Abstract
We and others have previously demonstrated that the acute release of progenitor cells in response to chemotherapy actually reduces the efficacy of the chemotherapy. Here, we take these data further and investigate the clinical relevance of circulating endothelial (progenitor) cells (CE(P)Cs) and modulatory cytokines in patients after chemotherapy with relation to progression-free and overall survival (PFS/OS). Patients treated with various chemotherapeutics were included. Blood sampling was performed at baseline, 4 hours, and 7 and 21 days after chemotherapy. The mononuclear cell fraction was analyzed for CE(P)C by FACS analysis. Plasma was analyzed for cytokines by ELISA or Luminex technique. CE(P)Cs were correlated with response and PFS/OS using Cox proportional hazard regression analysis. We measured CE(P)Cs and cytokines in 71 patients. Only patients treated with paclitaxel showed an immediate increase in endothelial progenitor cell 4 hours after start of treatment. These immediate changes did not correlate with response or survival. After 7 and 21 days of chemotherapy, a large and consistent increase in CE(P)C was found (P < .01), independent of the type of chemotherapy. Changes in CE(P)C levels at day 7 correlated with an increase in tumor volume after three cycles of chemotherapy and predicted PFS/OS, regardless of the tumor type or chemotherapy. These findings indicate that the late release of CE(P)C is a common phenomenon after chemotherapeutic treatment. The correlation with a clinical response and survival provides further support for the biologic relevance of these cells in patients' prognosis and stresses their possible use as a therapeutic target.
Introduction
In the past years, the concept of angiogenesis has evolved from a simple model of the formation of new blood vessels from the preexisting vasculature into a multifaceted process in which, beyond local activation and division of endothelial cells, bone marrow-derived endothelial progenitor cells (EPCs) contribute to neovascularization. It was postulated that EPCs are mobilized from the bone marrow into the circulation and subsequently home to sites of tumor neovascularization, where they differentiate into endothelial cells and contribute to angiogenesis [1–3]. However, controversy exists on the relative contribution of the EPC to the tumor vasculature, varying from less than 1% up to more than 50% [1,4–12]. Whereas the bone marrow does not seem to play an important role in supporting unperturbed tumor growth, an immediate and very effective release of progenitor cells is seen when the tumor or system is provoked by stress signals such as surgery or chemotherapy [14–16]. Recently, it was shown that EPCs egress the bone marrow and home to the tumor immediately after certain types of chemotherapy, predominantly paclitaxel. EPCs are mobilized from the bone marrow and home to sites of tumor neovascularization in response to various cytokines, such as stroma cell-derived factor-1 α (SDF-1α), matrix metalloproteinase-9, vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and granulocyte colony-stimulating factor (G-CSF) [1,2,13,15,18,21,35–39]. SDF-1α belongs to the chemokine family and binds to the CXCR-4 receptor. SDF-1α plays a key role in both the release and the homing process of EPCs; high concentrations in the bone marrow holds the stem cells in their niche. Various factors, including G-CSF, VEGF, and PlGF, deplete SDF-1α in the bone morrow and, subsequently, permit the egress of stem cells into the circulation. In turn, circulating stem cells, which express the SDF-1α receptor CXCR4, home toward SDF-1α. Within the tumor, the concentration of SDF-1α is increased in response to VEGF [38]. The acute mobilization after paclitaxel could be effectively inhibited by antibodies against the VEGF and CXCR-4 pathway, leading to enhanced antitumor efficacy particularly of these chemotherapeutics [15]. Besides EPC, mature circulating endothelial cells (CECs) are increased in the blood of cancer patients and correlate with angiogenesis and tumor volume [17–29]. CECs appear in the peripheral blood of cancer patients either because of release from the bone marrow, similar to EPC, or because of shedding from activated or damaged (tumor) vessels. Viable CECs may therefore reflect angiogenic activity, whereas apoptotic CECs may act as a surrogate marker for vascular damage [17,30]. These findings have provided new insight into the mechanism of tumor regrowth, resistance to chemotherapy, early recurrence, and metastasis formation during or after chemotherapy.
