Abstract
Across the boreal forest of North America, lynx populations undergo 10-year cycles. Analysis of 21 time series from 1821 to the present demonstrates that these fluctuations are generated by nonlinear processes with regulatory delays. Trophic interactions between lynx and hares cause delayed density-dependent regulation of lynx population growth. The nonlinearity, in contrast, appears to arise from phase dependencies in hunting success by lynx through the cycle. Using a combined approach of empirical, statistical, and mathematical modeling, we highlight how shifts in trophic interactions between the lynx and the hare generate the nonlinear process primarily by shifting functional response curves during the increase and the decrease phases.
Keywords: statistical modeling, nonlinearity, threshold autoregressive modeling, population dynamics
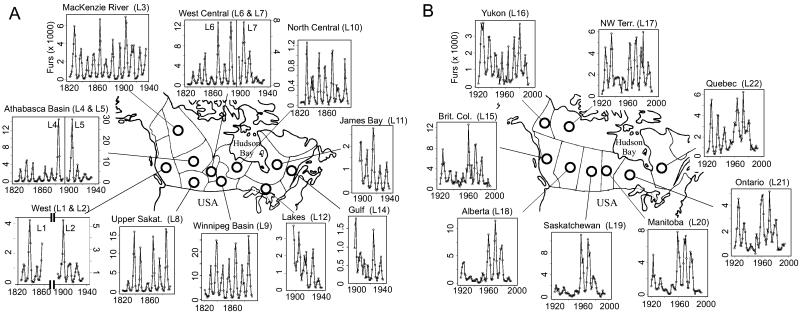
Periodic fluctuations in fur returns of the Canadian lynx (Lynx canadensis) have influenced ecological theory profoundly (1–29): these data have been used to examine concepts such as “the balance of nature,” predator–prey fluctuations, food web dynamics, chaos, and regional and community synchronization. The enigma of the lynx cycle has challenged observational and experimental (6–13), mathematical (5, 14–18, 26, 27), and statistical (25, 28, 29) ecology. Using a combined approach of empirical, statistical, and mathematical modeling (30–35), we here provide a comprehensive analysis of the lynx fur return data from the Hudson Bay Company (1) and more recent data (36, 37) from all of boreal Canada (Fig. 1 A and B). The resulting patterns are interpreted on the basis of recent experimental field studies on the snowshoe hare (Lepus americanus) and the lynx.
Figure 1.
The time series of fur returns of lynx (given on arithmetic scale) from the Canadian boreal forest (see Table 1). (A) The series L1-L12 and L14 represent the pelt counts between 1820 and 1940, as compiled by Elton and Nicholson (1). The borders on the map represent the demarcations of the regions. (B) The series L15-L22 represent data between 1920 and 1994 summarized by Statistics Canada (36, 37) for which the demarcation is given by the Canadian provincial borders. For the modern series (L15-L22), there were occasional zeros. We added one to the modern series before log-transformation.
Earlier statistical analyses of the Canadian lynx cycle have focused primarily on the MacKenzie River time series (series L3 of this paper; compare in Table 1). This series has been studied by using a variety of parametric and nonparametric models, including testing for the presence of nonlinearity, the determination of order and presence of significant lags, nonlinear prediction, and noisy chaos. It has, therefore, formed a benchmark data set for time series modeling (39, 45–57). Detailed scrutiny of a threshold autoregressive model has revealed a particularly good statistical fit to L3 (39, 49, 56–58).
Table 1.
Nonlinear autoregressive structure of old and modern time series on lynx in the Canadian boreal forest
| No. remaining | Time series | Years | Threshold, θ
|
||||
|---|---|---|---|---|---|---|---|
| NAIC
|
dopt optimal |
Overall θ d’s | Lag-2 | ||||
| d = 1 | d = 2 | ||||||
| L1 | West | 1825–1856 | −0.493 | −0.606 | 2 | 2 | 5.64 (0.56) |
| L2† | West | 1897–1934 | −1.789 | −1.892 | 2 | 2 | 6.56 (0.28) |
| L3* | MacKenzie River | 1821–1934 | −1.416 | −1.472 | 2 | 2 | 7.62 (0.29) |
| L4 | Athabasca Basin | 1821–1891 | −0.367 | −0.198 | 1 | 1 | 4.98 (0.67) |
| L5† | Athabasca Basin | 1897–1934 | −1.455 | −1.356 | 1 | 1 | 7.54 (0.45) |
| L6 | West Central | 1821–1891 | −1.621 | −1.717 | 2 | 2 | 6.18 (0.43) |
| L7† | West Central | 1897–1934 | −1.360 | −1.127 | 1 | 1 | 7.02 (0.36) |
| L8 | Upper Saskatchewan | 1821–1891 | −0.602 | −0.639 | 2 | 2 | 6.19 (0.75) |
| L9 | Winnipeg Basin | 1821–1891 | −1.656 | −1.709 | 2 | 2 | 8.26 (0.36) |
| L10 | North Central | 1821–1891 | −1.231 | −0.911 | 1 | 1 | 5.00 (0.33) |
| L11† | James Bay | 1895–1939 | −2.190 | −2.143 | 1 | 2 (1) | 6.55 (0.41) |
| L12† | Lakes | 1897–1939 | −1.890 | −1.978 | 2 | 2 | 6.59 (0.26) |
| L13† | James Bay and Lakes | 1897–1939 | −2.291 | −2.364 | 2 | 2 | 6.68 (0.32) |
| L14† | Gulf | 1897–1939 | −1.334 | −1.292 | 1 | 1 | 6.22 (0.40) |
| Overall weighted estimates for the Hudson Bay series§ | 6.646 (0.10) | ||||||
