Abstract
Background
Patient-reported outcomes are increasingly used to assess the efficacy of new treatments. Understanding relationships between these and clinical measures can facilitate their interpretation. We examined associations between patient-reported measures of health-related quality of life and clinical indicators of disease severity in a large, heterogeneous sample of patients with heart failure.
Methods
Patient-reported measures, including the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the EuroQol Visual Analog Scale (VAS), and clinical measures, including peak VO2, 6-minute walk distance, and New York Heart Association (NYHA) class, were assessed at baseline in 2331 patients with heart failure. We used general linear models to regress patient-reported measures on each clinical measure. Final models adjusted for significant sociodemographic variables and 2-way interactions.
Results
The KCCQ was correlated with peak VO2 (r = .21) and 6-minute walk distance (r = .27). The VAS was correlated with peak VO2 (r = .09) and 6-minute walk distance (r = .11). Using the KCCQ as the response variable, a 1 SD difference in peak VO2 (4.7 mL/kg/min) was associated with a 2.86-point difference in the VAS (95% confidence interval [CI], 1.98-3.74) and a 4.75-point difference in the KCCQ (95% CI, 3.78-5.72). A 1 SD difference in 6-minute walk distance (105 m) was associated with a 2.78-point difference in the VAS (95% CI, 1.92-3.64) and a 5.92-point difference in the KCCQ (95% CI, 4.98-6.87). NYHA class III was associated with an 8.26-point lower VAS (95% CI, 6.59-9.93) and a 12.73-point lower KCCQ (95% CI, 10.92-14.53) than NYHA class II.
Conclusions
These data may inform deliberations about how to best measure benefits of heart failure interventions, and they generally support the practice of considering a 5-point difference on the KCCQ and a 3-point difference on the VAS to be clinically meaningful.
Introduction
In addition to poor prognosis, patients with heart failure have poor health-related quality of life compared with patients with other chronic diseases.1 A major cause of this lower health-related quality of life is reduced exercise tolerance. As the disease progresses, patients become more incapacitated and deconditioned, unable to perform simple tasks without dyspnea and fatigue.2 Clinicians have increasingly come to recognize the role of patient-reported measures of health-related quality of life in the evaluation of new therapies and strategies. As mortality rates have declined, the quality of the added survival time has become important. A therapy that prolongs survival but produces severe morbidity may be viewed as undesirable by many patients and their physicians.3
There is little agreement about how to best characterize the impact of interventions on patients with heart failure, though there is increasing reliance upon patient-reported outcomes. The Food and Drug Administration recently stressed the importance of defining endpoint models that describe relationships among various outcome measures.4, 5 However, relatively few studies have formally assessed the relationships between patient-reported outcome measures and clinical measures of disease severity in large samples of patients with heart failure. Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) assessed the effects of exercise training on mortality, hospitalization, and health-related quality of life in a large, multinational sample of patients with heart failure. In this study, we used baseline data from HF-ACTION to characterize relationships among patient-reported outcome measures and clinical measures of the severity of heart failure. We also examined whether these relationships were consistent among subgroups of patients.
Methods
HF-ACTION was a multicenter, randomized controlled trial designed to test the long-term safety and efficacy of aerobic exercise training versus usual care in patients with left ventricular dysfunction and New York Heart Association (NYHA) class II to IV heart failure.6 Baseline assessments included health-related quality of life and health status measures and prerandomization cardiopulmonary exercise testing to determine aerobic capacity. Enrollment criteria included left ventricular ejection fraction of 35% or less, NYHA class II to IV heart failure, and ability and willingness to undergo exercise training. Patients were excluded if they were unable to exercise, were already exercising regularly, or had experienced a cardiovascular event in the preceding 6 weeks. Additional details have been described previously.6 The relevant institutional review boards, research ethics boards, and ethics committees of the participating centers and the coordinating center approved the protocol. This work was supported by grants from the National Heart, Lung, and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Patient-Reported Measures
The EuroQol Visual Analog Scale (VAS) has been used to measure health status in numerous patient populations, including patients with heart failure.7-9 The measure was used in HF-ACTION as a generic measure of health status. The VAS contains a single-item “feeling thermometer” on which respondents rate their health state from 0 (worst imaginable) to 100 (best imaginable).
We used the Kansas City Cardiomyopathy Questionnaire (KCCQ) as a disease-specific measure of health status. The KCCQ is a 23-item patient-reported measure that is responsive to underlying clinical changes in patients with heart failure.10 In addition to overall and clinical summary scores, the KCCQ has subscales for physical limitations, symptoms, social limitations, self-efficacy, and quality of life.11 The KCCQ is scored from 0 to 100, with higher scores representing better health. Missing values within a domain are assigned the average of the completed items within that domain.