However, little is known of EPC and CEC kinetics during chemotherapy in humans. The bone marrow depression and recovery, generally seen after chemotherapy, might influence the temporal changes in CEC and EPC and might be of importance when considering these cells as potential markers for therapy. Here we investigated the temporal changes in EPC and CEC and modulatory cytokines during the first cycle of chemotherapy. We show that the increase in EPC and CEC levels 21 days after start chemotherapy by far exceeds the change immediately after chemotherapy. Furthermore, we provide evidence that the magnitude of the increase in CEC and EPC levels after chemotherapy correlates with response and survival. These findings suggest that continuous suppression of EPC and CEC is important for optimizing treatment efficacy.
Patients and Methods
Characterization of Study Patients and Protocol
Blood samples were prospectively collected from cancer patients receiving maximum tolerated dose chemotherapy in a thrice weekly schedule either as (neo)adjuvant chemotherapy or as chemotherapy for metastatic disease. All patients with previous chemotherapy or surgery within 4 weeks were excluded. Patients were recruited between July 2006 and October 2008 in UMC Utrecht Cancer Center; follow-up ended on March 2009. The study was approved by the institutional ethics committee, and written informed consent was obtained from all patients. Blood sampling was performed before the first cycle of chemotherapy, 4 hours and 7 days thereafter and immediately before the second cycle (day 21). Response evaluation was performed after the third cycle of chemotherapy according to RECIST criteria. Progression-free survival (PFS) was defined as time from start chemotherapy to date of tumor progression according to RECIST. Time from start chemotherapy to date of patients' death was determined as overall survival (OS).
Plasma and Mononuclear Cell Isolation
EDTA plasma and mononuclear cells (MNCs) were collected using an EDTA vacutainer (Becton Dickinson, Mountain View, CA) and a cell preparation tube with sodium citrate (Becton Dickinson). Isolation of the MNCs occurred by centrifuging the cell preparation tubes at 1600g at room temperature for 30 minutes. The MNCs were washed once in RPMI and stored in 10% DMSO at −80°C until analysis. EDTA tubes were centrifuged at 800g at 4°C for 15 minutes. Plasma was stored immediately at −80°C.
Enumeration of CEC/CEPs by Flow Cytometry
CECs and EPCs were quantified by flow cytometry analysis according to the protocol described by Shaked et al. [15]. Briefly, a four-color FACS analysis (FACSCalibur; BD Biosciences, Franklin Lakes, NJ) was performed on MNCs. Mature CECs were defined as negative for hematopoietic marker CD45 (PerCP; BD Biosciences) and positive for endothelial cell markers CD31 (fluorescein isothiocyanate; BD Biosciences) and CD146 (phycoerythrin; BD Biosciences). EPCs were defined as negative for CD45 and positive for CD31 and stem cell marker CD133 (allophycocyanin; BD Biosciences). MNCs were stained according to standard methods [31,32]. Corresponding isotypes were used to correct for nonspecific binding. MNCs from healthy volunteers, human microvascular endothelial cells, and NT2 cells were used as positive controls. Gating and analysis were performed following standard protocols [31,32]. A minimum of 400,000 events was counted, and CECs and EPCs were calculated to number of cells per milliliter of blood using the mononuclear cell count of the original sample. CEC/EPC levels were normalized to the baseline values and expressed as percent change to minimize variability due to a large variation in baseline CEC and EPC levels.
Cytokine Analysis
Plasma G-CSF and SDF-1α were determined by commercially available ELISAs (Quantikine; R&DSystems, Abingdon, UK). VEGF, PlGF, and FGF were quantified by commercially available Luminex (R&D Systems) following the manufacturer's instructions.
Statistical Methodology
Statistical comparisons were performed using the (paired) t test and Pearson correlation when data were normally distributed and nonparametric analysis of Mann-Whitney and Wilcoxon signed rank test otherwise. To associate changes in CEC and EPC levels (separately or combined) for PFS and OS, variables were both tested as continuous variables applying univariable Cox regression proportional hazard (PH) analysis and dichotomized for Kaplan-Meier estimation. Differences were evaluated using the log-rank test, and hazard ratios were obtained. All results were analyzed using SPSS 15.0 (SPSS, Inc, Chicago, IL) and GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). Error bars shown are SEM. P < 0.05 (two-sided) was considered significant.