| Deduced phase dependency: β1,0 ≤ β2,0 β1,1 ≤ β2,1 β1,2 > β2,2 | |||||||
| Empirical Bayesian estimates for the Hudson Bay series¶ | |||||||
| Deduced phase dependency: β1,1 ≤ β2,1 β1,2 > β2,2 | |||||||
| L15 | British Columbia | 1920–1994 | −1.330 | −1.417 | 2 | 2 | 7.38 (0.26) |
| L16 | Yukon Territory | 1920–1994 | −1.084 | −1.188 | 2 | 2 | 7.25 (0.23) |
| L17 | Northwest Territory | 1920–1994 | −1.462 | −1.460 | 1 | 2 (1) | 7.13 (0.33) |
| L18 | Alberta | 1920–1994 | −1.161 | −1.134 | 1 | 2 (1) | 8.01 (0.43) |
| L19 | Saskatchewan | 1920–1994 | −0.646 | −0.697 | 2 | 2 | 6.51 (0.45) |
| L20 | Manitoba | 1920–1994 | −1.105 | −1.085 | 1 | 2 (1) | 6.39 (0.51) |
| L21 | Ontario | 1920–1994 | −1.960 | −1.928 | 1 | 2 (1) | 6.65 (0.29) |
| L22 | Quebec | 1920–1994 | −2.390 | −2.385 | 1 | 2 (1) | 7.19 (0.27) |
| Overall weighted estimates for the modern series§ | 7.128 (0.11) | ||||||
| Deduced phase dependency: β1,0 ≈ β2,0 β1,1 < β2,1 β1,2 > β2,2 | |||||||
| Empirical Bayesian estimates for the modern series¶ | |||||||
| Deduced phase dependency: β1,1 < β2,1 β1,2 > β2,2 | |||||||
| Grand total weighted estimates for all series§ | 6.858 (0.07) | ||||||
| Deduced phase dependency: β1,0 Η β2,0 β1,1 < β2,1 β1,2 > β2,2 | |||||||
| Empirical Bayesian estimates for all series¶ | |||||||
| Deduced phase dependency: β1,1 < β2,1 β1,2 > β2,2 | |||||||
| The lower regime of the SETAR model, increase phase
|
The upper regime of the SETAR model, decline phase
|
Any trend | ||||
|---|---|---|---|---|---|---|
| β1,0 (±SE) | β1,1 (±SE), direct DD | β1,2 (±SE), delayed DD | β2,0 (±SE) | β2,1 (±SE) direct DD | β2,2 (±SE) delayed DD | |
| 1.30 (1.07) | 1.02 (0.17) | −0.20 (0.30) | 5.83 (2.27) | 1.16 (0.25) | −1.04 (0.36) | No |
| 1.03 (1.09) | 0.91 (0.16) | −0.03 (0.24) | 2.54 (1.37) | 1.71 (0.18) | −1.08 (0.26) | No |
| 1.35 (0.31) | 1.27 (0.06) | −0.43 (0.07) | 2.68 (2.37) | 1.60 (0.13) | −1.01 (0.31) | No |
| 3.10 (2.30) | 0.53 (0.30) | −0.05 (0.44) | 3.52 (0.64) | 1.33 (0.10) | −0.86 (0.12) | No |
| 4.10 (1.08) | 1.36 (0.16) | −0.94 (0.18) | 5.39 (1.39) | 1.34 (0.16) | −0.99 (0.22) | No |
| 1.13 (0.62) | 1.28 (0.08) | −0.35 (0.13) | 1.71 (0.59) | 1.52 (0.08) | −0.81 (0.10) | No |
| 4.59 (1.73) | 0.80 (0.25) | −0.51 (0.26) | 3.97 (2.00) | 1.31 (0.19) | −0.83 (0.28) | No |
| −0.05 (1.36) | 1.08 (0.17) | 0.10 (0.92) | 2.90 (0.81) | 1.40 (0.10) | −0.81 (0.13) | No |
| 2.42 (0.80) | 1.37 (0.09) | −0.64 (0.13) | 1.91 (1.53) | 1.42 (0.13) | −0.67 (0.19) | No |
| 2.98 (1.31) | 0.76 (0.19) | −0.31 (0.25) | 0.48 (0.83) | 1.44 (0.11) | −0.59 (0.15) | No |
| 1.65 (0.46) | 1.45 (0.09) | −0.70 (0.11) | 1.60 (1.88) | 1.44 (0.18) | −0.73 (0.30) | No |
| 2.79 (0.95) | 1.29 (0.16) | −0.75 (0.20) | 4.79 (1.86) | 1.33 (0.19) | −0.99 (0.28) | No |
| 3.30 (1.02) | 1.30 (0.12) | −0.80 (0.18) | 3.68 (1.06) | 1.56 (0.14) | −1.05 (0.17) | No |
| 0.97 (1.07) | 1.05 (0.18) | −0.23 (0.23) | 2.09 (2.44) | 0.92 (0.24) | −0.31 (0.40) | No |
| 1.70 (0.19) | 1.24 (0.03) | −0.50 (0.04) | 2.63 (0.29) | 1.43 (0.04) | −0.82 (0.05) | |
| 1.25 (0.11) | −0.54 (0.13) | 1.40 (0.05) | −0.79 (0.07) | |||
| −1.15 (2.21) | 0.17 (0.17) | 0.76 (0.29) | 1.81 (1.28) | 0.96 (0.15) | −0.20 (0.20) | No |
| 3.20 (1.23) | 0.79 (0.16) | −0.26 (0.21) | 2.19 (1.82) | 1.25 (0.13) | −0.58 (0.26) | No |
| 6.32 (3.58) | 0.53 (0.28) | −0.42 (0.43) | 4.29 (0.98) | 1.07 (0.12) | −0.63 (0.15) | No |
| 1.97 (1.10) | 0.88 (0.16) | −0.14 (0.18) | 3.52 (1.18) | 1.45 (0.13) | −0.86 (0.17) | No |
| 4.90 (1.66) | 0.27 (0.22) | −0.11 (0.23) | 1.75 (0.63) | 1.27 (0.13) | −0.51 (0.14) | No |
| 2.86 (1.32) | 0.76 (0.22) | −0.27 (0.24) | 2.09 (0.60) | 1.29 (0.12) | −0.58 (0.13) | No |
| 5.76 (3.35) | 0.51 (0.28) | −0.40 (0.41) | 2.88 (0.67) | 1.26 (0.11) | −0.65 (0.13) | (<0.10) |
| 2.67 (1.33) | 1.31 (0.16) | −0.70 (0.22) | 4.13 (0.90) | 1.30 (0.11) | −0.83 (0.14) | No |
| 2.80 (0.54) | 0.75 (0.07) | −0.20 (0.09) | 2.66 (0.30) | 1.24 (0.04) | −0.63 (0.05) | |
| 0.92 (0.17) | −0.15 (0.12) | 1.21 (0.13) | −0.47 (0.20) | |||
| 1.83 (0.18) | 1.15 (0.03) | −0.45 (0.04) | 2.64 (0.20) | 1.36 (0.03) | −0.73 (0.04) | |
| 1.10 (0.20) | −0.37 (0.22) | 1.32 (0.12) | −0.66 (0.17) | |||