Clinical Measures
The physiological variables used in this study were peak VO2 and 6-minute walk distance. Patients performed a maximal exercise test with gas exchange measurements on a treadmill using the modified Naughton protocol.12 Patients who were unable to perform an exercise test on a treadmill underwent cycle ergometer testing using a 10 W/min ramp protocol. Patients also performed a 6-minute walk test.13
NYHA class is a physician-assigned functional classification system in which class I represents no limitation of physical activity, class II represents symptoms with usual physical activity, class III represents marked limitation of physical activity, and class IV means the patient is symptomatic at rest.14 In HF-ACTION, site-level investigators determined NYHA class at the time of patient enrollment.
Patient Characteristics
Sociodemographic characteristics used as covariates included sex, age, race, ethnicity, education level, annual household income, employment status, marital status, and health insurance. These variables were self-reported at baseline. We also report clinical history characteristics, including hypertension, diabetes mellitus, renal dysfunction, atrial fibrillation, ejection fraction, and heart failure etiology.
Statistical Analysis
We describe baseline patient characteristics using means and SDs for continuous variables and percentages for categorical variables. We describe distributions of clinical and patient-reported outcome measures using means, SDs, medians, and interquartile ranges for continuous variables and percentages for categorical variables. Side-by-side box plots depict observed differences in KCCQ and VAS scores between NYHA classes. We describe zero-order associations between patient-reported outcomes and continuous clinical measures using Pearson correlation coefficients. We also use partial correlations to describe the same relationships after adjusting for all sociodemographic characteristics that had a statistically significant relationship with the clinical measure. We used the Fisher transformation of Pearson correlation coefficients to calculate 95% confidence intervals (CIs).15
We created general linear univariable models in which the patient-reported outcome measure (KCCQ or VAS) was regressed on the clinical measure of severity (6-minute walk distance, peak VO2, or NYHA class), based on the Wilson and Cleary model that assumes patients' health-related quality of life is partially a function of patients' underlying physiological status.16 We report these parameter estimates with 95% CIs as unadjusted effects. We screened all sociodemographic characteristics using bivariable models, in which we added each sociodemographic characteristic to the univariable models described above. Using partial F tests, we evaluated all 2-way interactions between the clinical measure and the sociodemographic characteristics that were significant in the bivariable models. When significant, we tested each interaction separately. A significance level of α = 0.05 was used for all assessments, and all statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc, Cary, North Carolina).
The final models adjusted for all significant sociodemographic variables and interactions; we report parameter estimates and 95% CIs from the final models as adjusted effects. Because our aim was to describe relationships among clinical and patient-reported endpoints, both of which are assumed to reflect patients' clinical history in varying degrees, we did not adjust for clinical history variables. To facilitate interpretation, all continuous covariates are centered around their grand mean. We report parameter estimates for a 1 SD difference in 6-minute walk distance and peak VO2. Because so few participants in the sample were in NYHA class IV (n = 23), we developed models comparing NYHA classes II and III.
The patient-reported outcome measures used in this study were expected to be at least moderately correlated with each other, because both are patient-reported measures of health-related quality of life. We hypothesized that greater disease severity measured clinically—lower peak VO2, shorter 6-minute walk distance, and higher NYHA class—would be positively correlated with greater severity in patient reports, as measured by the KCCQ and the VAS. We expected that the heart failure–specific KCCQ would have a stronger relationship with the clinical measures than the general VAS. We also expected the KCCQ physical limitation subscale to have stronger relationships with peak VO2 and 6-minute walk distance than the other KCCQ subscales or the overall score, because it specifically targets patients' reports of their ability to walk 1 block, climb stairs, and jog, among other activities.
Results
HF-ACTION randomly assigned 2331 patients, of whom 2330 (99.9%) completed the KCCQ and 2274 (98%) completed the VAS. Participant characteristics are shown in Table 1. Table 2 shows the distributions of patient-reported and clinical outcome measures. Mean peak VO2 was 15 mL/kg/min, mean 6-minute walk distance was 365 m, and nearly two thirds of the patients were NYHA class II. The mean KCCQ score was 66, and the mean VAS score was 66.
Table 1.