Results
Patient Characteristics
Eighty-two patients with different malignancies, treated with various forms of chemotherapy, were enrolled in this study. Eleven patients did not finish the blood sampling owing to early withdrawal of informed consent, mainly because of the requirement of a second intravenous access. Seventy-one patients were evaluable for the analysis of changes in CEC, EPC, and growth factors after chemotherapy. Nine patients were treated with adjuvant chemotherapy and, by definition, not evaluable for response to treatment; nine more patients were not evaluable according to RECIST and were therefore excluded. Ultimately, 53 patients were evaluable to associate changes in CEC/EPC levels after chemotherapy with response to treatment. For 40 patients, the predictive value of the changes in CEC/EPC levels for PFS and OS was analyzed, as 13 patients receiving neoadjuvant chemotherapy were excluded from this analysis. Table 1 summarizes the demographics, follow-up, and tumor types of all patients.
Table 1.
Patients' Characteristics.
| Characteristic | |
| Sex, n (%) | |
| Male | 33 (47%) |
| Female | 38 (53%) |
| Age, years | |
| Median (range) | 62 (32–82) |
| Tumor type, n (%) | |
| Breast | 17 (24%) |
| Colorectal | 13 (18%) |
| Ovarian | 8 (11%) |
| Esophagus | 8 (11%) |
| Prostate | 6 (9%) |
| Head and neck | 5 (7%) |
| Sarcoma | 4 (6%) |
| Cervix | 4 (6%) |
| Other | 6 (8%) |
| Chemotherapy regimen, n (%) | |
| Taxane-based | 23 (32%) |
| Anthracyclin-based | 21 (30%) |
| 5-Fluorouracil-based | 11 (20%) |
| Platinum-based | 9 (13%) |
| Other | 4 (5%) |
| Response to chemotherapy after one cycle, n (%) | |
| Partial/complete remission | 21 (40%) |
| SD | 27 (51%) |
| PD | 5 (9%) |
| Follow-up, months | |
| Median follow-up (IQR) | 19 (12–28) |
| Median PFS (IQR) | 7 (4–11) |
| Median OS (IQR) | 14 (9–25) |
IQR indicates interquartile range.
Changes in CECs and EPCs during the First Cycle of Chemotherapy
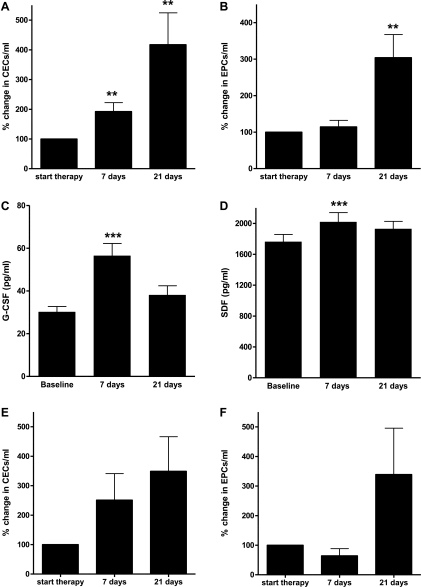
Overall, an increase was seen in CECs and EPCs after the first cycle of chemotherapy. Almost half of the patients (42%) already showed a moderate increase in CEC 4 hours after chemotherapy (mean, 176%; 95% confidence interval [CI], 76%–277%; NS). Regarding EPCs, as shown before [15], only patients treated with taxane-based chemotherapy showed an immediate increase of EPC (mean, 181%; 95% CI, 50%–311%; P < .05) compared with a mean decrease of 85% (95% CI, 48%–123%) in other chemotherapy groups (significant difference, P <.01).1 After 7 and 21 days, the increase of CECs and EPCs was substantially higher and consistently present after all types of chemotherapy. At day 7, CEC levels were increased to 192% (95% CI, 133%–252%; P < .01), and EPC levels were slightly increased to 114% (95% CI, 78%–151%; NS). On day 21, CEC and EPC levels were further increased to 418% (95% CI, 203%–632%; P < .01) and 304% (95% CI, 176%–1431%; P < .01; Figure 1, A and B), respectively.
Figure 1.
Kinetics of CECs, EPCs, and growth factors during the first cycle of chemotherapy. Overall, a significant increase was seen in CECs (A), EPCs (B), G-CSF (C), and SDF-1α (D) (n = 71, P < .01). The increase in CECs (E) and EPCs (F) seems also present in patients treated with adjuvant chemotherapy (n = 9, P = .08 and P = .18).