Assuming a SETAR(2;2,2) model, the NAIC [NAIC being AIC = −2ln(max likelihood) + 2(number of parameters) normalized by the effective number of observations] values for d = 1 and d = 2 are given together with the optimal d value, dopt, defined as the one minimizing the NAIC over d = 1 and d = 2; in cases that the NAIC values for d = 1 and d = 2 are insignificantly different {defined by [(NAIC(d−)−NAIC(dopt)]/[−NAIC(dopt)] < 0.025}, where d− is the nonoptimal d, both 1 and 2 are listed; d = 2 is given in bold because this is the overall most appropriate delay. The estimated parameters in the SETAR model (Eq. 1) for the lynx time series from Canada are provided by Elton and Nicholson (1) [L1-L14] and the time series provided by Dominion Bureau of Statistics and Statistics Canada (36, 37) [L15-L22]. Analyses are based on the original and not detrended data, for which the thresholds are estimated on the basis of the NAIC criterion; the same conclusions emerge if detrended data are analyzed. Detrending was done in S-plus by subtracting a fitted cubic B-spline with 4 degrees of freedom (38). The optimal threshold, θ, assuming a lag (d) equal to 2, was determined by NAIC (Ref. 39; p. 379); the threshold estimate is given together with the estimated bootstrap SE (40, 41). The column “Any trend” summarizes the results of testing the null hypothesis of a SETAR(2;2,2) model against the alternative of a “SETAR(2;2,2) + linear time trend” model. “No” indicates a rejection of the alternative at 5% level and hence suggests the adequacy of a SETAR model. The test is implemented via the method of Lagrange multiplier, also known as the score method (42). The overall weighted estimates were calculated as weighted means, Σ(μtwi)/Σwi, where μi are the estimated parameters for series i and wi = 1/(SEi)2. The overall SE is given as (1/Σwi)1/2. The empirical Bayes estimation (43) is done via the EM-algorithm (44). All series first are normalized (linearly) so that the 30 (70) percentiles become 0 (1). Only the mean lag-1 and lag-2 coefficient estimates are given in the table because the other parameters are not invariant under the scale change.
SE, standard error; NAIC, normalized kaike information criterion; DD, density dependent.
This series was analyzed by Tong (39).
Series has been interpolated for the missing observation in year 1914.
This combined series was studied by Stenseth et al. (28, 29) because this most closely corresponded to the snowshoe hare series they studied; this combined series is included here for comparative reasons but is excluded from the both sets of pooled estimates.
The weighted estimates are computed under the framework that the SETAR coefficients are the same for the all of the series in a particular panel of lynx data. The numbers in parentheses are the standard errors of the weighted estimates.
The empirical Bayesian estimates are computed based on a random coefficient model that for each series the SETAR coefficients are drawn from a super-population. The numbers in parentheses are the corresponding (between-region) standard deviations of the super-population (see main text).
We build on >40 years of statistical modeling of L3 to bridge the gap between statistical modeling of lynx data, mathematical modeling of the hare–lynx dynamics, and field studies on hares and lynx. We do so by synthesizing available time series data on the Canadian lynx within a nonlinear autoregressive statistical framework that is interpreted on the basis of an ecological model (Table 1). The statistical pattern engraved in these series is consistent with detailed experimental data from the 10-year “Kluane Boreal Forest Ecosystem Project” in the Yukon (10–13). This enables us to elaborate on the strengths and the functional forms of the interactions in the processes underlying the cycle. Earlier, Royama (25) discussed the monitoring data depicted in Fig. 1. Here, we substantially extend his analysis both by using more efficient statistical methods [the Threshold Auto-Regressive modeling approach (39)] and by interpreting the structure of the time series on the basis of new experimental findings. Statistical checks (59) on the fitted residuals of Royama’s model reveal that they cannot be seen as white noise. Thus, Royama’s original reservation about his model was well founded.
The Data
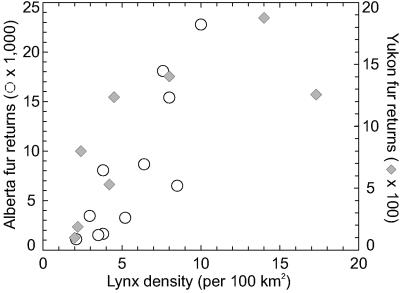
Our core data (Fig. 1) come from the Hudson Bay Company (1) and Statistics Canada (36, 37). They represent good proxies for the actual population abundances (Fig. 2). We estimatekthat, in peak years, trappers caught ≈5% of the total lynx population across Canada and, in trough years, ≈9%.
