Abstract
Introduction
Acute promyelocytic leukemia (APL) is a malignant disorder of the white blood cells. Arsenic trioxide (As2O3) has been used as a therapeutic agent to treat APL and other tumors. Studies suggest that ascorbic acid (AA) supplementation may improve the clinical outcome of As2O3 for APL patients. Our aim was to use human leukemia (HL-60) APL-cells as an in vitro test model to evaluate the effect of physiologic doses of AA on As2O3-induced toxicity and apoptosis of HL-60 cells.
Methods
HL-60 cells were treated either with a pharmacologic dose of As2O3 alone and with several physiologic doses of AA. Cell survival was determined by trypan blue exclusion test. The extent of oxidative cell/tissue damage was determined by measuring lipid hydroperoxide concentration by spectrophotometry. Cell apoptosis was measured by flow cytometry using Annexin-V and propidium iodide (PI) staining.
Results
AA treatment potentiates the cytotoxicity of As2O3 in HL-60 cells. Viability decreased from (58 ± 3)% in cells with As2O3 alone to (47 ± 2)% in cells treated with 100 µM AA and 6 µg/mL As2O3 with P < 0.05. There was a significant (P < 0.05) increase in lipid hydroperoxide concentrations in HL-60 cells co-treated with AA compared to As2O3 alone. Flow cytometry assessment (Annexin V FITC/PI) suggested that AA co-treatment induces more apoptosis of HL-60 cells than did As2O3 alone, but this was not statistically significant. Taken together, our experiment indicates that As2O3 induced in vitro cell death and apoptosis of HL-60 cells. Administration of physiologic doses of AA enhanced As2O3-induced cytotoxicity, oxidative cell/tissue damage, and apoptosis of HL-60 cells through externalization of phosphatidylserine.
Conclusions
These suggest that AA may enhance the cytotoxicity of As2O3, suggesting a possible future role of AA/As2O3 combination therapy in patients with APL.
Keywords: Arsenic Trioxide, Ascorbic Acid, Acute Promyelocytic Leukemia, Cell Viability, Apoptosis
I. Introduction
Acute promyelocytic leukemia (APL) is a malignant disorder of the white blood cells which can affect patients of all ages. Arsenic trioxide (As2O3) is been used as a therapeutic agent to treat APL [1] and other tumors [2]. In 2000, the U.S. Food and Drug Administration (FDA) approved the use of As2O3 (Trisenox) to treat relapsed APL [3]. In vitro studies have shown that As2O3 exerts a dual dose-dependent effect on APL cells by inducing partial differentiation at low concentrations and apoptosis at high concentrations [4,5]. Recently, we reported that the pharmacological effect of As2O3 as an effective anti-cancer drug is associated with its cytotoxic and genotoxic effects in human leukemia (HL-60) cells [6,7]. Current research in our laboratory indicates that As2O3 induces transcription of specific genes that affect mitogen response, cell cycle progression, programmed cell death, and cellular function in many ways in cultured human leukemia (HL-60) cells. Among these cellular responses to As2O3 in human leukemia (HL-60) cells are up-regulation of p53 tumor suppressor protein and repression of the c-fos transcription factor involved in cell cycle arrest or apoptosis, activation of cyclin D1 and cyclin A involved in cell cycle progression [8]. Other studies indicate that As2O3 induces the generation of reactive oxygen species that contribute significantly to cell killing [2,9,10] promotion of differentiation, and inhibition of growth [6].