This increase in CECs and EPCs was also seen in patients receiving adjuvant chemotherapy, although to a lesser extent and not reaching significance. At 7 and 21 days after chemotherapy, a mean increase in CECs of 250% (95% CI, 8%–486%; P = .16) and 275% (95% CI, 21%–987%; P = .08), respectively, was seen. EPCs were slightly decreased after 7 days (mean, 63%; 95% CI, 8%–128%; P = .2) but increased after 21 days to 231% (95% CI, 61%–1243%; P = .18). The levels of CECs and EPCs at days 7 and 21 in patients treated with adjuvant chemotherapy did not significantly differ from changes seen in patient with advanced disease (P = .8; Figure 1, E and F).
Cytokine Changes during the First Cycle of Chemotherapy
To determine the possible causes of the increase in CECs and EPCs, various cytokines were quantified in the patients' plasma. Four hours after chemotherapy, a significant increase in plasma SDF-1α was found in all patients treated with taxane-based chemotherapy (n = 6; mean, 135%; 95% CI, 108%–212%; P = .01); in all other patients, levels remained stable. In all patients, 4 hours after chemotherapy, a significant increase in PlGF levels (P < .05) was observed. In contrast, VEGF and FGF levels showed an immediate decrease within 4 hours after chemotherapy (P < .05). G-CSF levels remained stable. Seven days after chemotherapy, there was a significant increase in SDF-1α (P < .01) and G-CSF (P < .001; Figure 1, C and D). These increases were seen after all chemotherapeutic regimens. No significant changes could be found for PlGF, FGF, and VEGF 7 days after chemotherapy. At day 21, all growth factors returned to baseline level. For all patients, a significant inverse correlation between SDF-1α and CECs (Pearson R = 0.5; P < .001) and EPCs (Pearson R = 0.5; P < .001) at baseline and at 7 and 21 days was noticed. There was a significant positive correlation between EPC and SDF-1α 4 hours after chemotherapy (Pearson R = 0.3; P < .001) but not between CECs and SDF-1α (Pearson R = 0.09, P = .4). There was no correlation between other cytokines and CECs or EPCs at any time point.
Changes in CEC and EPC Levels after Chemotherapy Associated with Response, PFS, and OS
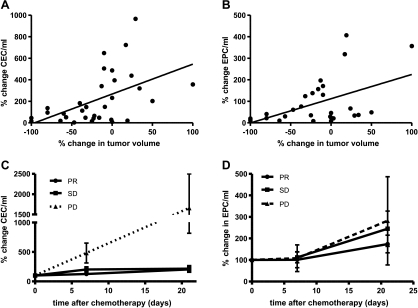
A significant correlation was found between changes in CEC and EPC 7 days after chemotherapy and percent tumor shrinkage according to RECIST after three cycles of chemotherapy (Pearson R = 0.5 and 0.4, P < .01, respectively). Changes in CEC or EPC after 4 hours and 21 days did not correlate with response to chemotherapy (Figure 2). The kinetics of the CEC 7 and 21 days after the first cycle of chemotherapy differed significantly between patients with partial remission/stable disease (PR/SD) and patients with progressive disease (PD) after three cycles of chemotherapy (P < .05). In patients with PD, a large increase in CEC was seen after both 7 and 21 days (mean increase, 386% and 1658%, respectively). In patients with PR/SD, a mean increase in CECs of 169% was found at day 7 (P < .05) and 210% after 21 days (P < .01). EPC levels did not differ significantly between patients with PD compared with patients with PR/SD.
Figure 2.
Significant correlation between the changes in CECs (A) and EPCs (B) 7 days after chemotherapy and the percent tumor shrinkage according to RECIST. n = 53, Pearson R = 0.5 and 0.4, respectively (P < .01). CEC levels, but not EPC levels, after 7 and 21 days discriminated between patients with PD (n = 5) and SD/PR (P <.05) (C and D).