Figure 2.
The relationship between lynx fur returns and lynx density estimates obtained from intensive field studies in Alberta [1964–74; from Keith and colleague (7–9)] and in Yukon [1987–94; from O’Donoghue et al. (60, 61)]. Provincial fur returns from Statistics Canada (37).
Statistical Modeling
Previous statistical analyses have demonstrated that L3 (on a logarithmic scalel)may be adequately fitted by a nonlinear autoregressive process of order two (28, 29, 42–46, 49, 51). The nonlinearity can be modeled as a threshold process of the form (42):
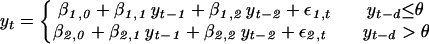 |
1 |
where yt is the log-transformed abundance of the lynx at year t [i.e., yt =ln(Yt)], βi,j are statistical parameters that determine the dynamic properties of the system (i = 1 and 2 correspond to the lower and the upper regimes of the model; j = 0, 1, and 2 correspond to the constant term and the lag-1 and lag-2 coefficients, respectively), θ is the threshold applicable to the density d years earlier, and ɛi,t are noise-terms with independent and normal distributions, N(0, σi2); this model is a SETAR(2;2, 2) model (39, 49–57), i.e., a self-exciting threshold autoregressive model. [The notation SETAR(m;p1,p2,… ,pm) stands for a SETAR model with m regimes, and the order of the jth regime equals pj]. As a form of local function approximation, SETAR modeling is a computationally efficient way of approximating complex and nonlinear structures, and its statistical properties are well documented (28, 29, 39). Several nonlinear ecological models can be approximated by a SETAR model on log-scale (64) and are characterized by the entire spectrum of dynamic behavior commonly found in ecological models (65).
The optimal thresholding lag, dopt, can be investigated statistically; Table 1 shows that dopt = 2 is the most parsimonious choice. The lag-2 is consistent with 16 of 21 series whereas lag-1 is consistent for 11 of 21 series. Furthermore, dopt = 2 is in agreement with the earlier reported approximate additive structure for these time series (28, 29). Analyzing the residuals demonstrates that a SETAR(2;2, 2) model provides an appropriate description of the datam.
Adopting the approach of empirical Bayes (43), and assuming a random coefficient model that specifies that the SETAR coefficients of each series are drawn randomly from a super-populationn, we may pool information across all of the older series (L1-L14), all of the modern series (L15-L22), and all series altogether (L1-L22). The empirical Bayes estimates are generally close to those obtained from simple weighted averaging (Table 1). However, for the modern series, there are some differences between the empirical Bayes estimates and the weighted average estimates of the lag-1 coefficient in the lower regime and the lag-2 coefficient in the upper regime. The between-region standard deviations of the upper regime of the older series are quite close to zero, suggesting common structure in that part of the modelo.
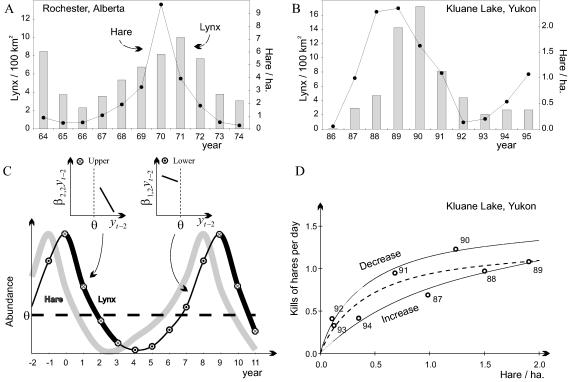
The ecological machinery behind the threshold structure of the lynx time series may be sought in the hare–lynx interaction. Fig. 3 depicts the hare–lynx cycle at Rochester, Alberta (Fig. 3A) and at Kluane Lake, Yukon (Fig. 3B); a more stylized hare–lynx cycle together with the threshold level separating the two regimes also is shown (Fig. 3C). The lower regime applies during the lynx increase, and the upper regime applies during the lynx (and hare) decrease. As a result, we call the lower regime the “increase phase” and the upper regime the “decline phase.”
Figure 3.
The pattern of fluctuation in the snowshoe hare (L. americanus Erxleben, 1777) and the Canadian lynx (L. canadensis Kerr, 1792) as recorded at Rochester, Alberta, from 1964 to 1974 (7–9) (A) and as recorded at Kluane Lake, Yukon, from 1986 to 1995 (10–13) (B). (C) The idealized pattern from the data in A and B with a schematic depiction of the phase dependency in β2,2yt−2 (see text) resulting from the predator–prey interaction. (D) The functional response curve of lynx feeding on snowshoe hares for Kluane Lake from O’Donoghue (60) and O’Donoghue et al. (66). Increase years (1987, 1988, 1989, and 1994) have a different functional response than decrease years (1990, 1991, 1992, and 1993), thus explaining the phase dependency in this system: the log likelihood increases by 9.19 when fitting two curves as compared with one common curve (the critical value being 3.00, i.e., 0.5*χ20.95(2); see ref. 67). The common model for the functional response curve {kill rate = s [hare density]/[1 + h (hare density)]} is given by the parameters s = 3.11 (±0.28) and h = 2.35 (±0.29) (RSS = 0.1937; 6 d.f.); for the decrease phase, the corresponding estimates are 3.92 (±0.38) and 2.47 (±0.36) (RSS = 0.01218; 2 d.f.) and, for the increase phase, they are 1.26 (±0.16) and 0.66 (±0.16) (RSS = 0.0073; 2 d.f.).