Patient Characteristics (N = 2331)*
| Characteristic | All Patients |
|---|---|
| Age, mean ± SD, y | 59.1 ± 12.6 |
| Female sex, No. (%) | 661 (28.4) |
| Race, No. (%) | |
| Black or African American | 749 (32.6) |
| White | 1426 (62.1) |
| Other | 121 (5.3) |
| Hispanic or Latino ethnicity, No. (%) | 88 (3.8) |
| Education level, No. (%) | |
| Less than high school | 285 (12.5) |
| High school graduate or equivalent | 635 (27.9) |
| Completed some college, but no degree | 608 (26.7) |
| Completed associate degree/diploma program | 201 (8.8) |
| College graduate | 356 (15.6) |
| Completed graduate school | 193 (8.5) |
| Annual income, No. (%) | |
| < $15,000 | 467 (22.5) |
| $15,000-$24,999 | 386 (18.6) |
| $25,000-$34,999 | 303 (14.6) |
| $35,000-$49,999 | 312 (15.1) |
| $50,000-$74,999 | 307 (14.8) |
| $75,000-$99,999 | 155 (7.5) |
| ≥ $100,000 | 143 (6.9) |
| Employment status, No. (%) | |
| Disabled | 711 (31.2) |
| Employed full-time | 416 (18.2) |
| Employed part-time | 131 (5.7) |
| Retired | 833 (36.5) |
| Other | 191 (8.4) |
| Marital status, No. (%) | |
| Divorced/separated | 417 (18.1) |
| Married/living with a partner | 1411 (61.1) |
| Single, never married | 261 (11.3) |
| Widowed | 221 (9.6) |
| Insurance, No. (%) | |
| Medicare Part A only | 157 (7.1) |
| Medicare Part A/B | 704 (31.9) |
| Non-Medicare patient | 1344 (61.0) |
| Comorbid conditions, No. (%) | |
| Atrial fibrillation/atrial flutter | 488 (20.9) |
| Diabetes mellitus | 748 (32.1) |
| Hypertension | 1388 (59.9) |
| Renal dysfunction | 31 (1.3) |
| Ejection fraction, mean ± SD, % | 25.2 (7.1) |
| Ischemic etiology of heart failure, No. (%) | 1197 (51.4) |
Some variables contain missing values.
Table 2.
Clinical and Patient-Reported Outcome Measures (N = 2331)
| Measure | Outcome |
|---|---|
| 6-minute walk distance, m | |
| Mean ± SD | 364.5 ± 104.7 |
| Median (IQR) | 370.6 (298.7-435.0) |
| Peak VO2, mL/kg/min | |
| Mean ± SD | 14.9 ± 4.7 |
| Median (IQR) | 14.4 (11.5-17.7) |
| NYHA class, No. (%) | |
| II | 1477 (63.4) |
| III | 831 (35.7) |
| IV | 23 (1.0) |
| EuroQol Visual Analog Scale | |
| Mean ± SD | 65.5 ± 19.0 |
| Median (IQR) | 70.0 (50.0-80.0) |
| KCCQ overall summary score | |
| Mean ± SD | 66.2 ± 20.6 |
| Median (IQR) | 68.0 (50.9-83.3) |
| KCCQ clinical summary score | |
| Mean ± SD | 71.3 ± 19.5 |
| Median (IQR) | 74.0 (57.8-86.7) |
| KCCQ physical limitation subscale | |
| Mean ± SD | 69.4 ± 21.9 |
| Median (IQR) | 70.8 (54.2-87.5) |
| KCCQ total symptom subscale | |
| Mean ± SD | 73.1 ± 20.8 |
| Median (IQR) | 77.1 (58.3-89.6) |
| KCCQ social limitation subscale | |
| Mean ± SD | 62.4 ± 27.5 |
| Median (IQR) | 62.5 (37.5-87.5) |
| KCCQ self-efficacy subscale | |
| Mean ± SD | 81.0 ± 20.3 |
| Median (IQR) | 87.5 (75.0-100.0) |
| KCCQ quality of life subscale | |
| Mean ± SD | 59.7 ± 24.7 |
| Median (IQR) | 58.3 (41.7-83.3) |
Abbreviations: IQR, interquartile range, NYHA, New York Heart Association Class; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Relationships Among Patient-Reported Outcomes and Clinical Measures
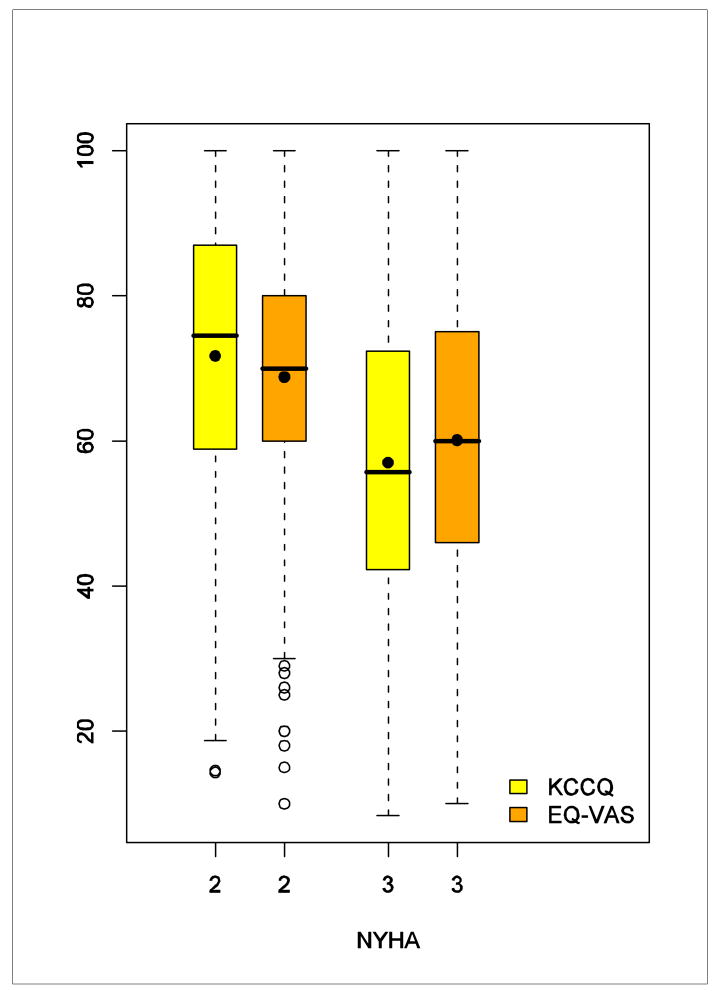
The correlation between the VAS and KCCQ scores was moderate (r = 0.53). Figure 1 shows the relationships between NYHA class and the patient-reported outcome measures. Patients in NYHA class II had higher VAS and KCCQ scores compared with patients in NYHA class III.