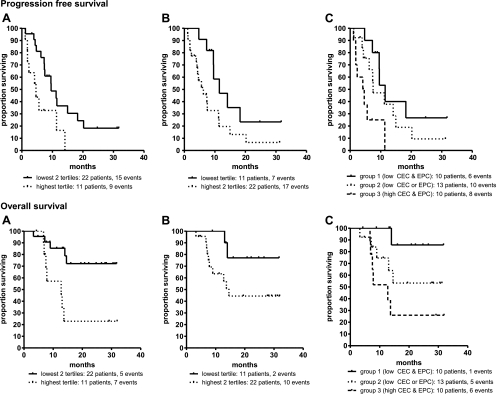
Subsequently, with univariable Cox PH regression, we determined whether CEC and EPC levels, at baseline and/or consecutive time points after chemotherapy could predict PFS and OS. At baseline and 4 hours after chemotherapy, no association between PFS/OS and CEC/EPC was observed (P > .15; data not shown). Furthermore, tumor type or chemotherapy regimen could not predict PFS or OS in univariable analysis (P > .5; data not shown). Interestingly, a large increase of CEC levels 7 days after chemotherapy showed a significant association with poor PFS (P = .007) and a trend toward poor OS (P = .09). Equally, at day 21, a large change in CEC levels was correlated with poor PFS and OS as well (P = .002 and P = .008, respectively). However, we focused on day 7 because this is the earliest time point that could be used as predictor for PFS/OS. Tertiles were chosen as objective cutoff points to dichotomize variables for Kaplan-Meier analysis. For CEC levels, the 67th tertile was chosen as cutoff to divide patients into two risk groups with distinct survival rates. Seven days after chemotherapy, a change in CEC levels of less than 193% (67th tertile) was associated with a significantly prolonged PFS (P = .01) and OS (P = .006; Figure 3, A and B, and Table 2). In univariate analysis, EPC levels were not significantly associated with PFS or OS at days 7 and 21. However, because both CECs and EPCs may play an important role in tumor progression, we investigated whether combining CEC and EPC levels in one risk model could improve the predictive accuracy. Therefore, we dichotomized the EPC levels at day 7 using a similar cutoff point as for CEC (33rd tertile). After dichotomization, EPC levels at day 7 were (borderline) significantly associated with PFS and OS (P = .046 and P = .06, respectively; Figure 3B and Table 2). Subsequently, patients were stratified in three risk groups with distinct survival rates based on CEC or EPC levels above or below their cutoff point. A favorable group without risk factors (low CEC and low EPC levels), an intermediate group with one risk factor (either low CEC levels or low EPC levels) and a poor risk group with both high CEC and high EPC levels were defined. This combination revealed an accurate risk model for PFS and OS (P = .006 and P = .02, respectively). For PFS, median survival times were 16, 11, and 5 months, respectively, for the favorable, intermediate, and poor risk groups. OS was 13 months for the poor risk group. For the favorable and intermediate risk groups, median survival times were not reached at the end of follow-up (Figure 3C and Table 2).
Figure 3.
PFS (upper panels) and OS (lower panels) of 33 patients by CECs (A) and EPCs (B) levels 7 days after chemotherapy individually and combined into three groups (C): (1) low CEC and low EPC levels, (2) either low CEC or low EPC levels, and (3) both high CEC and EPC levels.
Table 2.
Changes in CEC and EPC Levels 7 Days after Chemotherapy Associated with PFS and OS.
| Cut Point Used | Median PFS, months | Log-rank P | HR (95% CI) | Median OS, months | Log-rank P | HR (95% CI) | |
| CEC day 7 | |||||||
| Lowest two tertiles* | 193% | 9.6 | 0.01 | - | Not reached | 0.006 | - |
| Highest tertile | 4.6 | 2.9 (1.2–7.0) | 12.7 | 4.5 (1.4–14.6) | |||
| EPC day 7 | |||||||
| Lowest tertile* | 30% | 11.5 | 0.046 | - | Not reached | 0.06 | - |
| Highest two tertiles | 6.2 | 2.4 (1.0–5.9) | 14.6 | 3.8 (0.8–17.6) | |||
| EPC + CEC day 7 | |||||||
| Favorable* | Low EPC and CEC | 11 | 0.006 | - | Not reached | 0.02 | - |
| Intermediate | Either low EPC or low CEC | 7 | 1.7 (0.6–4.7) | Not reached | 5.3 (0.6–45.1) | ||
| Poor | High EPC and CEC | 4 | 5.5 (1.7–17.4) | 12.8 | 11.7 (1.4–98.4) |
HR indicates hazard ratio.
Risk group used as reference.
Discussion
Here, we showed that CECs and EPCs were increased in the blood of cancer patients after treatment with various chemotherapeutic regimens. This increase already started a few hours after chemotherapy [15], but the changes after 7 and 21 days after the start of chemotherapy exceeded the change immediately after chemotherapy and was not limited to specific types of chemotherapy. The increase in CECs and EPCs is seemingly unrelated to the presence of a tumor because adjuvant chemotherapy showed similar kinetics. This suggests that EPC and CEC release after chemotherapy is part of a reactive host response independent of tumor type and chemotherapy regimen. This response may very well be an important factor in determining the outcome of patients because EPC and CEC have been found to stimulate tumor growth, metastasis formation, and limit chemotherapeutic efficacy by prevention of necrosis [3,15]. Here, we showed that the magnitude of the increase of CECs and EPCs after chemotherapy was associated not only with response to chemotherapy after three cycles but also with PFS and OS. Although this is the first prospective analysis of the correlation between the changes in CECs/EPCs during the first cycle of MTD chemotherapy and response and survival, the correlation between CECs/EPCs and prognosis of patients is supported by others [21,32–34].
The chemotherapy-induced host response is likely to be mediated by the up-regulation of various cytokines that are known to be involved in progenitor cell recruitment, such as SDF-1α, VEGF, PlGF, and G-CSF [1,2,15,18,21,35–39]. Especially SDF-1α is known for its key role in both the release and the homing of EPC [38]. Previously, it was shown that certain types of chemotherapy can cause an acute up-regulation of SDF-1α, VEGF, and G-CSF [14,15,21,35,40]. In addition, inhibition of SDF-1α by neutralizing antibodies could inhibit the release of EPC and enhanced the antitumor efficacy of the chemotherapy [15]. We found a significant increase in SDF-1α and G-CSF 7 days after chemotherapy. The very consistent increase in G-CSF is not surprising given the important role in hematopoiesis and the recovery of the bone marrowafter chemotherapy. No correlation could be found between G-CSF and CEC or EPC. However, a significant correlation between CEC and EPC with SDF-1α was shown, suggestive for a role for SDF-1α in the recruitment and/or homing of these cells. In contrast, it should be noted that plasma SDF-1α levels already returned to baseline levels after 21 days, whereas the CECs and EPCs continued to increase. This may suggest that other growth factors are involved in the continued release of CECs and CEPs.
Preclinical evidence shows that antiangiogenic therapy could blunt the release of EPCs by (chemo)therapy [14,15]. Whether this is the case in patients is presently unclear; however, it has been shown that metronomic chemotherapy does inhibit the release of EPCs in patients [41]. Perhaps this inhibition of CECs and EPCs release provides an additional explanation for the synergistic efficacy of bevacizumab and chemotherapy. Conceptually, these findings point to an array of new therapeutic strategies by combining chemotherapy with agents capable of inhibiting the release of progenitor cells, such as SDF-1α/CXCR-4 antagonists, to enhance the therapeutic potential of conventional chemotherapy.
A potential limitation of this study is the heterogeneous population of chemotherapy and cancer types. However, we intended to test whether the host bone marrow response was a specific effect or a more generalized effect independent of the type of chemotherapy and found evidence for the latter. Furthermore, certain types of chemotherapy with a high response rate in certain forms of cancer could be a confounder in the analysis. Although, in univariate analysis, tumor type and chemotherapy regimen were both not predictive of survival, suggesting that our results are not influenced by a specific subgroup. There is still controversy on the definition of an EPC and CEC. No unique identifying markers have yet been reported, and functional characterization of the rare putative populations based on FACS phenotypes will be difficult to realize for a large data set. Here, we did not distinguish between viable and apoptotic CECs and EPCs. The true biologic meaning of the cells we quantified based on the selected phenotypes needs to be evaluated. However, using the selected phenotypical definitions, the data presented here support a general role of CECs and EPCs in the prognosis of cancer patients after chemotherapy and as a potential target for therapy.
In conclusion, we showed that chemotherapy evokes both an acute and a late systemic host response composed of the release of CECs, EPCs, growth factors, and chemokines. The extent of this release correlates with the response to therapy and the prognosis of patients. These findings provide new opportunities for further enhancing chemotherapy efficacy by inhibition of the released factors by combination treatment. Furthermore, this study paves the way for a prospective study in a uniformly treated patient population to determine whether CECs and EPCs might be used as a very early predictor of response to therapy.
Acknowledgments
The authors thank all medical doctors, research nurses, technicians, and students whowere involved in patient management and blood processing.