Fitting the SETAR model to all time series, we obtain (Table 1): (i) β1,0, measuring the average log-transformed abundance in the lower regime, is marginally smaller than or equal to β2,0; (ii) β1,1, measuring the lag-1 coefficient [sometimes called the coefficient of statistical direct density dependence (25)], is smaller than or equal to β2,1; and (iii) β1,2 is consistently greater than β2,2 (both negative). In absolute value, this implies a significantly weaker lag-2 effect (statistical delayed density dependence) during the increase phase than during the decrease phase. The βi,2yt-2-functions for regimes 1 and 2 are depicted in Fig. 3C. This is the component in which the major phase shift is located.
The full model (Eq. 1) is a density-dependent model because the lag-1 coefficient βi,1 and the lag-2 coefficient βi,2 are significantly different from 1 and 0, respectively (68–71). The fitted model is phase-dependent because the pattern of density dependence (β1,. and β2,.) varies across the cycle. The consistency across all panels of time series is conspicuous and indicates that the underlying ecological processes change through the cycle. This may be taken as a demonstration, based on time series analysis, of phase dependency in the population dynamics of the lynx; another form of phase dependency earlier has been demonstrated in the Norwegian lemming (Lemmus lemmus) (65, 72).
Mathematical Modeling
To interpret the statistical models (Table 1), we consider a predator–prey model for the lynx [Pt; yt = ln(Pt)] and the snowshoe hare [Ht; xt = ln(Ht)]. The following model may be seen as a Taylor-approximation to various, more specific model formulation (29) and is defined (26, 28, 29) as:
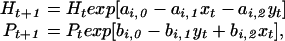 |
2 |
where ai,1 and bi,1 determine the degree of intraspecific regulation in the hare and the lynx (within phase or regime i), respectively; ai,2 and bi,2 determine the strength of the trophic interaction between the two species; and ai,0 and bi,0 represent the “intrinsic growth rate” (corresponding to conditions without any significant intraspecific interactions and in the absence of other species). Taking logs on both sides of Eq. 2, we may, under reasonable biological assumptions (K.-S.C., H.T., and N.C.S., unpublished work), write
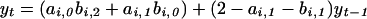 |
3 |
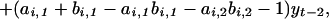 |
which is equivalent to the statistical model given by Eq. 1. This allows us to relate the ecological parameters of Eq. 2 to the autoregressive parameters of Eq. 1. The first-order autoregressive parameter, βi,1 = 2 − ai,1 − bi,1, is a function of the ecological density dependence in both the hare and the lynx (ai,1 and bi,1). The second-order autoregressive parameter, βi,2 = ai,1 + bi,1 − ai,1bi,1 − ai,2bi,2 − 1, incorporates the strength of the trophic interaction (i.e., ai,2bi,2). Note that the second-order coefficient βi,2 also may be represented as βi,2 = 1 − ( βi,1 + “intra”i) − “inter”i, where intrai and interi signify the strength of the multiplicative intra- and interspecific (trophic) interactions in the system. Thus, the second-order autoregressive parameter is influenced by trophic interactions as well as the ecological density dependencies within the interacting species. Assuming that the trophic model is appropriate, this suggests that the ecological mechanism underlying the observed nonlinearity is likely to be related to the interaction between the hare and the lynx.
Models and Field Observations
The ecological model allows a link between the population-level pattern and the mechanistic processes by putting constraints on permissible ecological interactions. We need to account for the following important patterns of density and phase dependence (Table 1): (i) The lag-1 coefficient is significantly positive, but less so during the increase phase; and (ii) the lag-2 coefficient is significantly negative, and more so during the decline phase. On the basis of Eq. 3, the pattern in the lag-1 coefficient (Table 1) implies that the sum of the strengths of intraspecific interactions (ai,1 + bi,1) is somewhat stronger during the increase phase than during the decline phase.
During the increase phase of the hare cycle, lynx appear to be territorial (60, 61, 74–76). This intraspecific regulation disappears as territoriality within the lynx population breaks down 2–3 years after the hare peak (76). Hares exhibit a dominance hierarchy; however, the only aggressive interactions apart from those related to breeding have been observed around concentrated food sources (77–81). Given that hares browse on widely distributed shrubs during the winter when food is most scarce, it is unlikely that individual hares effectively could be excluded from food. It is known that the reproductive rate of hares starts to fall in the late increase and reaches its lowest level in the decline and low (7–10). Thus, we can account for some, but not very strong, phase dependency in the intraspecific component of the dynamics of the two interacting species (i.e., as expressed in the statistical parameters, βi,1 and, as a result, in the ecological parameters, ai,1 and bi,1).
Turning to the trophic interaction, the phase dependency in the snowshoe hare is likely to arise from at least two biological processes: the hare’s change in habitat selection, which is itself a function of predation risk (82, 83), and the hare’s intrinsic responses either to predator-induced stress (10–13, 82–84) or to changes in its food supply (7–13). In lynx, it is likely to result from at least two biological processes: (i) changes in the foraging behavior of lynx as the relative abundances of hares and alternative prey fluctuate (60, 61), and (ii) shifts in the demographic structure of the lynx population. Reproductive output by lynx also declines rapidly to low levels after the hare peak (9, 10, 13), resulting in a gradual upward shift in the mean age of lynx as hares decline, and this is reversed only in the early increase (60, 61, 74–76, 85–89).
The phase dependency in the lag-2 coefficient (β1,2 > β2,2; Table 1) suggests that ai,2bi,2 is largest in absolute value during the decline phase (Eq. 3). New field observations (66) show that the kill rate per lynx at a given hare density is greater during the decline than during the increase (Fig. 3D). The functional responses of lynx were calculated based on measurements of kill rates/kilometer along lynx trails in snow, travel rates, and activity budgets (66). The probability that the four increase years all fall below the four decrease years, at given densities, is 0.014 (one-sided hypergeometric test). Separate functional response models for the increase and decrease phase also fit the data the best (see legend to Fig. 3D). This phase dependency may be caused by behavioral responses of lynx because lynx surviving into the low phase of the cycle switch to alternative prey and may have changed hunting tactics during the decrease phase. This behavioral change is known to persist into the subsequent early cyclic increase (60, 61, 77). Phase dependency in the kill rate also occurs in coyotes preying on hares (60) and thus may be a general feature of this predator–prey system. The inferred changes in ai,1, bi,1, ai,2, and bi,2 are all consistent with the estimated differences in βi,0 between the two regimes (Table 1).
By combining field observations with statistical and mathematical modeling, we have been able to detect and interpret new patterns within the boreal ecosystem. The interplay between theory and analyses of time series data (refs. 28 and 29, and this study) has shed light on a 25-year-old prediction of May (73) and suggests that, even though we need to consider both vegetation and the predators to understand the dynamics of the hare (10, 28, 29), we need only consider the hare to understand the dynamics of the lynx. In a community context, this makes the boreal forest ecosystem—within which the hare and the lynx are main actors—a particularly interesting system to study.
Conclusion
By combining statistical modeling of 21 time series spanning 175 years with mathematical and statistical modeling and experimental data, we have demonstrated: (i) The dynamic patterns (or structure) of the Canadian lynx is both phase- and density-dependent. The density dependence involves both direct and delayed effects. The structure of the dynamics is broadly similar over time (from the early part of the 19th century until the present) and space (the entire Canadian boreal forest region). (ii) The density-dependent structure of the lynx time series is consistent with the proposition that the dynamics in the Canadian lynx is governed by processes involved in the trophic interactions between the snowshoe hare. (iii) The phase-dependent structure of the lynx time series is primarily a result of the strength of the trophic interaction between snowshoe hare and lynx changing during the cycle. And, (iv), density dependence induces the regulatory delays whereas phase dependence induces the nonlinearity. The two act in concert to produce the observed lynx cycle. Altogether, we have clarified a long-lasting controversy in the ecology of cyclic vertebrates of the North, namely that concerning phase dependence and density dependence (6). For the Canadian lynx, it is clearly not a matter of one or the other, but both.
Acknowledgments
We appreciate the incisive comments we have received from Drs. T. F. Hansen and R. M. May as well as from three anonymous reviewers. We thank the following for financial support: the Norwegian Research Council and the University of Oslo (to N.C.S. and O.N.B.); European Union under the Human Capital Programme (CHRX-CT 94–0693), the Engineering and Physical Sciences Research Council of U.K. and the Hong Kong University Committee on Research and Conference Grants award (to H.T.); the National Science Foundation (DMS 9504798 to K.S.C.); the Natural Sciences and Engineering Research Council of Canada (to R.B., S.B., and C.J.K.); the Center for Advanced Study at the Norwegian Academy of Science and Letters (to N.C.S., R.B., and N.G.Y.); the University of Iowa (Faculty scholar award to K.S.C.), and the University of Lyon–Centre National de la Recherche Scientifique (to N.G.Y.).
ABBREVIATIONS
- SETAR model
self-exciting threshold autoregressive model
- SE
standard error
- NAIC
normalized kaike information criterion
- DD
density dependence
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
We assume that the average lynx density per 100 km2 varies over the hare cycle as given in Fig. 2, that the lynx removals are as given by the fur return records summarized in Fig. 1, and that the size of the boreal forest in Canada is ≈2.5 million km2.
Commonly, log-transformation of abundance data stabilizes the variance (62). Because population dynamics relate to multiplicative processes, a log-transformation is also appropriate (63).
Cumulative periodograms of residuals and the Ljung–Box test for whiteness (39) generally confirm that only white noise is left. Testing for linear trends in the normalized residuals, assuming the fitted SETAR model (Table 1), further shows that we may conclude safely that the fitted models have no residual linear trends; hence, the fitted models essentially have captured the temporal patterns in the data. This is supported further by the common structure of the series from the regions with temporally noncontinuous series (L1 and L2; L4 and L5; L6 and L7; unpublished results).
The series are individually normalized so that their 30 and 70 percentiles are 0 and 1, respectively, before doing the empirical Bayes pooling. This rescaling does not change the lag-1 and lag-2 coefficients, but other coefficients are affected. We set the super-population of the vector SETAR parameters (β1 = (β1,0,β1,1,β1,2), β2 = (β2,0,β2,1,β2,2), θ, σ12, σ22) to be the improper product distribution N(β1*,Σ1*)xN(β2*,Σ2*)xU(0, 1)xU(0,∞)xU(0, ∞), where N(..,) denotes a Normal distribution and U(v, w) denotes a uniform distribution over the interval [v, w]. The maximum likelihood estimates of these super-population parameters are estimated by using the expectation–maximization algorithm (44). The uniform distribution on the threshold reflects a partial belief that the threshold is distributed uniformly over the 30 to the 70 percentiles, at the original scale.
It is an interesting question to quantify the evidence for (partial) common dynamic structure across some series by testing whether some superparameters are nonrandom (or, equivalently, whether their marginal super-population distribution degenerates to a point probability mass). We may test for common structure by testing whether the standard deviations of the super-population distribution are zero. This problem requires a careful and extensive treatment, which is beyond the scope of this paper.