Figure 1. Side-by-Side Box Plot of the VAS and the KCCQ Overall Score by NYHA Class.
The upper and lower bounds of the boxes represent interquartile ranges. The lines dividing the boxes represent medians and the points inside represent means. The upper ends of the whiskers represent maximum observations; the lower ends represent minimum observations (NYHA = 3) or minimum observations above the lower fence of 1.5 interquartile range below the 25th percentile (NYHA = 2); and the open points represent extreme observations below the lower fence.
Table 3 shows zero-order and covariate-adjusted Pearson correlation coefficients comparing patient-reported and clinical measures. After adjustment for sociodemographic characteristics, there were small correlations between VAS scores and peak VO2 or 6-minute walk distance. The correlations between KCCQ score and peak VO2 or 6-minute walk distance were also modest, though they were significantly larger than the correlations with the VAS. The correlations between clinical measures and the KCCQ physical limitation subscale were significantly larger than correlations with the KCCQ overall score (P < .001). Other KCCQ subscales had smaller correlations with peak VO2 and 6-minute walk distance, though all correlations were statistically significant, except where noted in Table 3.
Table 3.
Correlations Between Patient-Reported Measures and Continuous Clinical Measures
| PRO Measure | Clinical Measure | Zero-Order Correlation (95% CI) | Adjusted Correlation (95% CI) |
|---|---|---|---|
| EuroQol VAS | Peak VO2 | 0.094 (0.052 to 0.135) | 0.137 (0.096 to 0.179) |
| Six-minute walk | 0.112 (0.071 to 0.153) | 0.137 (0.095 to 0.178) | |
| KCCQ overall score | Peak VO2 | 0.213 (0.173 to 0.252) | 0.222 (0.177 to 0.265) |
| Six-minute walk | 0.271 (0.232 to 0.308) | 0.278 (0.235 to 0.32) | |
| KCCQ clinical summary | Peak VO2 | 0.282 (0.244 to 0.320) | 0.274 (0.233 to 0.315) |
| Six-minute walk | 0.322 (0.285 to 0.358) | 0.317 (0.276 to 0.357) | |
| KCCQ subscales | |||
| Physical limitation | Peak VO2 | 0.309 (0.271 to 0.346) | 0.293 (0.252 to 0.334) |
| Six-minute walk | 0.345 (0.309 to 0.381) | 0.330 (0.288 to 0.369) | |
| Total symptom | Peak VO2 | 0.207 (0.168 to 0.246) | 0.207 (0.164 to 0.248) |
| Six-minute walk | 0.244 (0.205 to 0.282) | 0.239 (0.197 to 0.28) | |
| Social limitation | Peak VO2 | 0.152 (0.111 to 0.193) | 0.162 (0.118 to 0.205) |
| Six-minute walk | 0.209 (0.169 to 0.248) | 0.216 (0.172 to 0.26) | |
| Self-efficacy | Peak VO2 | 0.049 (0.008 to 0.090) | 0.031 (−0.011 to 0.072)† |
| Six-minute walk | 0.047 (0.006 to 0.088) | 0.028 (−0.014 to 0.07)‡ | |
| Quality-of-life | Peak VO2 | 0.101 (0.060 to 0.142) | 0.139 (0.094 to 0.183) |
| Six-minute walk | 0.166 (0.126 to 0.206) | 0.194 (0.149 to 0.237) |
Abbreviations: PRO, patient-reported outcome; CI, confidence interval; VAS, Visual Analog Scale; peak VO2, peak oxygen consumption during cardiopulmonary exercise testing; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Partial correlation coefficient after adjusting for significant patient characteristics listed in Table 1. All correlations are significantly different from zero with P < .05 except where noted.