As2O3 has also been used effectively in combination with other chemotherapeutic agents such as all-trans retinoic acid to treat APL [11]. Ascorbic acid (AA) is a natural supplement in our diet that has been studied for the prevention of human cancer and improvement of human health [12]. For many years, some scientists have claimed that use of high doses of ascorbic acid (>10 g/day) cure infections with common cold and treat AA can be used to treat cancers diseases because of the effect on the immune system [13]. Others researchers have reported that AA is effective in the prevention of cancer and protection against DNA damage through the neutralization of free radicals [14,15]. They also reported that AA may act as a pro-oxidant that helps the body's own free radical defense mechanism destroy tumors in their early stages [15,16]. Several recent studies have provided evidence that AA may extend the therapeutic spectrum of As2O3 in APL patients [17] and multiple myeloma patients [18]. However, little is known about the mechanisms of action of AA when combined with As2O3 for the treatment of APL. Therefore, the aim of this research was to use human leukemia (HL-60) APL-cells as an in vitro test model to determine the potential mechanism of action of AA on As2O3 chemotherapy of APL.
II. Materials and Methods
Chemicals and Test Media
Arsenic trioxide (As2O3), CASRN 1327-53-3, MW 197.84, with an active ingredient of 100% (w/v) arsenic in 10% nitric acid was purchased from Fisher Scientific in (Houston Texas). Growth medium RPMI 1640 containing 1 mmol/L L-glutamine was purchased from Gibco BRL products (Grand Island, NY). Fetal bovine serum (FBS), ascorbic acid, phosphate buffered saline (PBS), and MTT assay kit were obtained from Sigma Chemical Company (St. Louis, MO). Lipid peroxidation kit was purchased from Calbiochem-Novabiochem (San Diego, CA).
Tissue Culture
Human leukemia (HL-60) APL-cells, purchased from the American Type Culture Collection -ATCC (Manassas, VA), was thawed by gentle agitation of their containers (vials) for 2 minutes in a water bath at 37°C. After thawing, the content of each vial of cell was transferred to a 25 cm2 tissue culture flask, diluted with up to 10mL of RPMI 1640 containing 1 mmol/L L-glutamine (GIBCO/BRL, Gaithersburg, MD) and supplemented with 10% (v/v) fetal bovine serum (FBS), and 1% (w/v) penicillin/streptomycin. The 25 cm2 culture flasks, each containing 2 × 106 viable cells, were observed under the microscope, followed by incubation in a humidified 5% CO2 incubator at 37°C. Three times a week, they were diluted under same conditions to maintain a density of 5 × 105/mL, and harvested in the exponential phase of growth. The cell viability was assessed by the trypan blue exclusion test (Life Technologies) and manually counted using a hemocytometer.
Treatment and Measurement of Cell Viability
In a recently published experiment, we reported that physiologic doses of As2O3 increased cellular proliferation while pharmacologic doses of As2O3 were highly cytotoxic to HL-60 cells, showing a 24 hours LD50 of 6.4 ± 0.6 µg/mL [6]. Hence, to examine the effect of ascorbic acid (AA) on As2O3-induced cytotoxicity, cells exposed to physiologic doses of AA (25, 50, and 100 µM) 30 minutes prior were treated with 6 µg/mL As2O3 and incubated in humidified 5% CO2 incubator at 37°C for 24 hours. After the treatment period, the cell viability of human leukemia (HL-60) cells was determined by standard live-dead staining. To this end, ten µl of a 0.5% solution of the dye (trypan blue) was added to 100 µL of treated cells (1.0 × 105/mL). The number of viable (transparent) and dead (blue) cells was examined on a light microscopic analysis.
Measurement of Lipid Hydroperoxide
Lipid peroxidation is traditionally quantified by measuring malondialdehyde and 4-hydroxynonenal. These assays are nonspecific and often lead to mis-estimation of lipid peroxidation. A new lipid hydroperoxide assay kit [Calbiochem-Novabiochem, San Diego, CA] was used in this study to measure the hydroperoxide concentration by directly using the redox reactions with ferrous ions. The extraction procedure and measurement of the extracted lipid hydroperoxides was performed according to the manufacturer's instructions (Calbiochem-Novabiochem, San Diego, CA) [19,20].
