Abstract
Cytokines in the bone marrow of multiple myeloma patients activate Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathways in tumor cells and promote tumor growth, survival, and drug resistance. INCB16562 was developed as a novel, selective, and orally bioavailable small-molecule inhibitor of JAK1 and JAK2 markedly selective over JAK3. The specific cellular activity of the inhibitor was demonstrated by its potent and dose-dependent inhibition of cytokine-dependent JAK/STAT signaling and cell proliferation in the absence of effects on Bcr-Abl-expressing cells. Treatment of myeloma cells with INCB16562 potently inhibited interleukin-6 (IL-6)-induced phosphorylation of STAT3. Moreover, the proliferation and survival of myeloma cells dependent on IL-6 for growth, as well as the IL-6-induced growth of primary bone marrow-derived plasma cells from a multiple myeloma patient, were inhibited by INCB16562. Induction of caspase activation and apoptosis was observed and attributed, at least in part, to the suppression of Mcl-1 expression. Importantly, INCB16562 abrogated the protective effects of recombinant cytokines or bone marrow stromal cells and sensitized myeloma cells to cell death by exposure to dexamethasone, melphalan, or bortezomib. Oral administration of INCB16562 antagonized the growth of myeloma xenografts in mice and enhanced the antitumor activity of relevant agents in combination studies. Taken together, these data suggest that INCB16562 is a potent JAK1/2 inhibitor and that mitigation of JAK/STAT signaling by targeting JAK1 and JAK2 will be beneficial in the treatment of myeloma patients, particularly in combination with other agents.
Introduction
Multiple myeloma (MM or myeloma) is a clonal malignant B-cell disorder characterized by the accumulation of malignant plasma cells in the bone marrow, leading to osteolytic bone destruction and impaired hematopoiesis. MM accounts for approximately 10% of all hematologic cancers, and it is estimated that approximately 20,000 new cases will be diagnosed and that more than 10,000 patients will succumb to the disease annually in the United States alone [1]. Despite recent progress in the treatment of MM, there is still no cure for this disease, and most patients eventually develop advanced, relapsing disease that is resistant to the drug(s) to which they have had prolonged exposure. Therefore, new treatment approaches and novel drug combinations are needed [2].
The development and progression of MM is dependent on a variety of different cytokines that support myeloma cell proliferation in the bone marrow microenvironment. Cytokines released by bone marrow stromal cells (BMSCs) and/or MM cells that have been described to have this supportive potential include interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), insulin-like growth factor-1, basic fibroblast growth factor, IL-1, IL-10, IL-11, IL-15, IL-21, granulocyte macrophage colony-stimulation factor (GM-CSF), interferon-α, and leukemia inhibitory factor [3]. Among these cytokines, IL-6 has been most widely studied and is considered to play a pivotal role as a growth and survival factor for myeloma cells [4–7]. Evidence indicates that elevated IL-6 expression in the tumor microenvironment may be a major factor leading to drug resistance [8–10]. It is believed that BMSCs are a major source of IL-6 for the myeloma cells; however, the interaction between myeloma cells and BMSCs may be multifactorial [11,12]. Binding of IL-6 to the IL-6 receptor (IL-6R) on the myeloma cell surface induces dimerization with gp130 and activation of the receptor-associated Janus kinase (JAK) tyrosine kinases, JAK1, JAK2, and Tyk2 [13,14]. The activated JAKs trigger the phosphorylation of IL-6R and gp130, followed by activation of a number of downstream signaling molecules including signal transducer and activator of transcription-3 (STAT3), mitogen-activated protein kinase (MAPK), and Akt, thereby fostering the growth and/or survival of myeloma cells [13,15]. Similar to IL-6 signaling, the JAKs can be activated by many of the cytokines mentioned above whose receptors lack intrinsic kinase activity and therefore use the JAKs to transmit their extracellular signal into an intracellular response [16]. JAKs can also be aberrantly activated by either mutation, such as the JAK2V617F mutation that is found in myeloproliferative disease (MPD) or epigenetic inactivation of negative regulators such as SOCS1/3 and SHP-1 [17,18]. Regarding the latter, hypermethylation of SOCS1/3 and SHP-1 have been recently found in 63% and 80% of myeloma patients, respectively [19,20]. In addition, VEGF has been recently shown to play an important role in MM development. Although no JAK is directly associated with the VEGF receptor, it has been shown that IL-6 may be involved in promoting secretion of VEGF by MM cells and BMSCs [21]. Because the JAKs play critical roles in the signal transduction of IL-6 and many other cytokines that may be involved in promoting MM development, blockade of JAK signaling should diminish the supportive effects of aberrant JAK signaling in myeloma cells. Pharmacological inhibition of JAKs may therefore be a promising therapeutic strategy for treatment of myeloma.
We previously described the effects of INCB20, a pan-JAK inhibitor, in models relevant to MM [22]. However, INCB20 inhibits all JAK family members at similar potencies [22]. One concern of using such compounds is that inhibition of JAK3 may cause severe and undesirable immunosuppression in a patient population with an already compromised bone marrow function [23]. In addition, the pharmaceutical properties of INCB20 precluded oral dosing of animals. The present study describes a novel, orally bioavailable, and ATP-competitive JAK1/2 inhibitor, INCB16562, with potent enzyme and cellular activity. This compound is markedly selective for JAK1/2 over JAK3 and potently inhibits JAK/STAT signaling in a number of myeloma cell lines as well as primary MM cells. Moreover, INCB16562 affects the viability of IL-6-dependent myeloma cells in culture and in vivo by inducing caspase activation and apoptosis. For the first time, we show that selective JAK1/2 inhibition potentiates the effects of a variety of relevant therapeutics by mitigating the protective effects of IL-6 and the tumor microenvironment in tissue culture models and in vivo.