Significantly different from zero (P = .15).
Significantly different from zero (P = .20).
Table 4 shows the results of the regression models. In unadjusted results, a 1 SD difference in peak VO2 (4.7 mL/kg/min) was associated with a 1.78-point difference in VAS score (95% CI, 0.99-2.57) and a 4.38-point difference in KCCQ score (95% CI, 3.55-5.21). A 1 SD difference in 6-minute walk distance (105 m) was associated with a 2.15-point difference in VAS score (95% CI, 1.36-2.94) and a 5.57-point difference in KCCQ score (95% CI, 4.76-6.39). NYHA class II was associated with an 8.67-point lower VAS score (95% CI, 7.07-10.26) and a 14.67-point lower KCCQ score (95% CI, 13.04-16.31) compared to NYHA class III. Adjustment for sociodemographic characteristics resulted in slightly larger effect sizes. There were no interactions between patient characteristics and the associations of 6-minute walk distance or peak VO2 with VAS scores.
Table 4.
Predictors of Quality of Life in Patients With Heart Failure
| PRO Measure | Predictor | Unadjusted Effect β̂ (95% CI) | Adjusted Effect β̂ (95% CI) |
|---|---|---|---|
| EuroQol VAS | Peak VO2, 1 SD | 1.78 (0.99 to 2.57) | 2.86 (1.98 to 3.74) |
| Six-minute walk, 1 SD | 2.15 (1.36 to 2.94) | 2.78 (1.92 to 3.64) | |
| NYHA class II to III | −8.67 (−10.26 to −7.07) | −8.26 (−9.93 to −6.59) | |
| KCCQ overall score | Peak VO2, 1 SD | 4.38 (3.55 to 5.21) | 4.75 (3.78 to 5.72) |
| Six-minute walk, 1 SD | 5.57 (4.76 to 6.39) | 5.92 (4.98 to 6.87) | |
| NYHA class II to III | −14.67 (−16.31 to −13.04) | −12.73 (−14.53 to −10.92) | |
| KCCQ clinical summary | Peak VO2, 1 SD | 5.52 (4.75 to 6.29) | 5.41 (4.56 to 6.26) |
| Six-minute walk, 1 SD | 6.29 (5.53 to 7.05) | 6.37 (5.51 to 7.22) | |
| KCCQ subscales | |||
| Physical limitation | Peak VO2, 1 SD | 6.77 (5.91 to 7.63) | 6.44 (5.47 to 7.42)† |
| Six-minute walk, 1 SD | 7.59 (6.74 to 8.44) | 7.45 (6.48 to 8.43) | |
| Total symptom | Peak VO2, 1 SD | 4.32 (3.48 to 5.16) | 4.39 (3.47 to 5.30) |
| Six-minute walk, 1 SD | 5.07 (4.24 to 5.89) | 5.19 (4.26 to 6.13) | |
| Social limitation | Peak VO2, 1 SD | 4.18 (3.06 to 5.31) | 5.27 (2.69 to 7.86)‡ |
| Six-minute walk, 1 SD | 5.76 (4.65 to 6.88) | 6.21 (4.92 to 7.50) | |
| Self–efficacy | Peak VO2, 1 SD | 0.99 (0.16 to 1.82) | 0.62 (−0.22 to 1.47) |
| Six-minute walk, 1 SD | 0.95 (0.12 to 1.78) | 0.56 (−0.29 to 1.42) | |
| Quality–of–life | Peak VO2, 1 SD | 2.50 (1.49 to 3.51) | 5.11 (2.71 to 7.51)‡ |
| Six-minute walk, 1 SD | 4.10 (3.10 to 5.11) | 5.01 (3.85 to 6.16) |
Abbreviations: PRO, patient-reported outcome; CI, confidence interval; VAS, Visual Analog Scale; peak VO2, peak oxygen consumption during cardiopulmonary exercise testing; KCCQ, Kansas City Cardiomyopathy Questionnaire.
Adjusted for all significant patient characteristics listed in Table 1.
Significant interactions included peak VO2 with age and peak VO2 with ethnicity.
Significant interactions included peak VO2 with age, peak VO2 with employment status, and peak VO2 with marital status.