References
- 1.Elton C S, Nicholson M. J Animal Ecol. 1942;11:215–244. [Google Scholar]
- 2.Hewit C G. The Conservation of the Wildlife of Canada. New York: Schribner’s; 1921. [Google Scholar]
- 3.Elton C S. Br J Exp Biol. 1924;2:119–163. [Google Scholar]
- 4.Elton C S. Voles, Mice and Lemmings. Oxford: Clarendon; 1942. [Google Scholar]
- 5.Finerty J P. The Population Ecology of Cycles in Small Mammals: Mathematical Theory and Biological Fact. New Haven, CT: Yale Univ. Press; 1980. [Google Scholar]
- 6.Krebs C J. J Mammal. 1996;77:8–24. [Google Scholar]
- 7.Cary J R, Keith L B. Can J Zool. 1979;57:375–390. [Google Scholar]
- 8.Keith L B. Oikos. 1983;40:385–395. [Google Scholar]
- 9.Keith L B. Curr Mammal. 1990;2:119–195. [Google Scholar]
- 10.Krebs C J, Boutin S, Boonstra R, Sinclair A R E, Smith J N M, Dale M R T, Turkington R. Science. 1995;269:1112–1115. doi: 10.1126/science.269.5227.1112. [DOI] [PubMed] [Google Scholar]
- 11.Boutin S, Krebs C J, Boonstra R, Dale M T, Hannon S J, Martin K, Sinclair A R E, Smith J N M, Turkington R, Blower M, et al. Oikos. 1995;74:69–80. [Google Scholar]
- 12.Boonstra R, Singleton G R. Gen Comp Endocrinol. 1993;91:126–143. doi: 10.1006/gcen.1993.1113. [DOI] [PubMed] [Google Scholar]
- 13.Boonstra R, Hik D, Singleton G R, Tinnikov A. Ecol Monogr. 1998;68:371–394. [Google Scholar]
- 14.Volterra V. Nature (London) 1926;118:558–560. [Google Scholar]
- 15.Lotka A J. Elements of Physical Biology. Baltimore: Williams & Wilkins; 1925. [Google Scholar]
- 16.Moran P A P. Aust J Zool. 1953;1:163–173. [Google Scholar]
- 17.Cole L C. J Wildl Manage. 1954;18:2–24. [Google Scholar]
- 18.Bulmer M G. J Animal Ecol. 1974;43:701–718. [Google Scholar]
- 19.Egerton F N., III Q Rev Biol. 1973;48:322–350. [Google Scholar]
- 20.Gilpin M E. Am Nat. 1973;107:727–730. [Google Scholar]
- 21.Schaffer W M. Am Nat. 1984;124:798–820. [Google Scholar]
- 22.Pimm S L. The Balance of Nature? Chicago: Univ. of Chicago Press; 1981. [Google Scholar]
- 23.Smith C H, Davis J M. J Biogeography. 1981;8:27–35. [Google Scholar]
- 24.Sinclair A R E, Gosline J M, Holdsworth G, Krebs C J, Boutin S, Smith J N M, Boonstra R, Dale M. Am Nat. 1993;141:173–198. doi: 10.1086/285468. [DOI] [PubMed] [Google Scholar]
- 25.Royama T. Analytical Population Dynamics. London: Chapman & Hall; 1992. [Google Scholar]
- 26.May R M. Science. 1972;177:900–902. doi: 10.1126/science.177.4052.900. [DOI] [PubMed] [Google Scholar]
- 27.May R M. Stability and Complexity in Model Ecosystems. Princeton, NJ: Princeton Univ. Press; 1973. [PubMed] [Google Scholar]
- 28.Stenseth N C. Science. 1995;269:1061–1062. doi: 10.1126/science.269.5227.1061. [DOI] [PubMed] [Google Scholar]
- 29.Stenseth N C, Falck W, Bjørnstad O N, Krebs C J. Proc Natl Acad Sci. 1997;94:5147–5152. doi: 10.1073/pnas.94.10.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurney W S C, Blythe S P, Nisbet R M. Nature (London) 1980;287:17–21. [Google Scholar]
- 31.Gurney W S C, Nisbet R M, Lawton J M. J Animal Ecol. 1983;52:479–495. [Google Scholar]
- 32.Gurney W S C, Nisbet R M. Theor Popul Biol. 1985;28:150–180. [Google Scholar]
- 33.Dennis B, Desharnais R A, Cushing J M, Costantino R F. Ecol Monogr. 1995;65:261–281. [Google Scholar]
- 34.Costantino R F, Desharnais R A, Cushing J M, Dennis B. Science. 1996;275:389–391. doi: 10.1126/science.275.5298.389. [DOI] [PubMed] [Google Scholar]
- 35.Costantino R F, Cushing J M, Dennis B, Desharnais R A. Nature (London) 1995;375:227–230. [Google Scholar]
- 36.Dominion Bureau of Statistics. Catalogue 23–207: Fur Production. Ottawa: Dominion Bureau of Statistics; 1965. [Google Scholar]
- 37.Statistics Canada (1983–1995) [Statistics Canada (1893–1995), Ottawa].
- 38.Statistical Sciences. S-plus Guide to Statistical and Mathematical Analysis, Version 3.3. Seattle: Statistical Sciences; 1995. [Google Scholar]
- 39.Tong H. Non-linear Time Series: A Dynamical System Approach. Oxford: Clarendon; 1990. [Google Scholar]
- 40.Härdle W. Applied Nonparametric Regression. Cambridge, U.K.: Cambridge Univ. Press; 1990. [Google Scholar]
- 41.Hastie T, Tibshirani R. Generalized Additive Models: Monographs on Statistics and Applied Probability. London: Chapman & Hall; 1990. [Google Scholar]
- 42.Cox D R, Hinkley D V. Theoretical Statistics. New York: Chapman & Hall; 1979. [Google Scholar]
- 43.Maritz J S, Lwin T. Empirical Bayes Methods. New York: Chapman & Hall; 1989. [Google Scholar]
- 44.Dempster A P, Laird N M, Rubin D B. J R Stat Soc B. 1977;39:1–22. [Google Scholar]
- 45.Campbell M J, Walker A M. J R Stat Soc A. 1977;140:411–431. [Google Scholar]
- 46.Haggan V, Ozaki T. Biometrika. 1981;68:189–196. [Google Scholar]
- 47.Subba-Rao T, Gabr M M. J Time Series Analysis. 1980;1:145–158. [Google Scholar]
- 48.Tong H. J R Stat Soc A. 1977;140:432–436. [Google Scholar]
- 49.Chan K-S, Tong H. J R Stat Soc B. 1990;52:469–476. [Google Scholar]
- 50.Chan K-S, Tong H. J R Stat Soc B. 1994;56:301–311. [Google Scholar]
- 51.Teräsvirta T. Kybernetika. 1994;30:319–330. [Google Scholar]
- 52.Hjellvik V, Tjøstheim D. Biometrika. 1995;82:351–368. [Google Scholar]
- 53.Cheng B, Tong H. J R Stat Soc B. 1992;54:427–449. [Google Scholar]
- 54.Tjøstheim D, Auestad B H. J Am Stat Assoc. 1994;89:1410–1419. [Google Scholar]
- 55.Yao Q, Tong H. Philos Trans R Soc London A. 1994;348:357–369. [Google Scholar]
- 56.Tong H, Lim K S. J R Stat Soc B. 1980;42:245–292. [Google Scholar]
- 57.Tong H. Threshold Models in Non-Linear Time Series Analysis. New York: Springer; 1983. [Google Scholar]
- 58.Lin T C, Pourahmadi M. J R Stat Soc C. 1998;47:187–201. [Google Scholar]
- 59.Mah K P. Dissertation. Hong Kong: Univ. of Hong Kong; 1998. [Google Scholar]
- 60.O’Donoghue M. Ph.D. thesis. Vancouver, Canada: Univ. of British Columbia; 1997. [Google Scholar]
- 61.O’Donoghue M, Boutin S, Krebs C J, Hofer E J. Oikos. 1997;80:150–162. [Google Scholar]
- 62.Sen A, Srivastava M. Regression Analysis: Theory, Methods and Applications. Berlin: Springer; 1990. [Google Scholar]
- 63.Williamson M. The Analysis of Biological Populations. London: Edward Arnold; 1971. [Google Scholar]
- 64.Stenseth N C, Chan K S. Nature (London) 1998;394:620–621. [Google Scholar]
- 65.Stenseth N. C., Chan, K. S., Framstad, E. & Tong, H. (1998) Proc. R. Soc. London Ser. B, in press. [DOI] [PMC free article] [PubMed]
- 66.O’Donoghue M, Boutin S, Krebs C J, Zuleta G, Murray D L, Hofer E J. Ecology. 1998;79:1193–1208. [Google Scholar]
- 67.Weisberg S. Applied Linear Regression. New York: Wiley; 1985. [Google Scholar]
- 68.Hörnfeldt B. Ecology. 1994;75:791–806. [Google Scholar]
- 69.Bjørnstad O N, Falck W, Stenseth N C. Proc R Soc London Ser B. 1995;262:127–133. [Google Scholar]
- 70.Stenseth N C, Bjørnstad O N, Saitoh T. Proc R Soc London Ser B. 1996;263:1117–1126. doi: 10.1098/rspb.1996.0164. [DOI] [PubMed] [Google Scholar]
- 71.Stenseth N C, Bjørnstad O N, Falck W. Proc R Soc London Ser B. 1996;263:1423–1435. doi: 10.1098/rspb.1996.0208. [DOI] [PubMed] [Google Scholar]
- 72.Framstad E, Stenseth N C, Bjørnstad O N, Falck W. Proc R Soc London Ser B. 1997;264:31–38. [Google Scholar]
- 73.May R M. Ecology. 1973;54:315–325. [Google Scholar]
- 74.Ward R M P, Krebs C J. Can J Zool. 1985;63:2817–2824. [Google Scholar]
- 75.Poole K G. Can J Zool. 1995;73:632–641. [Google Scholar]
- 76.Slough B G, Mowat G. J Wildl Manage. 1996;60:946–961. [Google Scholar]
- 77.O’Donoghue M, Boutin S, Krebs C J, Murray D L, Hofer E J. Oikos. 1998;82:169–183. [Google Scholar]
- 78.Boutin S. Oecologia. 1984;62:393–400. doi: 10.1007/BF00384273. [DOI] [PubMed] [Google Scholar]
- 79.Graf R P. Can J Zool. 1985;63:468–474. [Google Scholar]
- 80.Boutin S. J Animal Ecol. 1984;53:623–637. [Google Scholar]
- 81.Boutin S, Krebs C J, Sinclair A R E, Smith J N M. Can J Zool. 1986;64:606–610. [Google Scholar]
- 82.Wolff J O. Ecol Monogr. 1980;50:111–130. [Google Scholar]
- 83.Hik D S. Wildl Res. 1995;22:115–129. [Google Scholar]
- 84.Christian J J. In: Biosocial Mechanisms in Population Regulation. Cohen M N, Malpass R S, Klein H G, editors. New Haven, CT: Yale Univ. Press; 1980. pp. 55–115. [Google Scholar]
- 85.Brand C J, Keith L B, Fischer C A. J Wildl Manage. 1976;40:416–428. [Google Scholar]
- 86.Brand C J, Keith L B. J Wildl Manage. 1979;43:827–849. [Google Scholar]
- 87.O’Connor R M. In: Cats of the World: Biology, Conservation and Management. Miller S D, Everett D D, editors. Washington, D. C.: National Wildlife Federation; 1986. pp. 311–325. [Google Scholar]
- 88.Poole K G. J Wildl Manage. 1994;58:608–618. [Google Scholar]
- 89.Mowat G, Slough B G, Boutin S. J Wildl Manage. 1996;60:441–452. [Google Scholar]