Briefly, untreated and treated HL-60 cells were washed twice with cold PBS and counted for the assay. A total volume of 20 × 106 cells/mL in cold PBS were mixed with 3 mL of chloroform/methanol (2:1) solution, vortexed and mixed well. The sample was centrifuged at 1000 × g for 5 minutes at 0°C until phase separation was achieved. The pasteur pipet was used carefully to collect 700 µL of chloroform layer in the bottom of the test tube and transferred to another test tube. Freshly prepared chromogen (50 µL) was added to each test tube, vortexed and mixed well. The sample was incubated at room temperature for 5 minutes. The absorbance of the sample was monitored at 500 nm, and the concentration of lipid hydroperoxide was determined from a standard curve.
Annexin V FITC/PI Binding Assay by Flow Cytometry
The response of HL-60 cells to arsenic trioxide (As2O3) alone and ascorbic acid (AA) plus As2O3 was assessed by flow cytometry using Annexin V FITC/PI staining kit. After 24 hours of exposure to either a pharmacologic dose of As2O3, or different physiologic doses of AA plus a pharmacologic dose of As2O3, 1 × 106 cells/mL were washed in PBS, re-suspended in binding buffer (10 mm Hepes/NaOH pH 7.4, 140 mm NaCl, 2.5 mM CaCl2), and stained with FITC-conjugated annexin V (Pharmingen, Becton Dickinson Co., San Diego, CA, USA). Then, cells were incubated for 15 minutes in the dark at room temperature, washed with binding buffer and analysed by flow cytometry (FACS Calibar; Becton-Dickinson) using CellQuest software.
Statistical Analysis
Experiments were performed at least in triplicates. Data were represented as means ± SDs. Where appropriate, one-way anova test or Student paired t-test was performed using SAS Software available in the Bio-statistics Core Laboratory at Jackson State University. P values less than 0.05 were considered statistically significant.
III. Results
Ascorbic Acid Enhances the Cytotoxicity of Arsenic Trioxide in HL-60 Cells
In the present study, HL-60 cells were treated either with a pharmacologic dose of As2O3, or with various physiologic doses of AA plus As2O3 as described in the trypan blue exclusion test. As shown in (Figure 1), As2O3 is highly cytotoxic to HL-60 cells at 6 µg/mL of exposure. Co-treatment of these cells using physiologic concentrations (25–100 µM) of AA and a pharmacologic dose (6 µg/mL) of As2O3 resulted in a higher level of cell death than did As2O3 alone. We found that the viability of HL-60 cells declined from (58 ± 3)% in cells treated with As2O3 alone to (47 ± 2)% in cells treated with 100 µM AA and 6 µg/mL As2O3 with P < 0.05.
Figure 1.
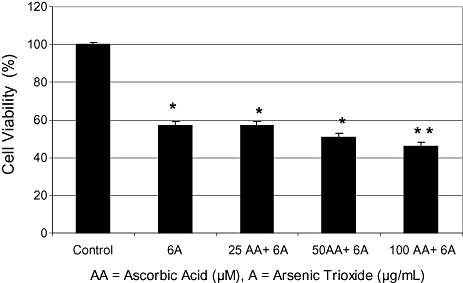
Potential effect of co-administration of ascorbic acid (AA) and arsenic trioxide (As2O3) to human leukemia (HL-60) cells. HL-60 cells were cultured in the absence or presence of AA and As2O3 or in combination of AA and As2O3 for 24 hr as indicated in the Materials and Methods. Cell viability was determined based on the trypan blue exclusion test. Each point represents a mean value and standard deviation of 3 experiments with 6 replicates per dose. *Significantly different from the control by anova Dunnett's test; P < 0.05. **Significantly different from As2O3 alone by anova Dunnett's test; P < 0.05.
