Abstract
The availability of an electrocardiogram (ECG) waveform in the adult mouse has permitted the measurement of fast, dynamic cardiac events where data acquisition is synchronized to the R-wave of the ECG waveform. These methods can easily attain one thousand frames/s at ultrasound frequencies greater than 20 MHz. With the heart being the first organ to develop, normal cardiovascular function is crucial to the viability of the developing embryo. Thus, translating such methodologies to analyze embryonic cardiovascular development would add crucial information in mouse models of congenital heart disease which are embryonic lethal. Obtaining an ECG signal from mouse embryos is impractical. Therefore, in this study, preliminary results are presented which derive a cardiac-trigger signal from Doppler blood-flow waveforms. A continuous wave 40 MHz Doppler ultrasound system was used to acquire the Doppler waveforms and a real-time algorithm was developed to process the Doppler waveforms and generate a trigger. Validation studies revealed that a heart rate can be reliably measured and that the Doppler trigger algorithm was robust enough to follow changes in the blood flow. Preliminary data showed that Doppler-derived triggers can be used for high-frame-rate prospective imaging of the early embryonic mouse heart.
I. Introduction
High-frequency (> 20 MHz) ultrasonic methods such as 2D echocardiography, M-mode, and pulsed wave Doppler have matured into valuable tools to analyze the cardiovascular system of the adult mouse [1] and the developing embryo [2], [3]. Recent technical developments in electrocardiogram (ECG) gated imaging have allowed for high temporal resolution measurements in the adult mouse such as pulse-wave velocity [4], myocardial elastography [5], and high-frame-rate echocardiography using annular arrays [6]. These techniques do not translate directly to the mouse embryo because of the technical challenges to obtain the ECG reading from the mouse embryo in utero.
Doppler signals have been used as a surrogate for the ECG signal in 3D cardiac imaging of sheep embryos [7] and human fetuses [8] using 5 MHz clinical scanners. In those studies, the envelope of the Doppler blood flow waveform was detected and converted into a cardiac cycle trigger signal. In order to develop this technique for the mouse embryo, high-frequency Doppler ultrasound is necessary because blood flow velocity in the embryonic mouse is an order of magnitude less than that of the adult mouse. High-frequency Doppler ultrasound is a robust, noninvasive technique which can unambiguously visualize and quantify the periodic motion of the embryonic heart, as well as other cardiac events [3]. Here we present preliminary results that show stable cardiac-trigger signals can be derived from high frequency Doppler ultrasound waveforms, and that these triggers can be used to image the embryonic mouse heart cycle with high temporal resolution.
II. Methods
A. Doppler transducer fabrication
Continuous-wave (CW), unfocused Doppler transducers were fabricated as previously reported [9]. Briefly, two matched 1.5 mm diameter lithium niobate disks were individually air-back mounted on SMA connectors with each transducer having a center frequency of 43 MHz. The transducers were then mounted on a custom-machined plexiglass holder such that the angle between the transmit and receive ultrasound beams was 30° (Fig. 1). The 6-dB axial and lateral beamwidths were 1 mm and 0.25 mm, respectively, with a 5.3 mm Doppler sample volume depth.
Fig. 1.
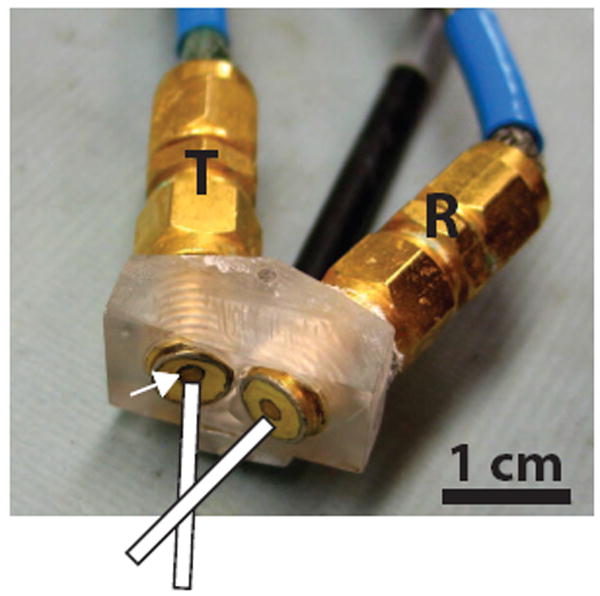
CW Doppler transducer consisting of a pair of single-element lithium niobate transducers individually mounted on SMA connectors. The mounting angle is fixed at 30°. The Doppler sample volume is defined by the intersection of the transmit (T) and receive (R) ultrasound beams. White arrow points to one of the 1.5 mm lithium niobate elements.