Abbreviations
- CEC
circulating endothelial cell
- EPC
endothelial progenitor cell
- MNC
mononuclear cell
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial remission
- SD
stable disease
Footnotes
Parts of these results were presented as oral presentation at the ASCO Annual Meeting 2009 and the Keystone Angiogenesis Conference 2009.
The results from four of six patients were already published in Shaked et al. 15.
References
- 1.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der ZR, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 4.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 5.Davidoff AM, Ng CY, Brown P, Leary MA, Spurbeck WW, Zhou J, Horwitz E, Vanin EF, Nienhuis AW. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin Cancer Res. 2001;7:2870–2879. [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 10.Duda DG, Cohen KS, Kozin SV, Perentes JY, Fukumura D, Scadden DT, Jain RK. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–2776. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, Manova K, Mittal V, Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 12.Yu D, Sun X, Qiu Y, Zhou J, Wu Y, Zhuang L, Chen J, Ding Y. Identification and clinical significance of mobilized endothelial progenitor cells in tumor vasculogenesis of hepatocellular carcinoma. Clin Cancer Res. 2007;13:3814–3824. doi: 10.1158/1078-0432.CCR-06-2594. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 15.Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, Daenen LG, Man S, Xu P, Emmenegger U, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farace F, Massard C, Borghi E, Bidart JM, Soria JC. Vascular disrupting therapy-induced mobilization of circulating endothelial progenitor cells. Ann Oncol. 2007;18:1421–1422. doi: 10.1093/annonc/mdm367. [DOI] [PubMed] [Google Scholar]
- 17.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 18.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- 19.Beaudry P, Force J, Naumov GN, Wang A, Baker CH, Ryan A, Soker S, Johnson BE, Folkman J, Heymach JV. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;1:3514–3522. doi: 10.1158/1078-0432.CCR-04-2271. [DOI] [PubMed] [Google Scholar]
- 20.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Furstenberger G, von MR, Lucas R, Thurlimann B, Senn HJ, Hamacher J, Boneberg EM. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goon PKY, Lip GYH, Stonelake PS, Blann AD. Circulating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia. 2009;11:771–779. doi: 10.1593/neo.09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell'Agnola C, Gobbi A, Martinelli G, Bertolini F. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 2001;61:4341–4344. [PubMed] [Google Scholar]
- 24.Schuch G, Heymach JV, Nomi M, Machluf M, Force J, Atala A, Eder JP, Jr, Folkman J, Soker S. Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res. 2003;63:8345–8350. [PubMed] [Google Scholar]
- 25.Zhang H, Vakil V, Braunstein M, Smith EL, Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U, et al. Circulating endothelial progenitor cells in multiple myeloma: implications and significance. Blood. 2005;105:3286–3294. doi: 10.1182/blood-2004-06-2101. [DOI] [PubMed] [Google Scholar]
- 26.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett CG, Boucher Y, di TE, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett CG, Boucher Y, Duda DG, di TE, Munn LL, Tong RT, Kozin SV, Petit L, Jain RK, Chung DC, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 29.Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- 30.Chang YS, di TE, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, et al. Circulating endothelial cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 34.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 35.Nathan PD, Judson I, Padhani A, Harris A, Carden CP, Smythe J, Collins D, Leach M, Walicke P, Rustin GJ. A phase I study of combretastatin A4 phosphate (CA4P) and bevacizumab in subjects with advanced solid tumors. J Clin Oncol. 2008;26(suppl) Abstract 3550. [Google Scholar]
- 36.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, Young PP. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 38.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Renzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 40.Lemoli RM, Catani L, Talarico S, Loggi E, Gramenzi A, Baccarani U, Fogli M, Grazi GL, Aluigi M, Marzocchi G, et al. Mobilization of bone marrow-derived hematopoietic and endothelial stem cells after orthotopic liver transplantation and liver resection. Stem Cells. 2006;24:2817–2825. doi: 10.1634/stemcells.2006-0333. [DOI] [PubMed] [Google Scholar]
- 41.Stoelting S, Trefzer T, Kisro J, Steinke A, Wagner T, Peters SO. Low-dose oral metronomic chemotherapy prevents mobilization of endothelial progenitor cells into the blood of cancer patients. In Vivo. 2008;22:831–836. [PubMed] [Google Scholar]