Ascorbic Acid Enhances Lipid Hydroperoxide Generation in Arsenic Trioxide-treated HL-60 Cells
A high level of lipid hydroperoxide concentration was detected in HL-60 cells after 24 hours of As2O3 treatment compared to control cells (Figure 2). A concentration-dependent increase in lipid hydroperoxide generation was observed in HL-60 cells co-treated with ascorbic acid (AA) and As2O3 compared to As2O3 alone. Taken together, co-administration of AA and As2O3 in culture cells caused significant (P < 0.05) increase of lipid hydroperoxide concentration resulting from oxidation of fatty acids and/or degradation products of poly-unsaturated fatty acids. Findings from this experiment suggest that the pro-oxidant property of AA in vitro may increase reactive oxygen species (ROS) formation that potentiates the cytotoxicity of As2O3.
Figure 2.
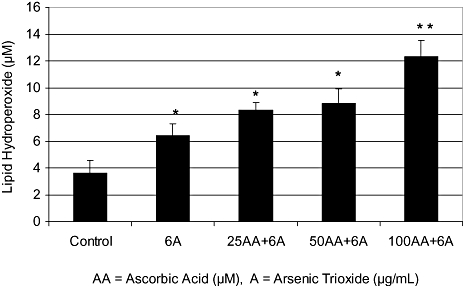
AA potentiation on As2O3-induced oxidative stress in HL-60 cells. Cells were incubated for 24 hr with 6 µg/mL As2O3 and various concentrations of AA (25, 50, and 100 µM). Lipid hydroperoxide formation was determined as described in Materials and Methods. *Significantly different from the control by anova Dunnett's test; P < 0.05. **Significantly different from As2O3 alone by anova Dunnett's test; P < 0.05. Data are representative of 3 independent experiments.
Ascorbic Acid Enhances Arsenic Trioxide-induced Apoptosis in HL-60 Cells
To determine whether physiologic doses of ascorbic acid (AA) could sensitize arsenic trioxide (As2O3)-mediated apoptosis, HL-60 cells were treated for 24 hours, subsequently stained with annexin V/PI, and analyzed by flow cytometry. As shown in (Figures 3 and 4), AA enhanced the percentage of cells positive for annexin V, but this was not statistical significant. The percentage of cells positive for annexin V was (40 ± 5)% in cells treated with As2O3 alone and (46 ± 4)% in those treated with 100 µM AA and 6 µg/mL As2O3 with P > 0.05.
Figure 3.

Representative flow cytometry analysis data from Annexin V-FITC staining. The histogram shows a comparison of the distribution of annexin V negative cells (M1) and annexin V positive cells (M2) after 24 h incubation in HL-60 cells. A: control; B: 6 µg/mL As2O3; C: 25 µM AA + 6 µg/mL As2O3; D: 50 µM AA + 6 µg/mL As2O3; E: 100 µM AA + 6 µg/mL As2O3.
Figure 4.
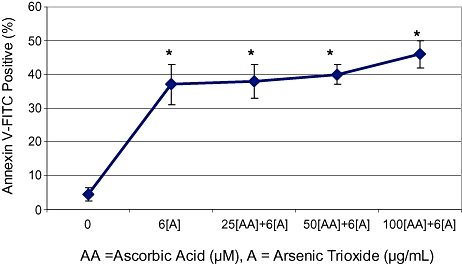
Annexin V-FITC positive cells induced by either arsenic trioxide alone or ascorbic acid and arsenic trioxide combination in HL-60 cells. Each point represents the mean value and the standard deviation of three experiments, showing similar results. *Significantly different from control (0 µg/mL), P < 0.05.
IV. Discussion
Ascorbic Acid Enhances the Cytotoxicity of Arsenic Trioxide in HL-60 Cells
In this study, we investigated the cellular effect of ascorbic acid (AA) in conjunction with arsenic trioxide (As2O3) in human leukemia (HL-60) cells. Our data showed increased cell death in the human leukemia (HL-60) cells at physiologic doses (25–100 µM) of AA and pharmacologic dose (6 µg/mL) of As2O3, indicating potentiation effect between AA and As2O3. We found that the combination of physiologic doses of AA and pharmacologic dose of As2O3 is more cytotoxic to HL-60 cells compared to As2O3 alone. We previously demonstrated that low or physiologic doses (25–100 µM) of AA were not cytotoxic, suggesting that AA has the potential to be safe and acts as effective chemosensitizing agent in As2O3-based chemotherapy [21]. Zhang and his co-workers have reported the role of physiologic doses of AA in gastric cancer cells [22]. Preclinical studies have shown the efficacy of As2O3 on various cultured human (HL-60, HepG2, Jurkat) cancer cell lines [7]. Current research has reported many possible treatments for APL patients [23,24]. However, a common treatment remains As2O3, or perhaps a combination of AA and As2O3[18]. Interestingly, finding from our present studies suggest that the combination of these two compounds could be a more proficient treatment in killing cancer cells compared to As2O3 alone. Similar to our findings, previous studies have shown that AA potentiates As2O3-mediated cytotoxicity in U266 and multiple myeloma cells [18,25]. The use of AA alone has a controversial history in cancer treatment [26]. Cameron and Pauling reported that AA or ascorbate, given in pharmacologic doses of 10 g/day, is effective in treating some cancers and improving patient well-being [12]. One the contrary, Moertel and his co-workers reported that the same dose of AA had no effect on patient well-being and survival in two double-blind placebo-controlled trials [27].
Ascorbic Acid Enhances Lipid Hydroperoxide Generation in Arsenic Trioxide-treated HL-60 Cells
To investigate the hypothesis that ascorbic acid (AA) enhances lipid hydroperoxide generation in arsenic trioxide (As2O3)-treated cells. HL-60 cells were exposed to different physiologic doses (25–100 µM) of AA and a pharmacologic dose (6 µg/mL) of As2O3 for 24 hours. Our results indicate that the treatment of HL-60 cells with As2O3 alone produces a significantly higher level of lipid hydroperoxide, a major mediator of oxidative stress and cellular injury that often leads to cell death. This significant increase in lipid hydroperoxide concentrations was further exacerbated by AA co-treatment. Based on these results, it is evident that AA acts as a pro-oxidant in As2O3-treated HL-60 cells. Our findings are in agreement with a previous report indicating that AA exhibits pro-oxidant activity in the presence of free transition metals [28]. The relatively higher sensitivity of tumor cells to the pro-oxidant action of AA may be related to its lower antioxidant defense and to the presence of transition metals [29,30]. On the contrary, AA has an antioxidant effect in the absence of metals, but becomes a pro-oxidant when they are present [31,32]. Because AA potentiated As2O3-mediated cell death, it is possible that As2O3 treatment increased reactive oxygen species (ROS) production. Consistent with this finding, published reports indicate that arsenic induces the generation of reactive oxygen species (ROS) that contribute significantly to cell killing [10,33]. Another study indicates that the cytotoxic and genotoxic effects of As2O3 are mediated through oxidative stress [7].
Ascorbic Acid Enhances Arsenic Trioxide-induced Apoptosis in HL-60 Cells
Annexin-V is a specific phosphatidylserine-binding protein used to detect apoptotic cells by providing an assessment of the progression from living cells (annexin−/PI−) towards apoptotic stage (annexin+/PI−) and postapoptotic cell death (annexin+/PI+). Our data show a progressive non-significant increase of apoptotic cells which reach the highest value in the presence of AA and As2O3 (Figures 3 and 4). This finding was not statistically significant, but a limitation of the study was the small number of experiments performed. Recent literature has indicated that low concentrations of As2O3 (2 µM) induces apoptosis in HPV 16 DNA-immortalized human cervical epithelial cells and that its molecular pathways leading to apoptosis may be associated with down-regulation of viral oncogene expression [34]. Others have reported that As2O3 selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway (10). Using the trypan blue exclusion test and the flow cytometry analysis, we have shown in the present study that As2O3 causes substantial cell death and apoptosis of HL-60 cells. Administration of physiologic doses of AA was sufficient to enhance As2O3-induced cytotoxicity, oxidative cell/tissue damage, and apoptosis of HL-60 cells. These findings highlight the potential effect of AA in promoting the pharmacologic effect of As2O3, suggesting a possible future role of AA/As2O3 combination therapy in patients with APL.