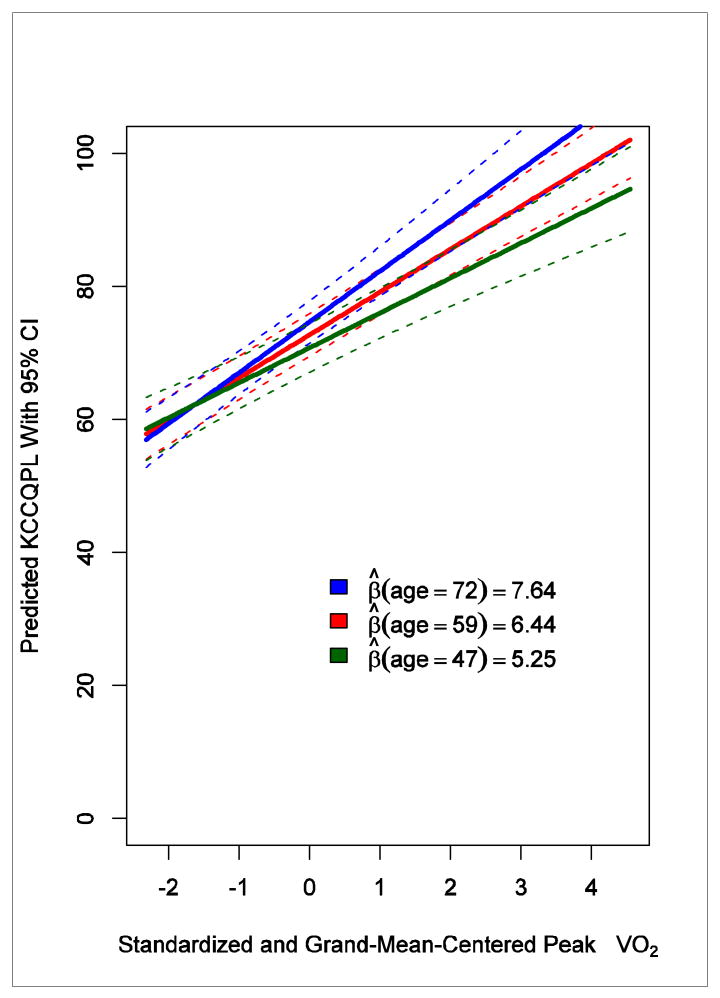
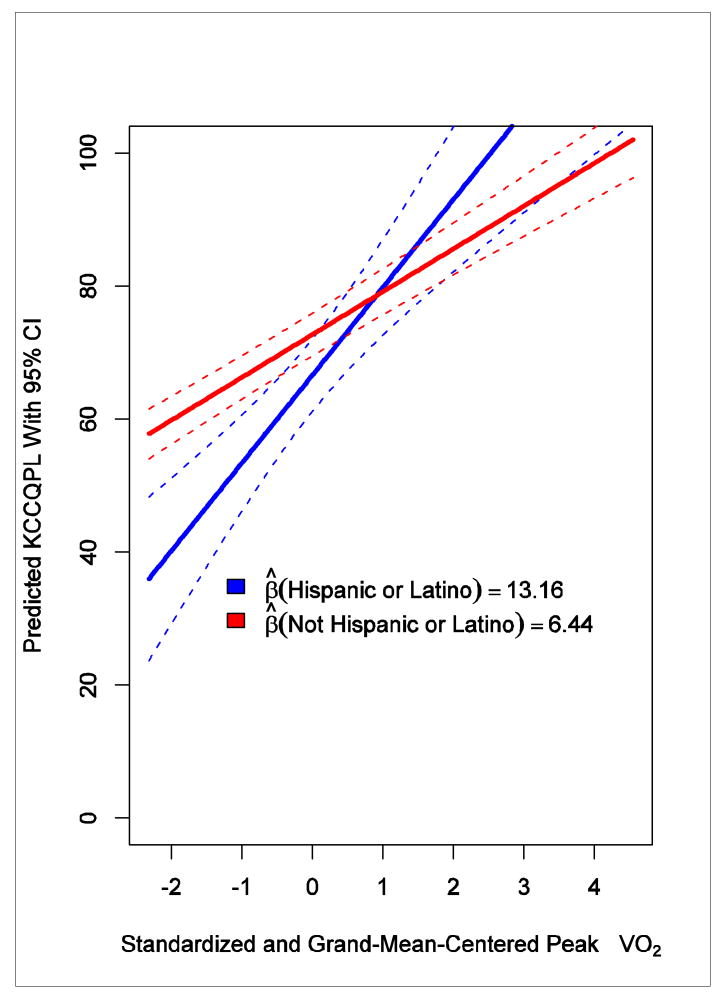
A 1 SD difference in peak VO2 was associated with a 6.77-point difference in KCCQ physical limitation subscale score (95% CI, 5.91-7.63), and a 1 SD difference in 6-minute walk distance was associated with a 7.59-point difference in KCCQ physical limitation subscale score (95% CI, 6.74-8.44). Although adjustment for sociodemographic characteristics did not result in much difference, there was an interaction effect with peak VO2 and age (P = .008) and peak VO2 and ethnicity (P = .006). At higher aerobic capacity, patient-reported physical functioning was greater for older patients versus younger patients and for Hispanic or Latino patients versus non–Hispanic or Latino patients (Figures 2 and 3).