Materials and Methods
Kinase Enzyme Assays
INCB16562, as a novel JAK inhibitor, was discovered and synthesized at Incyte. Its ability to inhibit the activity of kinases of the JAK family was measured using in vitro enzyme assays as previously described [22]. Briefly, the enzymes used in the assays were partially purified and N-terminal FLAG-tagged recombinant proteins consisting of the catalytic domains of human JAK1, JAK2, JAK3, or Tyk2. These enzymes catalyzed the phosphorylation of the peptide biotin-EQEDEPEGDY-FEWLE and the HTRF fluorescent signal was then measured on a plate reader. The IC50 was calculated and reported as the compound concentration required for inhibition of 50% of the fluorescent signal. The ATP concentrations used in each enzyme reactions were 90, 30, 3, and 20 µM for JAK1, JAK2, JAK3, and Tyk2, respectively, equivalent to the Km for ATP for the corresponding enzyme. Assays were also conducted using an ATP concentration of 1 mM comparable to cellular levels of ATP, on JAK1, JAK2, and JAK3 to confirm the selectivity of INCB16562 among the JAK family members. To determine the selectivity of INCB16562 against other kinases, the compound was tested at a concentration of 100 nM for the ability to inhibit kinase activities of a commercial panel of 36 protein kinases at Upstate (Charlottesville, VA). The results were calculated and listed in Table 2.
Table 2.
Selective Inhibition of JAKs by INCB16562.
| Enzyme | % Inhibition |
| Alk | 36 |
| AurA | 46 |
| CDK1/CycB | 1 |
| CDK2/CycE | 3 |
| CHK1 | 0 |
| CK2 | 10 |
| C-Raf | 5 |
| CK2 | 10 |
| EGFR | 9 |
| EphB4 | 8 |
| FGFR3 | 0 |
| FLT-1 | 8 |
| FLT-3 | 0 |
| GSK3a | 9 |
| HER2 | 0 |
| HER4 | 0 |
| IGF-1R | 0 |
| IKKa | 6 |
| IKKb | 0 |
| IR | 3 |
| JAK1 | 100 |
| JAK2 | 100 |
| JNK1 | 0 |
| Lck | 54 |
| MAPK1 | 0 |
| MAPK2 | 0 |
| MEK1 | 0 |
| Met | 0 |
| p70S6K | 0 |
| PDGFR | 0 |
| PDK1 | 10 |
| PKA | 0 |
| PKBa | 0 |
| PKCa | 0 |
| PLK3 | 11 |
| SGK | 0 |
Cell Culture
Human MM cell lines H929, U266, and RPMI8226 were purchased from the American Type Culture Collection (ATCC, Rockville, MD), and Dex-sensitive MM1.S and IL-6-dependent INA-6 cell lines were kindly provided by Dr. R. Burger [24] (Dana-Farber Cancer Institute, Boston, MA). A complete medium of RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine was used to maintain these cell lines at 37°C in 5% CO2 atmosphere. For INA-6 only, 1 ng/ml of human recombinant IL-6 (R&D System, Minneapolis, MN) was added to the medium. The parental cytokine dependent human erythroleukemic cell line TF-1 was obtained from ATCC, and a cytokine-independent TF-1-Bcr-Abl cell line was developed by transfection and stable overexpression of the human Bcr-Abl gene in the TF-1 cells. Both cells were cultured in the same medium with the added presence of 2 ng/ml human granulocyte macrophage colony-stimulating factor (GM-CSF) (R&D System) for the TF-1 cell culture. Primary bone marrow CD138+ plasma cells from a newly diagnosed MM patient were purchased from All cells (Emeryville, CA). The cells were cultured in the same medium used for above MM cells based on the protocol suggested by the manufacturer. Human BMSCs were purchased from Cambrex (Walkersville, MD) and initially grown in a Dulbecco's modified Eagle medium containing 20% fetal bovine serum, 1 mM Na-pyruvate (Hyclone, Logan, UT), 1 ng/ml epidermal growth factor (R&D System), and 2 mM l-glutamine. The medium was then switched to the same medium used for MM cells in experiments.
Cell Viability Assay
Suspensions of INA-6, TF-1, TF-1-Bcr-Abl, U266, H929, RPMI8226, MM1.S, or primary CD138+ plasma cells in medium supplemented with 1 ng/ml IL-6 for INA-6 or 2 ng/ml of GM-CSF for TF-1 were equally distributed into 96-well flat-bottomed plates. Triplicate wells were treated with INCB16562 at various concentrations or DMSO (Sigma, St. Louis, MO) as control. Plates were incubated at 37°C in 5% CO2 atmosphere for 72 hours. Cell viability or proliferation was measured using the CellTiter-Glo (Promega, Madison, WI) reagent according to the manufacturer's protocol or using Trypan blue exclusion tests. The IC50 was calculated as the compound concentration to inhibit 50% of the signal from DMSO-treated cells, and the percent inhibition of growth was also calculated relative to DMSO-treated cells.
Proliferation Assay in Coculture with Bone Marrow Stromal Cells
Stromal cells were seeded in flat bottom 96-well culture plates at confluence in the RPMI 1640 medium and incubated for 1 day. INA-6 or MM1.S cells were added to the stromal cells in the same medium. Dexamethasone (Sigma), melphalan (Sigma), bortezomib (Millennium Pharmaceuticals, Cambridge, MA), and INCB16562, either as single compound or in combination, were then added at the final concentrations indicated in the corresponding figures. The plates were incubated at 37°C in 5% CO2 atmosphere for 72 hours, and then 0.25 µCi of [3H]-thymidine (PerkinElmer, Boston, MA) per well was added and incubated for an additional 7 hours. The cultures were harvested onto GF-B 96-well filter plates using a FilterMate Harvester (PerkinElmer). Incorporated radioactivity was counted on a TopCount NXT (PerkinElmer) with the scintillant MicroScint-20 (PerkinElmer). The percent inhibition of cell growth was calculated based on the negative control, the DMSO-treated cells.
Cell Cycle Analysis
Cell cycle distribution was determined by staining cells with propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ). Briefly, INA-6 cells were equally distributed into six-well plates in medium in the presence of 1 ng/ml of IL-6. Cells were treated with either INCB16562 at 800 nM or an equal volume of DMSO and then incubated at 37°C in 5% CO2 atmosphere for 20 hours. Approximately 1 x 106 cells were collected and fixed in 70% ethanol and then stained with PI for 30 minutes at room temperature according to the manufacturer's protocol. The percentage of cells in the different phases of the cell cycle was analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Apoptosis Analysis
INCB16562-induced apoptosis in INA-6 cells was assayed by annexin V/PI staining and caspase activation. Cells were equally distributed into 6-well or 96-well culture plates in medium in the presence of 1 ng/ml of IL-6. Cells were treated with INCB16562 at various concentrations as indicated in the figures or with DMSO as a control and then incubated at 37°C in 5% CO2 atmosphere for 24 hours. For annexin V/PI staining, an aliquot of cells was removed from the six-well plate and stained with annexin V-fluorescein isothiocyanate and PI according to the manufacturer's directions (Apoptosis Detection Kit; Sigma) and analyzed using a FACSCalibur flow cytometer (BD Biosciences). For caspase activation assays, cell lysis reagents and specific substrates of caspase-3/7, caspase-8, or caspase-9 were directly added into cell cultures in the 96-well plates, and the fluorescent signals (RFLU) of rhodamine-110 groups released from the substrates on activation of caspases were analyzed based on the manufacturer's protocols (Promega).