Conclusions
Ascorbic acid (AA) and arsenic trioxide (As2O3) co-treatment exerts dual effects on human leukemia (HL-60) cells by inducing oxidative stress and subsequent inhibition of cell growth and induction of apoptosis. The trypan blue exclusion test results indicated that AA and As2O3 combination significantly (P < 0.05) reduced cell viability of human leukemia (HL-60) cells stronger than did As2O3 alone. Although the mechanism by which AA enhances As2O3-mediated cytotoxicity in HL-60 cells remains unknown, here we provide evidence that AA potentiates As2O3-induced toxicity through oxidative cell/tissue damage and perhaps via apoptosis in human leukemia (HL-60) cells. Based on this knowledge, the elucidation of the synergy and mechanisms of action between AA/As2O3 may eventually lead to a more effective approach for the management of patients with APL.
Acknowledgments
This research was financially supported partly by a grant from the NIH-EARDA (Grant No. 5G11HD046519-05), and in part by a grant from the National Institutes of Health (Grant No. 2G12RR13459-11), through the RCMI-Center for Environmental Health at Jackson State University, Jackson, MS. A poster based on this paper was presented at the American Association for Cancer Research (AACR) Annual Meeting. April 18–22, 2009 held at Colorado Convention Center Denver, CO.
Conflict of Interest:
No authors have any conflict of interest relevant to this work. CG Yedjou has presented this work at the 2009 American Association for Cancer Research (AACR) Annual Meeting. L Thuisseu, C Tchounwou and M Gomes are students. They have performed the experiment and drafted the manuscript that was reviewed by all authors. C Howard has assisted in performing the statistical analysis and data interpretation. P Tchounwou has supervised the experiment and reviewed the manuscript for submission.
References
- 1.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60. [PubMed] [Google Scholar]
- 2.Huang HS, Chang WC, Chen CJ. Involvement of reactive oxygen species in arsenite-induced downregulation of phospholipid hydroperoxide glutathione peroxidase in human epidermoid carcinoma A431 cells. Free Radic Biol Med. 2002;33:864. doi: 10.1016/s0891-5849(02)00983-8. [DOI] [PubMed] [Google Scholar]
- 3.Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 4.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of arsenic trioxide in the treatment of acute promyelocytic leukemia (APL). Arsenic trioxide exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–53. [PubMed] [Google Scholar]
- 5.Yedjou CG, Moore P, Tchounwou PB. Dose and time-dependent response of human acute promyelocytic leukemia (HL-60) cells to arsenic trioxide treatment. Int J Environ Res Public Health. 2006;2:136–40. doi: 10.3390/ijerph2006030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yedjou CG, Tchounwou PB. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoreis (comet) assays. Mol Cell Biochem. 2007;301:123–30. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yedjou CG, Tchounwou PB. Oxidative stress in human leukemia (HL-60), human liver carcinoma (HepG2), and human Jurkat-T cells exposed to arsenic trioxide. Metal Ions Biol Med. 2006;9:293–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Yedjou CG, Tchounwou PB. Modulation of p53, c-fos, RARE, cyclin A, and cyclin D1 expression in human leukemia (HL-60) cells exposed to arsenic trioxide. Mol Cell Biochem. 2009 doi: 10.1007/s11010-009-0160-z. May 15. [Epub ahead of print] doi:10.1007/s11010-009-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11. [PubMed] [Google Scholar]
- 11.Quezada G, Kopp L, Estey E, Wells RJ. All-trans-retinoic acid and arsenic trioxide as initial therapy for acute promyelocytic leukemia. Pediatric Blood Cancer. 2008;51:133. doi: 10.1002/pbc.21529. [DOI] [PubMed] [Google Scholar]
