E, Margolis B. The phosphotyrosine interaction domains of X11 and FE65 bind to distinct sites on the YENPTY motif of amyloid precursor protein. Mol. Cell Biol. 1996;16:6229–6241. doi: 10.1128/mcb.16.11.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radzimanowski J, Simon B, Sattler M, Beyreuther K, Sinning I, Wild K. Structure of the intracellular domain of the amyloid precursor protein in complex with Fe65-PTB2. EMBO Rep. 2008;9:1134–1140. doi: 10.1038/embor.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor- containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 61. Muresan V, Varvel NH, Lamb BT, Muresan Z. The cleavage products of amyloid-β precursor protein are sorted to distinct carrier vesicles that are independently transported within neurites. J. Neurosci. 2009;29:3565–3578. doi: 10.1523/JNEUROSCI.2558-08.2009. ▪ Demonstrates that axonal transport of APP mostly occurs after its cleavage into functional polypeptides, and that these polypeptides are sorted into separate cargo vesicles that are independently transported by kinesin-1 on distinct microtubule tracks to different destination within the neurites.
- 62.Muresan Z, Muresan V. A phosphorylated, carboxy-terminal fragment of β-amyloid precursor protein localizes to the splicing factor compartment. Hum. Mol. Genet. 2004;13:475–488. doi: 10.1093/hmg/ddh054. [DOI] [PubMed] [Google Scholar]
- 63. Salehi A, Delcroix JD, Belichenko PV, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. ▪ Demonstrates that increased expression of APP in transgenic mice leads to decreased axonal transport of select proteins, via a mechanism that likely does not involve Aβ.
- 64.Stokin GB, Almenar-Queralt A, Gunawardena S, et al. Amyloid precursor protein-induced axonopathies are independent of amyloid-β peptides. Hum. Mol. Genet. 2008;17:3474–3486. doi: 10.1093/hmg/ddn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 66.Kasa P, Papp H, Kovacs I, Forgon M, Penke B, Yamaguchi H. Human amyloid-β1–42 applied in vivo inhibits the fast axonal transport of proteins in the sciatic nerve of rat. Neurosci. Lett. 2000;278:117–119. doi: 10.1016/s0304-3940(99)00863-0. [DOI] [PubMed] [Google Scholar]
- 67.Hiruma H, Katakura T, Takahashi S, Ichikawa T, Kawakami T. Glutamate and amyloid β-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J. Neurosci. 2003;23:8967–8977. doi: 10.1523/JNEUROSCI.23-26-08967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by β-amyloid peptides in hippocampal neurons. J. Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. ▪ Demonstrates that exposure of hippocampal neurons to Aβ causes acute inhibition of mitochondrial transport, via a signaling pathway involving protein kinase A and glycogen synthase kinase 3β.
- 69. Pigino G, Morfini G, Atagi Y, et al. Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid-β. Proc. Natl Acad. Sci. USA. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. ▪ Demonstrates that perfusion of squid axoplasm with Aβ oligomers causes inhibition of bidirectional axonal transport. This inhibition occurs through activation of casein kinase 2, which leads to the phosphorylation of the light chains of kinesin-1 and release of the motor from its cargoes.
- 70.Muresan V, Muresan Z. No conventional function for the conventional kinesin? Traffic. 2008;9:1823–1827. doi: 10.1111/j.1600-0854.2008.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takahashi RH, Almeida CG, Kearney PF, et al. Oligomerization of Alzheimer’s β-amyloid within processes and synapses of cultured neurons and brain. J. Neurosci. 2004;24:3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muresan Z, Muresan V. Neuritic deposits of amyloid-β peptide in a subpopulation of central nervous system-derived neuronal cells. Mol. Cell Biol. 2006;26:4982–4997. doi: 10.1128/MCB.00371-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muresan Z, Muresan V. Seeding neuritic plaques from the distance: a possible role for brainstem neurons in the development of Alzheimer’s disease pathology. Neurodegenerative Dis. 2008;5:250–253. doi: 10.1159/000113716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai D, Leem JY, Greenfield JP, et al. Presenilin-1 regulates intracellular trafficking and cell surface delivery of β-amyloid precursor protein. J. Biol. Chem. 2003;278:3446–3454. doi: 10.1074/jbc.M209065200. [DOI] [PubMed] [Google Scholar]
- 75.Naruse S, Thinakaran G, Luo JJ, et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron. 1998;21:1213–1221. doi: 10.1016/s0896-6273(00)80637-6. [DOI] [PubMed] [Google Scholar]
- 76.Wang R, Tang P, Wang P, Boissy RE, Zheng H. Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer’s disease mutation. Proc. Natl Acad. Sci. USA. 2006;103:353–358. doi: 10.1073/pnas.0509822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lazarov O, Morfini GA, Pigino G, et al. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s disease-linked mutant presenilin 1. J. Neurosci. 2007;27:7011–7020. doi: 10.1523/JNEUROSCI.4272-06.2007. ▪ Demonstrates that expression of presenilin 1 variants linked to familial forms of Alzheimer’s disease in transgenic mice leads to impaired axonal transport of APP and Trk receptors, increased phosphorylation of cytoskeletal proteins, and motor neuron functional deficits.
- 78. Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. ▪ Identifies a mechanism whereby PS1 variants linked to familial forms of Alzheimer’s disease lead to impairment of axonal transport; the mutant PS1 triggers activation of GSK3β, which phosphorylates the light chains of kinesin-1 and releases the motor from its cargoes.