Western Blot Analysis
Cells were treated with INCB16562 or DMSO at concentrations and for periods as indicated in the figures. After treatment, cells were washed with ice-cold PBS and resuspended in a cell extraction buffer (Invitrogen, Carlsbad, CA) and lysed based on the manufacturer's protocols. Equivalent amounts of protein (∼70 µg) from each lysate were resolved in 4% to 12% SDS-PAGE (Invitrogen) and transferred to polyvinylidene difluoride membranes (Invitrogen). The primary antibodies specific for the following proteins were used at the indicated dilutions: phospho-STAT3 (1:1000), STAT3 (1:1000), STAT5 (1:1000), phospho-JAK2 (1:1000), and JAK2 (1:1000; all from Cell Signaling, Beverly, MA); phospho-STAT5 (1:1000; Millipore, Temecula, CA); Mcl-1 (1:100), poly (ADP-ribose) polymerase (PARP; 1:100), Bcl-2 (1:100), Bcl-XL (1:100), α-actin (1:100; all from Santa Cruz Biotechnology, Santa Cruz, CA). After incubating with the antibody, the immunoreactive bands were detected with a chemiluminescent substrate (SuperSignal; Pierce, Rockford, IL).
Tumor Xenografts
Animal studies were performed under Animal Welfare Regulation Guidelines in a facility at the DuPont Experimental Station, Wilmington, DE, accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Studies were performed as described previously [22]. Briefly, 6- to 8-week-old severe combined immunodeficient mice (Charles River, Wilmington, MA) were injected subcutaneously with approximately 1 x 106 viable INA-6.Tu1 cells freshly harvested from a tumor-bearing mouse. Animals were monitored daily for signs of tumor growth and measured with calipers two to three times each week after visible tumor was detected. Tumor volume was calculated as (length x width2) / 2. When tumors were well established (>125 mm3), animals were assigned into treatment groups with similar median tumor volumes. Mice were dosed orally, twice daily, with vehicle (0.5% methocellulose and 5% N,N-dimethylacetamide) or INCB16562. Melphalan and bortezomib were formulated in sterile saline and were dosed twice each week, i.p., beginning 3 days after onset of treatment with INCB16562. Animals were weighed regularly to adjust dose levels and to monitor for gross signs of toxicity. Percent tumor growth inhibition was calculated as follows: [1 - (mean tumor volume, treated group ÷ mean tumor volume, vehicle group)] x 100. Statistical significance between mean tumor volumes in various treatment groups was assessed using Student's t test.
Results
Enzymatic Potency of INCB16562
The biochemical potency of INCB16562 (Figure 1) for the inhibition of JAKs was determined in enzymatic assays using recombinant proteins containing the catalytic domain of each human JAK family member. Assays were conducted at an ATP concentration equivalent to the Km for each enzyme. INCB16562 was determined to be a low-nanomolar inhibitor of JAKs with IC50 values of 2.2, 0.25, 10.1, and 2.7 nM for JAK1, JAK2, JAK3, and TYK2, respectively (Table 1). Because this inhibitor was found to be a reversible ATP-competitive kinase inhibitor (unpublished data), the calculated IC50 values taking into account the high concentration of ATP in cells (using 1 mM ATP as typical of the cell concentration [25]) predict that this compound would have a relative selectivity for JAK2 and JAK1 over TYK2 and a marked selectivity over JAK3 inside cells. This predicted selectivity of JAK1/2 over JAK3 was experimentally confirmed by running enzymatic assays at 1 mM ATP concentration (Table 1).
Figure 1.
Chemical structure of INCB16562.
Table 1.
INCB16562 Is a Potent JAK1/2 Inhibitor.
| Enzyme | IC50 (nM)* (ATP = Km) | IC50 (nM)† (ATP = 1 mM) |
| JAK1 | 2.2 ± 0.77 | 9.1 ± 2.5 |
| JAK2 | 0.25 ± 0.17 | 2.1 ± 1.1 |
| JAK3 | 10.1 ± 3.6 | 1663 ± 876 |
| TYK2 | 2.7 ± 0.9 | 68‡ |
All IC50 values were assayed at Km for ATP.
All IC50 values were assayed at 1 mM of ATP.
Calculated IC50.
To more broadly characterize the selectivity of INCB16562 among other human kinases, we tested this compound against a commercial panel of 36 kinases at 100 nM, a concentration approximately 75x the average IC50 value for JAK1 and JAK2 (Table 2). INCB16562 demonstrated no significant inhibition (<20% inhibition) for most of the kinases tested. Modest inhibitory effects (36%–54%) against Lck, Aurora-A, and Alk kinases were observed at this relatively high concentration of inhibitor.