Figure 2. Interaction Between Age and Peak VO2 in Relationship With the KCCQ Physical Limitation Subscale.
The solid lines represent predicted regression lines at the mean age (59 years, in red), 1 SD above the mean age (72 years, in blue), and 1 SD below the mean age (47 years, in green), with all categorical variables (ie, education level, annual income, employment status, marital status, and ethnicity) held at their modes. The dashed lines represent 95% confidence intervals around the fitted lines. β̂ denotes predicted slope.
Figure 3. Interaction Between Ethnicity and Peak VO2 in Relationship With the KCCQ Physical Limitation Subscale.
The solid lines represent predicted regression lines for patients of Hispanic or Latino ethnicity and for patients who are not Hispanic or Latino, with age held at the mean (59 years) and all categorical variables (ie, education level, annual income, employment status, and marital status) held at their modes. The dashed lines represent 95% confidence intervals around the fitted lines. β̂ denotes predicted slope.
In the models that included a single interaction term each, there were also significant interactions between peak VO2 and age, peak VO2 and employment status, and peak VO2 and marital status when predicting the KCCQ quality-of-life and social limitation subscales. However, due to considerable correlations among age, employment status, and marital status, these interaction terms were not statistically significant when entered into the same model.
Discussion
We used baseline data from HF-ACTION to characterize relationships among important patient-reported health-related quality-of-life and clinical measures and to examine whether the relationships differed among subgroups. As expected, the patient-reported outcome measures (ie, VAS and KCCQ) were moderately correlated with each other, and the heart failure–specific KCCQ had stronger correlations with exercise capacity than the more general VAS. The KCCQ physical limitation subscale, which was designed to quantify patients' exertional capacity, had the largest, though still modest, correlation with clinical measures of exercise capacity, a pattern previously found for this subscale and 6-minute walk distance.17 Consistent with other disease domains,16 none of the patient-reported outcome measures were highly correlated with peak VO2 or 6-minute walk distance. These imperfect correlations with clinical measures reflect the unique information that patient-reported outcome measures add. Thus, the findings underscore the importance of directly assessing patients' health status from self-report rather than relying on traditional assessments of function to quantify patients' perspectives of their disease.16, 17
Most relationships between clinical and patient-reported outcome measures did not vary by sociodemographic characteristics. Moderate correlations among several sociodemographic characteristics made it difficult to isolate individual interactions between these variables and aerobic capacity in predicting patient-reported outcome scores; however, the data suggest that the KCCQ subscales for physical limitation, quality of life, and social limitation varied in their relationships with peak VO2 by age, such that differences in peak VO2 translated into larger differences in the KCCQ scores for older adults. A similar finding has been reported for the KCCQ quality-of-life subscale.18 It is questionable whether these differences by age are clinically significant, however (ie, 1 SD differences in age [12 years] correspond to differences of about 1 point on the KCCQ physical limitation subscale).
The large sample size of HF-ACTION permitted a strong corroboration of the relationship between KCCQ scores and clinical endpoints that had been reported in previous studies.8, 11, 17, 19, 20 Our findings also help to characterize differences in KCCQ scores in terms of clinically meaningful differences in health status. In a prior study, a mean within-person change of 5 or more points corresponded to cardiologists' assessments of clinically significant changes in patients with heart failure during a 6-week period.10 Our findings indicate that a 5-point difference between people may correspond to clinically meaningful differences between people, as a 1 SD difference in peak VO2 and 6-minute walk distance each corresponded to roughly a 5-point difference in KCCQ score. Without consensus about what constitutes clinically significant differences in 6-minute walk distance and peak VO2, we chose 1 SD because it clearly represents a meaningful difference between patients with heart failure. Previous studies have considered far less stringent criteria than the 1 SD difference we used for 6-minute walk distance8, 18 and peak VO2.21-23
Comparing NYHA class II to III showed much larger differences in KCCQ and VAS scores, 15 and 9 points respectively. These results confirm previous work that suggested a 15-point difference in KCCQ corresponds to a large change as evaluated by cardiologists during a 6-week period10 as well as a 9-point difference on a “ladder of life” (VAS) corresponding to the difference between NYHA classes II and III.24 These large differences are congruent with the coarse nature of the categorical NYHA class. As shown in Figure 1, each class (assigned by the physician rather than the patient) encompasses patients with a broad range of self-reported symptoms, function, and health-related quality of life.