- 79.Drubin DG, Kirschner MW. Tau protein function in living cells. J. Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002;21:4896–4905. doi: 10.1093/emboj/cdf503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vershinin M, Xu J, Razafsky DS, King SJ, Gross SP. Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic. 2008;9:882–892. doi: 10.1111/j.1600-0854.2008.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khatoon S, Grundke-Iqbal I, Iqbal K. Brain levels of microtubule-associated protein tau are elevated in Alzheimer’s disease: a radioimmuno-slot-blot assay for nanograms of the protein. J. Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 85.Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3β influences tau binding to microtubules and microtubule organisation. J. Cell Sci. 1996;109(Pt 6):1537–1543. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 86.Lovestone S, Reynolds CH, Latimer D, et al. Alzheimer’s disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr. Biol. 1994;4:1077–1086. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 87.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 88.Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer’s disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- 89.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 90.Velasco ME, Smith MA, Siedlak SL, Nunomura A, Perry G. Striation is the characteristic neuritic abnormality in Alzheimer disease. Brain Res. 1998;813:329–333. doi: 10.1016/s0006-8993(98)01034-8. [DOI] [PubMed] [Google Scholar]
- 91.Chee FC, Mudher A, Cuttle MF, et al. Over-expression of tau results in defective synaptic transmission in Drosophila neuromuscular junctions. Neurobiol Dis. 2005;20:918–928. doi: 10.1016/j.nbd.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 92.Mudher A, Shepherd D, Newman TA, et al. GSK-3β inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry. 2004;9:522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- 93.Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J. Neurosci. Res. 2007;85:2620–2630. doi: 10.1002/jnr.21154. [DOI] [PubMed] [Google Scholar]
- 94.Yuan A, Kumar A, Peterhoff C, Duff K, Nixon RA. Axonal transport rates in vivo are unaffected by tau deletion or overexpression in mice. J. Neurosci. 2008;28:1682–1687. doi: 10.1523/JNEUROSCI.5242-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King ME, Kan HM, Baas PW, Erisir A, Glabe CG, Bloom GS. Tau-dependent microtubule disassembly initiated by prefibrillar β-amyloid. J. Cell Biol. 2006;175:541–546. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cruz JC, Kim D, Moy LY, et al. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid-β in vivo. J. Neurosci. 2006;26:10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 98.Falzone TL, Stokin GB, Lillo C, et al. Axonal stress kinase activation and tau misbehavior induced by kinesin-1 transport defects. J. Neurosci. 2009;29:5758–5767. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 100.Higuchi M, Lee VM, Trojanowski JQ. Tau and axonopathy in neurodegenerative disorders. Neuromolecular Med. 2002;2:131–150. doi: 10.1385/NMM:2:2:131. [DOI] [PubMed] [Google Scholar]
- 101.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tesseur I, Van Dorpe J, Bruynseels K, et al. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am. J. Pathol. 2000;157:1495–1510. doi: 10.1016/S0002-9440(10)64788-8. ▪ Demonstrates that neuronal expression in human APOE4 transgenic mice, a risk factor in Alzheimer’s disease, leads to a complex pathology consistent with impaired axonal transport.
- 103.Tesseur I, Van Dorpe J, Spittaels K, Van den Haute C, Moechars D, Van Leuven F. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 2000;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gotthardt M, Trommsdorff M, Nevitt MF, et al. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 105.Yamazaki H, Bujo H, Kusunoki J, et al. Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J. Biol. Chem. 1996;271:24761–24768. doi: 10.1074/jbc.271.40.24761. [DOI] [PubMed] [Google Scholar]
- 106.Andersen OM, Reiche J, Schmidt V, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andersen OM, Schmidt V, Spoelgen R, et al. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry. 2006;45:2618–2628. doi: 10.1021/bi052120v. [DOI] [PubMed] [Google Scholar]
- 108.Taira K, Bujo H, Hirayama S, et al. LR11, a mosaic LDL receptor family member, mediates the uptake of ApoE-rich lipoproteins in vitro. Arterioscler. Thromb. Vasc. Biol. 2001;21:1501–1506. doi: 10.1161/hq0901.094500. [DOI] [PubMed] [Google Scholar]
- 109.Muresan Z, Muresan V. The amyloid-β precursor protein is phosphorylated via distinct pathways during differentiation, mitosis, stress, and degeneration. Mol. Biol. Cell. 2007;18:3835–3844. doi: 10.1091/mbc.E06-07-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 111.Misgeld T, Kerschensteiner M. In vivo imaging of the diseased nervous system. Nat. Rev. Neurosci. 2006;7:449–463. doi: 10.1038/nrn1905. [DOI] [PubMed] [Google Scholar]
- 112.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat. Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- 113.Misgeld T, Nikic I, Kerschensteiner M. In vivo imaging of single axons in the mouse spinal cord. Nat. Protoc. 2007;2:263–268. doi: 10.1038/nprot.2007.24. [DOI] [PubMed] [Google Scholar]
- 114.Duncan JE, Goldstein LS. The genetics of axonal transport and axonal transport disorders. PLoS Genet. 2006;2:e124. doi: 10.1371/journal.pgen.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dompierre JP, Godin JD, Charrin BC, et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J. Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peris L, Wagenbach M, Lafanechere L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell. Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J. Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]