B. B-mode image acquisition
B-mode images of mouse embryos were acquired using a high-frequency ultrasound scanner similar to one previously described [6]. The imaging system consisted of three main components: imaging hardware, motion control, and radiofrequency (RF) signal acquisition. A spherically focused, 40 MHz polyvinylidene difluoride (PVDF) single-element transducer with a 5 mm aperture and 10 mm focal length was acoustically excited with a mono-cycle pulser (Avtech AVB2-TA-C-CVA, Ottawa, Ontario, Canada). The transducer was mounted and mechanically scanned with a fast linear actuator (LAS35, SMAC, Carlsbad, CA) controlled via a motion control card (PXI-7532, National Instruments, Austin, TX). The return RF echoes were digitized at 250 MHz using an 8 bit digitizer (PXI-5152, National Instruments). The parameters and synchronization of each of the components were controlled via a custom graphical user interface developed in LabVIEW (National Instruments).
C. CW Doppler waveform detection and acquisition
When ultrasound at a frequency fo impinges on a scatterer moving at a velocity vb, the reflected signal is shifted in frequency by an amount fd given by the Doppler equation
| (1) |
where ϕ is the angle between the transmit and receive ultrasound beams, θ is the angle between the flow and the incident ultrasound beam direction, and c is the speed of sound in the medium.
For our experiments, the transmit and receive channels from the CW Doppler transducer were connected to a custom built quadrature demodulation electronic box in order to detect the Doppler frequency shifts (fd) [9]. Briefly, a quadrature synthesizer (ADS-432-1176A, Sciteq Electronics, San Diego, CA) was tuned to the transmit transducer’s resonant frequency and the transmit was excited with a one volt CW signal. The receive signal was demodulated with both quadrature reference signals from the synthesizer to generate in-phase (I) and quadrature (Q) Doppler signals. The I and Q signals were then amplified and stored in digital audio tape (DAT) (Tascam DA-45HR, Teac America, Montebello, CA) for later analysis. The DAT tape also converts the Doppler frequency shifts into audible signals, and is used to monitor the blood flow in the Doppler sample volume. The Doppler signals could also be streamed directly to the data acquisition system for real-time analysis. A 25 Hz clutter filter was used to minimize the modulation of the Doppler signals from the mother’s breathing.
D. Doppler processing
With the acquired Doppler signals falling in the audio range, 16 ms time segments of I and Q Doppler signals were continuously digitized at a sampling frequency of 16 kHz using a data acquisition card (PXI-6229, National Instruments). The I and Q time domain waveforms were cast into complex form I+ jQ and windowed with an 8 ms Hanning window. The segment was then Fourier transformed and mapped to the first line of a time-frequency spectrogram where the y-axis represented Doppler frequency shifts and magnitude was mapped to a grey-scale color table. The time window was shifted by 4 ms, and the previous steps were repeated until the whole 16 ms segment was processed. The resulting spectrogram visualizes the blood flow within the interrogated vessel where the time resolution of each line corresponded to 4 ms.
E. Real-time Doppler trigger
The algorithm for generating the trigger signals from the Doppler waveforms was implemented in LabVIEW. Real-time performance was achieved by using a queue structure that assigned data acquisition and data processing to separate processor cores. As each line of the spectrogram was generated, its maximum frequency above a set amplitude threshold was stored. The time evolution of these maximal frequencies traced out the envelope of the Doppler waveform which was then filtered using a fifth-order Butterworth low-pass filter. With proper positioning of the interrogation site [3], the Doppler envelope waveform traced out peaks and valleys which were precisely synchronized to the heart cycle. A search algorithm that found the local peaks of the Doppler envelope was implemented into the data processing loop, and a software trigger was generated at each local peak.
F. Mouse imaging and Doppler sample volume placement
A semi-invasive approach was used to visualize individual embryos [3]. Briefly, an anesthetized, pregnant mouse was fastened to an imaging stage, and an intact twelve-day old embryo was surgically exposed into a Petri dish filled with saline solution kept at physiologic temperature. The imaging system was set for real-time B-mode imaging at 12 frames per second, and the embryonic heart was positioned in the middle of the imaging plane. Subsequently, with the Doppler transducer mounted on a micromanipulator, the Doppler sample volume was moved until the audio signals from the DAT tape indicated blood flow.
III. Results
A. Image guided Doppler acquisition
With the scanner set to real-time B-mode imaging, the embryonic heart appeared as a rhythmically contracting structure easily differentiated from the rest of the embryonic anatomy. Having set the imaging plane, the Doppler blood flow waveforms were displayed in real-time, and the Doppler transducer was moved until the sample volume was over the descending aorta. The spectrogram from the descending aorta (Fig. 2b) showed periodic, highly pulsatile blood flow with clear peaks, and time intervals with no blood flow. The mean cycle length over six cycles was 230:2 ± 7:3 ms giving a calculated heart rate of 261 beats/min, which was consistent with previous measurements on similarly staged embryos [10], [11]. The blood flow from the descending aorta was found in 100% of the embryos imaged.
Fig. 2.

(a) Time Doppler waveform from the descending aorta of a twelve-day old mouse embryo. (b) The spectrogram revealed the pulsatile nature of the blood flow where physiologic cardiac parameters, such as cycle length (CL), ejection time (ET) and peak flow velocity (PV), were easily identified and quantified.
The Doppler envelope from the descending aorta was acquired over a period of 10 minutes for several embryos. When embryonic movement was limited to vertical displacements from the mother’s respiratory motion, the trigger points (Fig. 4b) were reproducible and stable over the examination time. Because the real-time B-mode imaging and Doppler envelope were acquired simultaneously, the change in shape of the Doppler envelope in some of the embryos was correlated with embryonic motion out of the imaging plane which indicated that the Doppler sample volume was isonating a different part of the embryonic cardiovascular system. These changes have to be taken into account when using Doppler triggers for high-frame-rate prospective image acquisition.
Fig. 4.
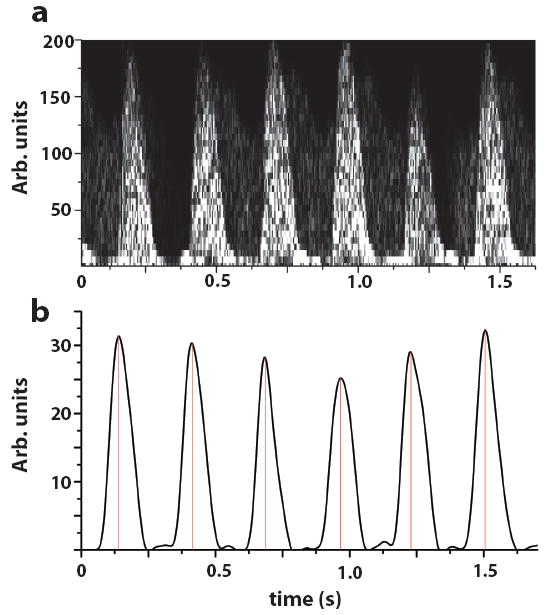
(a) Spectrogram from six beats windowed from the time trace in Fig. 2a. (b) After Doppler envelope detection and low-pass filtering, a clean Doppler envelope served as the input to the gating algorithm. Vertical lines represent software triggers used to initiate data acquisition for prospective high-frame-rate imaging.
B. Doppler-triggered high-frame-rate prospective imaging
Prospective imaging was implemented in a similar fashion as previously reported [6]. Briefly, a set of M-modes, each consisting of A-lines digitized at a fixed pulse repetition frequency (PRF), were acquired at equidistant positions over the span of the embryonic heart. Each M-mode was synchronized to the same phase of the heart cycle, which permitted the parsing of the data into a cine-loop of B-mode images at a frame rate equal to the M-mode acquisition PRF. M-mode data were gated to the resting phase of the mouse’s respiration cycle [6] in order to eliminate motion artifacts. To validate prospective imaging using Doppler-derived cardiac-triggers, ten-day old mouse embryos were imaged as mentioned previously. Figure 5a shows a reference B-mode image of an embryonic heart where the cardiac chambers were clearly defined. The prospective imaging was set to acquire 101 M-mode data sets, each containing 250 lines acquired at a PRF of 500 Hz. Figure 5b shows a frame from the prospective imaging cine loop confirming that our Doppler-triggering algorithm was able to synchronize the M-modes to the embryonic heart cycle. Although the heart chambers appeared to agree with that of the pre-scan image, there are significant motion artifacts corrupting the image making the clear definition of the embryonic anatomy difficult. Figures 5c and 5d show data from a second ten-day old mouse embryo imaged on a different day. Here, respiratory gating eliminated most of the motion artifacts. The high temporal resolution of the cine loop revealed blood flow into the cardiac chambers and in other vessels present in the field of view.
Fig. 5.
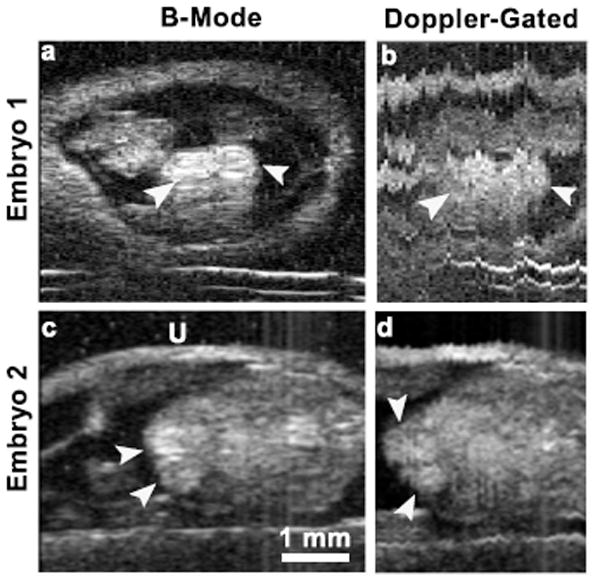
B-mode images (a), (c) and frames from Doppler-gated high-frame-rate cine loops (b), (d) from two different ten-day old mouse embryos. B-mode images show two chambers of the embryonic heart (white arrows). Acquisition with (Embryo 2) and without (Embryo 1) respiratory gating. U=uterus.
IV. Conclusions
We have shown that trigger signals can be obtained from Doppler blood velocity waveforms from in utero mouse embryos. These robust signals were shown to be stable enough to measure the embryonic heart cycle with an accuracy of 7 ms. This technique can also be adapted to pulsed-wave (PW) Doppler ultrasound which gives the added advantage of range gating the Doppler sample volume. Because of the much smaller Doppler sample volume in PW Doppler, the trigger waveforms will be more susceptible to respiratory artifacts or to small out-of-plane shifts of the blood vessel being isonated. The trigger algorithm was explicitly written in LabVIEW as a self contained module which directly plugged into our existing annular-array image acquisition software [6]. Future work will use Doppler-derived triggers with our 5-element, 40 MHz annular-array system to image the embryonic heart at high temporal resolution and with an increased depth of field. For in utero imaging, coded excitation could also be implemented to take advantage of the increased penetration depth [12]. This Doppler-triggered high-frame-rate imaging will permit crucial measurements in the normal and abnormal development of the embryonic cardiovascular system.
Fig. 3.
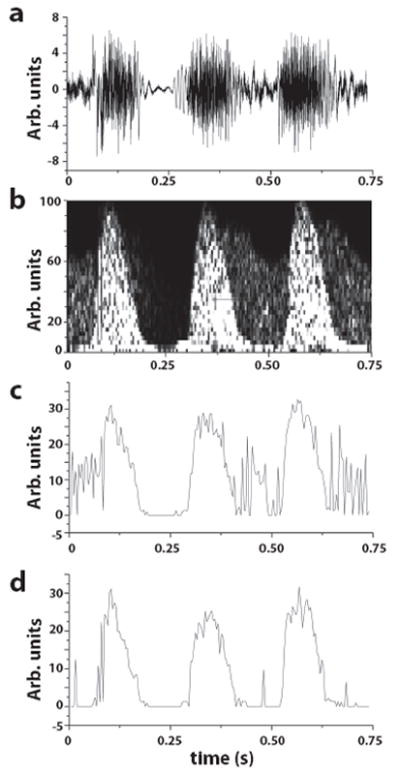
(a) Time trace of three heart beats corresponding to the section between the red cursors in Fig. 2a. (b) Using a low threshold setting, background venous blood flow was detected within the same Doppler sample volume. Doppler envelopes detected for a low (c) and high (d) threshold setting in the Doppler envelope detection algorithm. With a low threshold, the detected Doppler waveform envelope was noisy due to the venous blood flow. (d) With a higher threshold, the noise from the venous blood flow was almost completely eliminated.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (EB008606).
References
- 1.Zhou YQ, Foster FS, Nieman BJ, Davidson L, Chen XJ, Henkelman RM. Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics. 2004;18:232–244. doi: 10.1152/physiolgenomics.00026.2004. [DOI] [PubMed] [Google Scholar]
- 2.Mu J, Slevin JC, Qu D, McCormick S, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol. 2008;6:34. doi: 10.1186/1477-7827-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genomics. 2003;14:3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- 4.Williams R, Needles A, Cherin E, Zhou YQ, Henkelman RM, Adamson SL, Foster FS. Noninvasive ultrasonic measurement of regional and local pulse-wave velocity in mice. Ultrasound Med Biol. 2007;33:1368–1375. doi: 10.1016/j.ultrasmedbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Konofagou EE. High-frame rate, full-view myocardial elastography with automated contour tracking in murine left ventricles in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:240–248. doi: 10.1109/TUFFC.2008.633. [DOI] [PubMed] [Google Scholar]
- 6.Ketterling JA, Aristizábal O. Prospective ECG-gated mouse cardiac imaging with a 34-Mhz annular array transducer. IEEE Trans Ultrason Ferroelecrt Freq Contr. 2009;56:1394–1404. doi: 10.1109/TUFFC.2009.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J, Birkett AG, Kalache KD, Hanson MA, Peebles DM, Linney AD, Lees WR, Rodeck CH. Conversion of umbilical arterial Doppler waveforms to cardiac cycle triggering signals: a preparatory study for online motion-gated three-dimensional fetal echocardiography. Ultrasound Med Biol. 2001;27:51–59. doi: 10.1016/s0301-5629(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 8.Deng J, Rodeck CH. New fetal cardiac imaging techniques. Prenat Diagn. 2004;24:1092–1103. doi: 10.1002/pd.1066. [DOI] [PubMed] [Google Scholar]
- 9.Aristizábal O, Christopher DA, Foster FS, Turnbull DH. 40-MHZ echocardiography scanner for cardiovascular assessment of mouse embryos. Ultrasound Med Biol. 1998;24:1407–1417. doi: 10.1016/s0301-5629(98)00132-x. [DOI] [PubMed] [Google Scholar]
- 10.Phoon CK, Aristizábal O, Turnbull DH. Spatial velocity profile in mouse embryonic aorta and Doppler-derived volumetric flow: a preliminary model. Am J Physiol Heart Circ Physiol. 2002;283:H908–916. doi: 10.1152/ajpheart.00869.2001. [DOI] [PubMed] [Google Scholar]
- 11.Yu Q, Leatherbury L, Tian X, Lo CW. Cardiovascular assessment of fetal mice by in utero echocardiography. Ultrasound Med Biol. 2008;34:741–752. doi: 10.1016/j.ultrasmedbio.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamou J, Aristizábal O, Silverman RH, Ketterling JA, Turnbull DH. High-Frequency Chirp Ultrasound Imaging with an Annular Array for Ophthalmologic and Small-Animal Imaging. Ultrasound Med Biol. 2009 Apr 24; doi: 10.1016/j.ultrasmedbio.2008.12.017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