Limitations
This study has important limitations. First, the data come from a clinical trial population, and thus men and younger patients were overrepresented compared to the typical heart failure population. A limited number of patients in NYHA class IV enrolled in the trial, so our results may not be applicable to all patients with heart failure, especially those with severe symptoms. Also, some caution is advised in interpreting the associations where the number of subjects is small, such as the interaction between peak VO2 and patient-reported physical limitation by ethnicity. Second, the present analyses were based on cross-sectional data and describe the minimum important difference between patients. This study examined cross-sectional relationships, and although the analyses provide information about meaningful differences in patient-reported outcome measures between individuals, longitudinal data are needed to assess meaningful changes within individuals. Follow-up of the HF-ACTION patient population will allow such assessment of prospective associations.
Finally, to test the sensitivity of our results to the modeling assumption that patients' health-related quality of life is partially a function of patients' underlying physiological status, we tested a symmetric model (using orthogonal regression) in which no variable was considered the independent variable. This resulted in a 1 SD difference in peak VO2 corresponding to a 91.8 point difference in KCCQ and a 191.5 point difference in the VAS. (Stated another way, a 5-point difference in KCCQ corresponded to a 0.26 mL/min/kq difference in peak VO2 and a 3-point difference in the VAS corresponded to a 0.07 mL/min/kq difference in peak VO2.) A 1 SD difference in 6-minute walk corresponded to a 5.8 point difference in KCCQ and 2.2 point change in the VAS. (A 5-point difference in KCCQ corresponded to a 90 m difference in 6-minute walk and a 3-point difference in the VAS corresponded to a 141 m difference in 6-minute walk.) This sensitivity analysis supports the practice of considering a 5-point difference on the KCCQ and a 3-point difference on the VAS to be clinically meaningful when considering 6-minute walk but not peak VO2. These sensitivity analyses underscore how lower correlations between patient-reported outcomes and clinical endpoints lead to greater uncertainty about the precise mapping of one metric onto another. We intend to fully explore this issue in a separate, methodological manuscript.
Conclusions
Differences in patient-reported outcome measures (including the KCCQ, its subscales, and the VAS) are associated with differences in clinical measures (including peak VO2, 6-minute walk distance, and NYHA class). The VAS, a general patient-reported measure, had smaller correlations with exercise capacity than the disease-specific KCCQ. With a few exceptions, these relationships are consistent across different groups of patients with heart failure. While the small size of the correlations introduces significant uncertainty, the findings generally support the current practice of considering a 5-point difference between individuals on the KCCQ to be clinically meaningful and provide some support for using a 3-point difference between individuals on the VAS as clinically meaningful.
Acknowledgments
We thank Susan Kay Roll, RN, University of Cincinnati, for her role as an author on the abstract and Damon M. Seils, MA, Duke University, for assistance with manuscript preparation. Ms Roll and Mr Seils did not receive compensation for their assistance apart from their employment at institutions where the study was conducted.
A complete list of the HF-ACTION investigators is available as the last item in this supplement. This research was supported by National Institutes of Health grants: 5U01HL063747, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, 5U01HL064264, 5U01HL066461, R37AG18915, P60AG10484.
Funding/Support: HF-ACTION was funded by grants 5U01HL063747 (Duke University, C. O'Connor, coordinating center), 5U01HL066461 (Duke University, K. Schulman, economics and quality of life), 5U01HL068973 (Boston Medical Center, W. Colucci), 5U01HL066501 (Case Western Reserve University, I. Piña), 5U01HL066482 (Emory University, A. Smith), 5U01HL064250 (Henry Ford Hospital, S. Keteyian), 5U01HL066494 (Ohio State University, W. Abraham), 5U01HL064257 (Oregon Health & Science University, R. Hershberger), 5U01HL066497 (University of Alabama at Birmingham, V. Bittner), 5U01HL068980 (University of California, Los Angeles, G. Fonarow), 5U01HL064265 (University of Colorado Health Sciences Center, E. Wolfel), 5U01HL066491 (Wake Forest University, D. Kitzman), and 5U01HL064264 (Washington University in St. Louis, G. Ewald) from the National Heart, Lung, and Blood Institute.
Footnotes
ClinicalTrials.gov Identifier: NCT00047437
Financial Disclosures: Dr Ellis reports receiving grants from GE Medical. Dr Spertus owns the copyright to the Kansas City Cardiomyopathy Questionnaire. Dr Whellan reports receiving grants or funding from GE Medical and the National Institutes of Health. Dr Piña reports receiving grants or funding from the National Institutes of Health; receiving personal income for consulting from the Food and Drug Administration; and receiving honoraria from AstraZeneca, Innovia, Merck, Novartis, Sanofi-Aventis, and Solvay. Dr Schulman reports receiving research support from Actelion Pharmaceuticals, