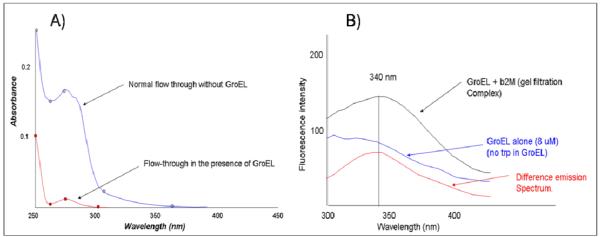
Figure 6.
Initially folded β-2- microglobulin can partition onto the GroEL chaperonin. In Panel A, the difference in a matched set of ultrafiltration experiments illustrate that the microglobulin is retained in the Amicon Centricons (50 kDa cutoff) that contain the chaperonin GroEL. A similar sample subjected to the same ultrafiltration procedure without GroEL is not retained. In Panel B, the fluorescence of a GroEL sample that has been incubated with β-2- microglobulin and isolated via gel filtration (S-300 column) shows a clear increase in tryptophan fluorescence compared with an equivalent amount of GroEL alone isolated in a separate gel filtration run. Since GroEL does not contain tryptophan, the total spectra and the difference spectra reveal the presence of tryptophan fluorescence indicating the contribution from GroEL bound β-2- microglobulin. A similar run with β-2- microglobulin alone did not show any co-migration with the GroEL peak.