Cellular Effects of INCB16562
Whereas IL-6 has been implicated in the pathogenesis of myeloma, the reliance of established myeloma cell cultures on exogenous cytokines may not be conserved, depending on the culture conditions used to establish and maintain them [26]. Therefore, we analyzed the effects of INCB16562 in both cytokine-dependent and cytokine-responsive myeloma cells. We first chose the human INA-6 MM cell line to study the effects of INCB16562 on JAK1 and/or JAK2 activities because these cells require exogenous IL-6 for in vitro growth and survival [24]. It has been previously demonstrated that activation of JAK/STAT3 in these cells is dependent on the presence of IL-6 and inactivation of JAK/STAT3 by either withdrawal of IL-6 or prevention of IL-6 binding to the receptor induces cell death through apoptosis [27]. Moreover, using a commercially available pan-JAK inhibitor, these cells have been shown to be responsive to JAK inhibition that results in a concordant reduction in the levels of phosphorylated STAT3 (p-STAT3) [28]. Therefore, the cellular activity of INCB16562 could be assessed by examining inhibition of STAT3 phosphorylation and cell growth in INA-6 cells. As shown in Figure 2A, the compound potently inhibited STAT3 phosphorylation with almost complete inhibition at concentrations of 300 nM or greater. As a control, the total STAT3 level was not significantly changed. Because INA-6 cells require JAK activating cytokines for survival, we determined the effects of INCB16562 on the viable number of cells during a 3-day period. A dose-dependent reduction in viable cells was observed with an average IC50 of 191 ± 50 nM (Figure 2B), consistent with the observed potency on STAT3 phosphorylation. In addition, we also measured the potency shift of INCB16562 in response to the addition of different concentrations (0.5–100 ng/ml) of IL-6 to INA-6 cells, considering the variation of IL-6 concentrations in the BM microenvironments of MM patients. As assessed by STAT3 phosphorylation and cell proliferation, higher concentrations of IL-6 did cause a rightward shift in IC50 value when compared with lower concentrations. However, the fold shift was small and within a two-fold variation range (data not shown), suggesting that this compound should remain potent even in the presence of very high concentrations of IL-6, and this effect should be extended to other cytokines as well.
Figure 2.
INCB16562 inhibits cytokine-induced JAK/STAT signaling activities and cell growth. (A) INA-6 cells were cultured in the presence of 1 ng/ml of human IL-6 and treated with INCB16562 at indicated concentrations for 3 hours. Total lysates were subjected to Western blot analysis for p-STAT3. The same membrane was washed and reprobed for STAT3. (B) INA-6 cells were grown in the presence of 1 ng/ml of IL-6 as well as the drug at various concentrations for 3 days. Cell growth inhibition was calculated as a percentage of control from vehicle-treated cells. This is a representative curve from an assay run in triplicate assay, and the IC50 value is averaged from 20 independent assays. (C) MM1. S, H929, U266, and RPMI8226 cells were serum-stared for 4 hours and then pretreated with the drug at 0.3 or 1 µ M for 20 minutes. Cells were stimulated with 10 ng/ml of IL-6 for 10 minutes, and total lysates were subjected to Western blot analysis for p-STAT3. Membranes were washed and reprobed for STAT3. (D) MM1.S, H929, U266, and RPMI8226 cells were grown in regular culture medium in the presence of the drug at 0.3 or 1 µ M for 3 days. The cell viability was determined and expressed as the percentage of inhibition relative to the DMSO control. (E) The primary bone myeloma CD138+ plasma cells from a MM patient were seeded in the presence of 10 ng/ml of IL-6 and were incubated with INCB16562 at the indicated concentrations for 3 days. In addition, cells treated with DMSO in the absence of IL-6 served as negative controls. The cell viability was determined by CellTiter-Glo. The average fluorescent value from the negative controls was subtracted from all the treated values indicated in the figure. The growth inhibition was expressed as percentage relative to the DMSO-treated cells. (F) Parental TF-1 cells were serum-starved for 4 hours and then stimulated with human GM-CSF (2 ng/ml) for 10 minutes after a 20-minute preincubation with INCB16562 at 0.3 or 1 µM. Total lysates were used for Western blot analysis for p-STAT5. The same membrane was washed and reprobed for STAT5. (G) Growing GM-CSF (2 ng/ml) stimulated parental TF-1 cells or TF-1-Bcr-Abl cells were cultured with the drug at various concentrations for 3 days. Percent inhibition of cell proliferation was calculated relative to vehicle-treated cells. Error bars in all figures represent the SD of triplicates from one representative assay.
The ability of INCB16562 to inhibit JAK/STAT3 activation in myeloma cells was confirmed using a panel of cell lines that have been selected for IL-6 independence but remain cytokine responsive: MM1.S, H929, U266, and RPMI8226. Each of these cell lines demonstrated robust activation of JAK signaling on addition of IL-6, as shown by markedly increased levels of p-STAT3 (Figure 2C). Importantly, INCB16562 potently and dose-dependently reduced p-STAT3 levels stimulated by IL-6 in all these cell lines without affecting the total STAT3 present in these cells. Possibly because of the higher intracellular ATP levels, higher concentrations of INCB16562 were required to completely inhibit the STAT3 phosphorylation in some cell lines. Although remaining IL-6-responsive, the growth of these cells was not significantly affected by exogenously added IL-6 (data not shown). To evaluate any effects of INCB16562 on the growth of these cell lines, cells were incubated with the compound at pharmacologically active concentrations in regular culture medium for 3 days, and the cell viability was analyzed. It was found that INCB16562 did not inhibit the growth of MM1.S, RPMI8226, and H929 cells, but it partially inhibited the growth of U266 cells (Figure 2D). The data are consistent with previous reports that the growth of U266, but not the other three cell lines, is partially dependent on JAK/STAT activation through the autocrine IL-6 signaling pathway [28,29].
The cellular activity of INCB16562 was also examined in primary CD138+ plasma cells from the bone marrow of a newly diagnosed MM patient. The primary cells were incubated with INCB16562 at various concentrations in the absence or presence of IL-6 for 3 days, and the cell viability was determined. We found that INCB16562 only had marginally inhibitory effects on the growth of these cells at 1 µM in the absence of IL-6, but we observed an approximately 70% increase in cell growth in the DMSO-treated cells in the presence (vs absence) of IL-6 (data not shown). However, the increased growth was completely inhibited by INCB16562 in a dose-dependent manner (Figure 2E), indicating that inhibition of the JAK/STAT signaling has significant effects on the cytokine-stimulated growth of primary myeloma cells. No significant effects of INCB16562 on the viability of normal B cells and peripheral blood mononuclear cells were observed over the same dose range as was tested in the plasma cells (data not shown).
To evaluate the cell-based selectivity of INCB16562, we compared its effect on viable cell number in a pair of isogenic cell lines, parental versus Bcr-Abl-transduced TF-1 cells. Parental TF-1 cells are a cytokine-dependent human erythroleukemic cell line. Human GM-CSF supports proliferation and viability of the parental TF-1 cells through activation of the JAK2/STAT signaling pathway. Bcr-Abl expression in these cells (TF-1-Bcr-Abl) renders them cytokine-independent because their proliferation and survival are driven by the constitutively active Abl kinase. Figure 2F shows that 300 nM of INCB16562 completely prevented STAT5 phosphorylation stimulated by the addition of 2 ng/ml of human GM-CSF to TF-1 cells. As a result, the growth of the parental TF-1 cells in the presence of GM-CSF was potently inhibited by INCB16562 with an IC50 of 102 ± 36 nM, whereas the compound had no effect on TF-1-Bcr-Abl cell growth (Figure 2G). Only at concentrations exceeding 4000 nM was a significant effect (>30% inhibition) observed. These results indicate that this compound is cell selective for JAKs over the Abl kinase. The results also suggest that, at concentrations less than 4000 nM, INCB16562 does not significantly inhibit other kinases or nonkinase enzymes that are critical for cell growth or survival. Collectively, the cellular data, along with the enzyme data in Tables 1 and 2, demonstrate that INCB16562 is a potent and selective inhibitor of the JAK1 and JAK2 kinases in cells.
INCB16562 Induces Cell Death through Apoptosis in INA-6 Cells
The cellular assays described above are unable to discern whether the observed effects on viable cell number were due to decreased cell proliferation, increased cell death, or both. Therefore, we determined the effects of INCB16562 on the cellular DNA content by flow cytometry analysis in IL-6-dependent INA-6 cells. As shown in Figure 3A, the data indicate that INCB16562 alters the cell cycle distribution and induces a modest G2/M arrest in INA-6 cells treated with the compound for 20 hours at a concentration sufficient to completely inhibit STAT3 phosphorylation in these cells. Moreover, consistent with published data that abrogation of the IL-6/JAK/STAT3 signaling pathway induces apoptosis in INA-6 cells [26,27], we observed an increase in the population of cells with a sub-G1 DNA content, indicative of apoptosis. Looking more closely at the apoptotic effects of INCB16562, we then treated INA-6 cells with increasing concentrations of the compound and determined the percentage of apoptotic cells by flow cytometric analysis of annexin V and PI-stained cells. As shown in Figure 3B, the compound induced apoptosis in cells in a dose-dependent manner suggesting the effects on viable cell number were due to both decreased proliferation and increased cell death.
Figure 3.
INCB16562 induces apoptosis and modest G2/M delay in INA-6 cells. All cells were grown in the presence of IL-6 (1 ng/ml). (A) Cells were incubated with 0.8 µM of INCB16562 for 20 hours and then stained with PI for cell cycle analysis by flow cytometry. (B and C) Cells were incubated with the drug at indicated concentrations for 24 hours. Apoptosis was then determined by flow cytometric analysis of annexin V/PI staining and activation of caspase-3/7, -8, and -9. (D) Cells were treated with either 1 µM of INCB16562 or DMSO for 9 hours. Total lysates were analyzed by Western blot for p-STAT3, cleavage of PARP (lower band), Mcl-1, Bcl-2, Bcl-XL, and β-actin (as control for protein loading).
To explore the apoptotic mechanisms induced by blocking JAK/STAT activation, we measured the activities of the apical caspases, caspase-8 and -9, as well as the effector caspases, caspase-3 and -7. A robust dose-dependent activation of caspase-3/7 activity was observed after treatment with INCB16562, in agreement with the annexin V data (Figure 3C). Using isoform-specific assays, we observed that caspase-9 activity was markedly increased with INCB16562 treatment compared with minimal activation of caspase-8. These data clearly implicate activation of the intrinsic apoptotic pathway in the death of INCB16562-treated myeloma cells and suggest that unbalancing of the Bcl-2 family may contribute to the observed effects. Therefore, we next analyzed the levels of protein expression of various Bcl-2 family members in INA-6 cells treated with 1 µM of INCB16562. As expected, the compound markedly reduced p-STAT3 levels and induced cleavage of PARP, another marker of caspase-dependent cell death (Figure 3D). Although we observed no significant changes in Bcl-2 or Bcl-XL expression, Mcl-1 levels were dramatically reduced with INCB16562 treatment (Figure 3D). Because it was previously demonstrated that IL-6-activated STAT3 can directly bind to the promoter and transcriptionally upregulate Mcl-1 expression [30–32], the data here suggest that reduced levels of this antiapoptotic protein caused by inhibition of STAT3 activity may have been at least partially responsible for the observed apoptosis in INCB16562 treated INA-6 cells. By searching for potential effects of INCB16562 on other signaling pathways, we found that the compound at 1 µM did not inhibit phosphorylation of ERK1/2 and Akt and had no effects on IκB-α phosphorylation or degradation (data not shown), indicating that signaling through MAPK, Akt, or nuclear factor-κB is unlikely to be directly involved in INCB16562-mediated apoptosis in INA-6 cells. Thus, blockade of IL-6-induced JAK/STAT signaling by INCB16562 led to significant apoptosis in combination with a small G2/M delay in INA-6 cells.
INCB16562 Abrogates the Protective Effects of IL-6 and Bone Marrow Stromal Cells
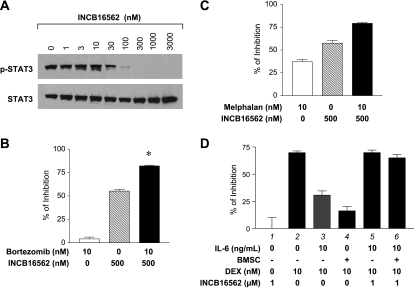
The bone marrow microenvironment is rich in supportive growth factors such as cytokines that are involved in support of the growth and survival of myeloma cells [3]. We hypothesized that IL-6 and other JAK-dependent cytokines were central to these protective effects. To test this, we used an in vitro coculture model system assessing proliferation of INA-6 cells on a confluent layer of human BMSCs [22,28]. Our previous data demonstrated that the IC50 value of INCB16562 in blocking INA-6 cell proliferation when cocultured with BMSCs was approximately 1.3- to 1.5-fold higher than the value obtained when the cells were grown in the presence of 1 ng/ml of IL-6 alone (data not shown), indicating that the compound had the ability to potently inhibit JAK activity even in the presence of BMSCs. We first confirmed that INCB16562 can potently inhibit STAT3 phosphorylation in the INA-6 cells in the coculture system with BMSCs (Figure 4A). We next used this coculture assay system to examine the effect of combination of INCB16562 with other agents (bortezomib or melphalan) that have demonstrated utility in treatment of myeloma. In a representative experiment, 500 nM INCB16562 inhibited proliferation of INA-6 cells by 55% in the presence of human BMSCs, whereas 10 nM of bortezomib had only a slight (5%) inhibitory effect (Figure 4B). However, in combination, the proliferation was inhibited up to 82% suggesting a synergistic response. A similar pattern of enhanced effect was also observed in the combination between melphalan and INCB16562 (Figure 4C), although the single-agent activity of melphalan was more impressive. These results demonstrate that the combination of bortezomib or melphalan with INCB16562 can inhibit proliferation of the myeloma cells more robustly than either drug alone in the presence of BMSCs.
Figure 4.
INCB16562 enhances the effects of bortezomib and melphalan in INA-6 cells and sensitizes MM1.S to Dex in the presence of BMSCs. (A) INA-6 cells (washed to remove IL-6) were seeded on top of confluent BMSCs and treated with various concentrations of INCB16562. After 3 hours of incubation, the INA-6 cells were collected and the total lysates were analyzed by Western blot for p-STAT3. The same membrane was washed and reprobed for STAT3. (B and C) INA-6 cells (washed to remove IL-6) were seeded on top of confluent BMSCs. Cells were then treated for 3 days with the drugs listed in the figures, and proliferation of the INA-6 cells was determined by [3H]-thymidine uptake (see Materials and Methods). The t test P value of the combination versus the sum of the net individual effect was calculated. *P ≤ .05 and statistically significant. (D) MM1.S cells were cultured in the absence or presence of either IL-6 or BMSCs for 3 days with concomitant exposure to Dex in the presence or absence of INCB16562. Cell proliferation was determined by [3H]-thymidine uptake in MM1.S cells. Error bars represent SD of triplicates in representative experiments.
To better understand the nature of the potentiation of INCB16562 in antagonizing the protective effects of IL-6 or BMSCs, we moved to another coculture model system in which JAK inhibition alone has limited effects on tumor cell proliferation. Dexamethasone (Dex) is widely used in the treatment of MM, and the human MM1.S myeloma cell line is responsive to treatment with Dex in culture. However, it has been shown that Dex-induced myeloma cell death can be abrogated by addition of IL-6 or coculture with BMSCs [9,33]. We hypothesized that some, if not all, of the protective effects of coculture with BMSCs was mediated by JAK-activating cytokines (e.g., IL-6), and we tested this hypothesis by assessing growth inhibition of MM1.S cells in response to Dex +/- INCB16562 in the presence or absence of IL-6 or BMSCs. Previously, we demonstrated responsiveness of MM1.S cells to IL-6 by showing that the cells have low constitutive levels of p-STAT3 but respond to IL-6 with a robust activation of JAK/STAT and, importantly, that this is reversed by addition of INCB16562 (Figure 2C). In a representative experiment, shown in Figure 4D, we first confirmed that JAK/STAT activation was sufficient to convey resistance to Dex-treated MM1.S cells. Under standard cell culture conditions, Dex alone inhibited MM1.S proliferation by approximately 70% compared with vehicle-treated cells (lane 2). This growth inhibition was dramatically decreased to approximately 30% when exogenous IL-6 was added to the cell culture (lane 3), confirming that IL-6 provides a protective effect to Dex-treated MM1.S cells. In a similar fashion, coculture with BMSCs also protected cells from Dex-induced growth inhibition (lane 4; ∼16% inhibition). Although the addition of pharmacologically active (Figure 2C) levels of INCB16562 had no significant effect on the proliferation of MM1.S cells (lane 1), it did completely revert the MM1.S cells to a Dex-sensitive state when grown with either IL-6 (lane 5; ∼70% inhibition) or BMSC (lane 6; ∼66% inhibition). In aggregate, the results suggest that activation of the JAK/STAT signaling by IL-6 and/or other cytokines in the bone marrow microenvironment protects myeloma cells from the antiproliferative effects of a variety of therapeutics and that JAK1/2 inhibition can abrogate such protective mechanisms.
JAK Inhibition Potentiates the Growth Inhibitory Effects of Bortezomib and Melphalan In Vivo
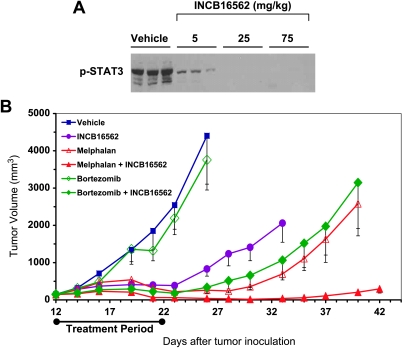
We have previously demonstrated that the INA-6.Tu1 myeloma xenograft model—a tumorigenic subclone of the INA-6 line—is responsive to a pan-JAK inhibitor in vivo [22]. Here, we evaluated the ability of INCB16562 to improve therapeutic responses to clinically relevant therapies using this tumor model. First, we established INA-6. Tu1 tumor xenografts in immunocompromised mice and assigned them into treatment groups with similar mean tumor volumes. In the initial experiment, treatment consisted of a single oral dose of vehicle or three different dose levels of INCB16562 (5, 25, and 75 mg/kg). Tumors were harvested 4 hours after dosing and analyzed for levels of p-STAT3 after normalizing samples for total protein (Figure 5A). Results from this experiment demonstrated that a dose of 5 mg/kg was sufficient to modestly reduce p-STAT3 levels in tumor tissue. A dose of 25 mg/kg was determined to be the lowest dose tested that provided a marked inhibition of JAK/STAT in tumors for 4 hours or longer per dose. This dose level was therefore chosen for subsequent experiments. Next, we treated similar cohorts of tumor-bearing mice (n = 7 or 8 animals/group) with INCB16562 (25 mg/kg, twice a day), melphalan (5 mg/kg, twice a week), bortezomib (1.5 mg/kg, twice a week), or combinations of these agents and compared tumor growth to vehicle-treated animals (Figure 5B). As a single agent, INCB16562 resulted in 85% inhibition of tumor growth (day 23). Melphalan and bortezomib, administered at or near their maximally tolerated dose levels, caused 91%and 14%growth inhibition, respectively. The addition of INCB16562 resulted in a near-complete inhibition of tumor growth when combined with either melphalan (98%) or bortezomib (93%), demonstrating the ability of a selective JAK1/2 inhibitor to potentiate the antitumor effects of these relevant therapies in vivo. Importantly, the addition of a selective JAK inhibitor to either treatment regiment was well tolerated, as assessed by clinical observation and gross body weights (data not shown).
Figure 5.
INCB16562 inhibits growth of INA-6.Tu1 xenografts and enhances toxicity of bortezomib or melphalan in tumors. (A) Severe combined immunodeficient mice were engrafted with INA-6.Tu1 cells. When tumors were established, animals were orally given a signal dose of vehicle or INCB16562 at 5, 25, or 75 mg/kg (n = 3 animals/group). Tumors were harvested in 4 hours after dosing, and total lysates were prepared from each tumor for Western analysis of p-STAT3. (B) Similarly, after INA-6.Tu1 tumors were established in mice, animals were assigned into different groups (n = 7 or 8 animals/group) and treated with vehicle, INCB16562 (25 mg/kg, twice a day), melphalan (5 mg/kg, twice a week), bortezomib (1.5 mg/kg, twice a week), or combination of these reagents for 12 days. Bortezomib or melphalan was given on days 3, 5, 8, 10, and 12 after dosing of INCB16562. Antitumor effects of each drug alone or drugs in combination and individual body weight of mice were measured and assessed two to three times each week. Error bars represent SD in each treatment group.
Discussion
Multiple lines of evidence support an important role for JAK signaling in the initiation and progression of myeloma. In mice, constitutive expression of IL-6—a JAK-dependent cytokine—is sufficient to induce plasmacytomas [6,34]; conversely, IL-6 knockout mice are resistant to tumor induction in an induced model of B-cell neoplasms [5]. These data are complemented by the following observations: (1) studies in myeloma patients demonstrate the presence of elevated levels of IL-6 and/or its soluble receptor (sIL6R) [35], (2) BMSCs support the growth and survival of myeloma cells, at least in part, by secreting a number of JAK activating cytokines [3], and (3) cell autonomous dysregulation of key regulatory feedback loops (e.g., SOCS-1 and SHP-1) has been described in most myeloma patients [19,20], consistent with the frequent finding of STAT3 activation in tumor samples [36,37]. In aggregate, the evidence supports a fundamental role for JAK signaling in the pathobiology of myeloma. JAK inhibitors can disrupt such signaling cascades, and therefore, they may directly cause inhibition of myeloma cell survival and/or proliferation and abrogate the protective environment resulting in sensitization of myeloma cells to relevant drugs such as Dex, melphalan, or bortezomib.
AG490 has been described and used as a JAK2 inhibitor in the literature for a long period, but our internal data and recent results from Pedranzini et al. [28] strongly suggest that this compound is not a potent or selective JAK inhibitor. Pyridone-6 and INCB20 are two recently identified JAK inhibitors; however, these molecules are pan-JAK inhibitors that potently inhibit not only JAK1/2 but also JAK3 and/or Tyk2, [22,28]. CP-690550 was described as an ATP-competitive JAK3 inhibitor developed clinically as an immune suppressive agent for the treatment of organ transplant recipients [38], but this compound was recently found to have potent JAK1 and JAK2 activities in enzyme assays as well as in cells [39,40]. In an effort to develop JAK2 selective compounds for the treatment of MPDs, TG-101348 and XL-019 have been recently described and are currently in clinical trials for MPDs. Both inhibitors demonstrate a selectivity for JAK2 over JAK1, JAK3, and Tyk2 [41,42], but their ability to effectively block JAK signaling by cytokines such as IL-6 in myeloma cells may be hampered by their lack of JAK1 activity. CYP387 is another newly characterized JAK inhibitor with modest selectivity (∼10-fold) for JAK1/2 over JAK3 in enzyme assays, and it has been shown to inhibit wild type JAK2 as well as JAK2V617F in cellular assays [42], but this compound has yet to be evaluated in myeloma models. Here, we describe the biochemical and cellular activities of INCB16562, a novel, orally bioavailable, and potent JAK1/2 selective inhibitor. We believe that, for the treatment of myeloma and a number of other neoplasias, JAK1/2 inhibition may be the favored selectivity profile for a JAK inhibitor. This is based on the reliance of either or both JAK1 and JAK2 in a number of homodimeric or heterodimeric signaling complexes associated with different cytokine and growth factors along with the potential liability of immune suppression associated with JAK3 inhibition. Using this novel tool, we investigated the role of JAK1/2 signaling in myeloma cell growth, survival, and resistance to therapeutic treatment. INCB16562 potently inhibits JAK1 and JAK2 at very low or subnanomolar concentrations and demonstrates excellent selectivity within the JAK family (>300-fold selective against JAK3 at 1 mM ATP) and against a broad panel of additional kinases (Tables 1 and 2). The biochemical selectivity of INCB16562 was maintained in cells as demonstrated by its growth inhibitory potency when tested in the cytokine/JAK-dependent INA-6 cells (Figure 2B) and TF-1 cells compared with the isogenic TF-1-Bcr-Abl cells (Figure 2G) in which proliferation is supported by the Abl oncogene. Characterization of the response of INA-6 cells to JAK inhibition revealed effects on intracellular signaling pathways (Figure 2A), proliferation (Figure 3A), and apoptosis (Figure 3, B–D), each occurring within the same relative concentration range of INCB16562. The data implicate the intrinsic/mitochondrial apoptotic program (caspase-3, -7, and -9) as the major effector pathway in the observed cell death. Mechanistically, we observed a significant decrease in the expression levels of Mcl-1, a prosurvival member of the Bcl-2 family, consistent with activation of the intrinsic apoptotic machinery. As Mcl-1 is a reported STAT3 target gene and an important regulator of cell survival [30–32], we surmise this effect contributes to the observed caspase-dependent cell death. We have been unable to completely rule out a role of the extrinsic pathway owing to the detectable though modest increases in caspase-8 activity (Figure 3C).
Importantly, we find that the ability of INCB16562 to inhibit STAT phosphorylation in myeloma cells is not limited to the INA-6 cells. Indeed, four additional myeloma lines were studied and, although they lacked high levels of basal p-STAT3, INCB16562 potently inhibited IL-6 stimulation of STAT3 phosphorylation (Figure 2C). Although treatment of these cells with INCB16562 had limited or partial effects on their survival, consistent with other reports [28], this is not unexpected because the process of isolating and maintaining cell lines under various culture conditions can influence reliance on various growth factors and their signaling pathways [26]. Nonetheless, these data demonstrated that the myeloma cells can respond to cytokines in the environment, such as in the bone marrow milieu, by activating STAT signaling pathways in a JAK1/2-dependent manner. The relevance of this cytokine-induced JAK signaling was demonstrated in experiments in which myeloma cells were cultured either in the presence of BMSC or recombinant IL-6 and then treated with clinically relevant therapeutics in the presence or absence of INCB16562. These experiments show that inhibition of JAK1/2 in either setting potentiates the effects of drug treatment by antagonizing the protective effects of JAK/STAT signaling and suggest that suboptimal clinical responses to treatment may be limited by JAK activation. Indeed, we demonstrate for the first time that inhibition of JAK1/2 improves the antitumor activity of two common myeloma therapies, melphalan and bortezomib in an in vivo model of myeloma.
Although there have been great strides made in the treatment of myeloma during the past decade, there remains a need for new agents. Accumulating data in the literature and our data described here suggest that the benefit of multiple treatment regimens may be blunted because of the activation of survival pathways such as JAK/STAT. Clearly, exploration of different drug combination regiments with a selective JAK inhibitor is warranted.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang XG, Bataille R, Widjenes J, Klein B. Interleukin-6 dependence of advanced malignant plasma cell dyscrasias. Cancer. 1992;69:1373–1376. doi: 10.1002/1097-0142(19920315)69:6<1373::aid-cncr2820690612>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Lattanzio G, Libert C, Aquilina M, Cappelletti M, Ciliberto G, Musiani P, Poli V. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am J Pathol. 1997;151:689–696. [PMC free article] [PubMed] [Google Scholar]
- 6.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano MM, Mihara K, Huang N, Tsujimoto T, Kuramoto A. Differentiation of early plasma cells on bone marrow stromal cells requires interleukin-6 for escaping from apoptosis. Blood. 1995;85:487–494. [PubMed] [Google Scholar]
- 8.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872. [PubMed] [Google Scholar]
- 9.Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 10.Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein A, Berenson J, Norman D, Chang MP, Carlile A. Production of cytokines by bone marrow cells obtained from patients with multiple myeloma. Blood. 1989;74:1266–1273. [PubMed] [Google Scholar]
- 12.Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 13.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, Schlossman RL, Anderson KC. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 14.Berger LC, Hawley TS, Lust JA, Goldman SJ, Hawley RG. Tyrosine phosphorylation of JAK-TYK kinases in malignant plasma cell lines-growth stimulated by interleukins 6 and 11. Biochem Biophys Res Commun. 1994;202:596–605. doi: 10.1006/bbrc.1994.1970. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 16.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 17.Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673–683. doi: 10.1038/nrc2210. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galm O, Yoshikawa H, Esteller M, Osieka R, Herman JG. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood. 2003;101:2784–2788. doi: 10.1182/blood-2002-06-1735. [DOI] [PubMed] [Google Scholar]
- 20.Chim CS, Fung TK, Cheung WC, Liang R, Kwong YL. SOCS1 and SHP1 hypermethylation in multiple myeloma: implications for epigenetic activation of the Jak/STAT pathway. Blood. 2004;103:4630–4635. doi: 10.1182/blood-2003-06-2007. [DOI] [PubMed] [Google Scholar]
- 21.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 22.Burger R, Le Gouill S, Tai YT, Shringarpure R, Tassone P, Neri P, Podar K, Catley L, Hideshima T, Chauhan D. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol Cancer Ther. 2009;8:26–35. doi: 10.1158/1535-7163.MCT-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Shea JJ, Husa M, Li D, Hofmann SR, Watford W, Roberts JL, Buckley RH, Changelian P, Candotti F. Jak3 and the pathogenesis of severe combined immunodeficiency. Mol Immunol. 2004;41:727–737. doi: 10.1016/j.molimm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Burger R, Günther A, Bakker F, Schmalzing M, Bernand S, Baum W, Duerr B, Hocke GM, Steininger H, Gebhart E, et al. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J. 2001;2:42–53. doi: 10.1038/sj.thj.6200075. [DOI] [PubMed] [Google Scholar]
- 25.Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- 26.Rawat R, Rainey GJ, Thompson CD, Frazier-Jessen MR, Brown RT, Nordan RP. Constitutive activation of STAT3 is associated with the acquisition of an interleukin 6-independent phenotype by murine plasmacytomas and hybridomas. Blood. 2000;96:3514–3521. [PubMed] [Google Scholar]
- 27.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden JR, Gramatzki M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. [PubMed] [Google Scholar]
- 28.Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66:9714–9721. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- 29.Schwab G, Siegall CB, Aarden LA, Neckers LM, Nordan RP. Characterization of an interleukin-6-mediated autocrine growth loop in the human multiple myeloma cell line, U266. Blood. 1991;77:587–593. [PubMed] [Google Scholar]
- 30.Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK/STAT rather than ras/MAP kinase pathway. Eur J Immunol. 1999;29:3945–3950. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–1338. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 33.Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Exp Hematol. 1998;26:597–603. [PubMed] [Google Scholar]
- 34.Dedera DA, Urashima M, Chauhan D, LeBrun DP, Bronson RT, Anderson KC. Interleukin-6 is required for pristane-induced plasma cell hyperplasia in mice. Br J Haematol. 1996;94:53–61. doi: 10.1046/j.1365-2141.1996.6282074.x. [DOI] [PubMed] [Google Scholar]
- 35.Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nuñez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 37.Quintanilla-Martinez L, Kremer M, Specht K, Calzada-Wack J, Nathrath M, Schaich R, Höfler H, Fend F. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003;162:1449–1461. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 39.Williams NK, Bamert RS, Patel R, Wang C, Walden PM, Wilks AF, Fantino E, Rossjohn J, Lucet IS. Dissecting specificity in the Janus kinases: the structures of JAK-specific inhibitors complexed to the JAK1 and JAK2 protein tyrosine kinase domains. J Mol Biol. 2009;387:219–232. doi: 10.1016/j.jmb.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Manshouri T, Quintás-Cardama A, Nussenzveig RH, Gaikwad A, Estrov Z, Prchal J, Cortes JE, Kantarjian HM, Verstovsek S. The JAK kinase inhibitor CP-690,550 suppresses the growth of human polycythemia vera cells carrying the JAK2V617F mutation. Cancer Sci. 2008;99:1265–1273. doi: 10.1111/j.1349-7006.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, Gozo M, McDowell EP, Levine RL, Doukas J, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]