- 12.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978:4538–42. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2000;2:CD000980. doi: 10.1002/14651858.CD000980. [DOI] [PubMed] [Google Scholar]
- 14.Frei B. Reactive oxygen species and antioxidant vitamins: Mechanisms of action. Am J Med. 1994;97:5S–13S. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 15.Uddin S, Ahmad S. Antioxidants protection against cancer and other human diseases. Compr Ther. 1995;21:41–5. [PubMed] [Google Scholar]
- 16.Schwartz JL. The dual roles of nutrients as antioxidants and prooxidants: Their effects on tumor cell growth. J Nutr. 1996;126:1221–7. doi: 10.1093/jn/126.suppl_4.1221S. [DOI] [PubMed] [Google Scholar]
- 17.Bachleitner-Hofmann T, Gisslinger B, Grumbeck E, Gisslinger H. Arsenic trioxide and ascorbic acid: synergy with potential implications for the treatment of acute myeloid leukaemia? Br J Haematol. 2001;112:783–6. doi: 10.1046/j.1365-2141.2001.02608.x. [DOI] [PubMed] [Google Scholar]
- 18.Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood. 2001;98:805–13. doi: 10.1182/blood.v98.3.805. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 21.Yedjou CG, Rogers C, Brown E, Tchounwou PB. Differential effect of ascorbic acid and n-acetyl-l-cysteine on arsenic trioxide mediated oxidative stress in human leukemia (HL-60) cells. Biochem Mol Toxicol. 2008;22:85–9. doi: 10.1002/jbt.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZW, Abdullahi M, Farthing MJG. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut. 2002;50:165–9. doi: 10.1136/gut.50.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raffoux E, Rousselot P, Poupon J, Daniel MT, Cassinat B, Delarue R, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. JCO. 2003;21:2326–34. doi: 10.1200/JCO.2003.01.149. [DOI] [PubMed] [Google Scholar]
- 24.Berenson JR, Boccia R, Siegel D, Bozdech M, Bessudo A, Stadtmauer E, et al. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006;135:174–83. doi: 10.1111/j.1365-2141.2006.06280.x. [DOI] [PubMed] [Google Scholar]
- 25.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–77. [PubMed] [Google Scholar]
- 26.Padayatty SJ, Levine M. Reevaluation of Ascorbate in cancer treatment: Emerging evidence, open minds and serendipity. J Am Coll Nutr. 2000;19:423–5. doi: 10.1080/07315724.2000.10718941. [DOI] [PubMed] [Google Scholar]
- 27.Moertel CG, Fleming TR, Creagan ET, Rubin J, O'Connell MJ, Ames MM. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths L. Ascorbic acid in the 21st century-more than a simple antioxidant. Environ Toxicol Pharmacol. 2001;10:173–82. doi: 10.1016/s1382-6689(01)00081-3. [DOI] [PubMed] [Google Scholar]
- 29.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. London: Clarendon Press; 1985. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendiratta S, Qu ZC, May JM. Erythrocyte defenses against hydrogenperoxide: the role of ascorbic acid. Bioch Bioph Acta. 1998;1380:389–95. doi: 10.1016/s0304-4165(98)00005-1. [DOI] [PubMed] [Google Scholar]
- 32.Van den Bergh JJ, Kuypers FA, Roelofsen B, Op den Kamp JA. The cooperative action of vitamins E and C in the protection against peroxidation of parinaric acid in human erythrocyte membranes. Chem Phys Lipids. 1990;53:309–20. doi: 10.1016/0009-3084(90)90028-p. [DOI] [PubMed] [Google Scholar]
- 33.Park CH. The biological nature of the effect of ascorbic acid on the growth of human leukemic cells. Cancer Res. 1985;45:3969–73. [PubMed] [Google Scholar]
- 34.Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999;82:286–92. doi: 10.1002/(sici)1097-0215(19990719)82:2<286::aid-ijc21>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]


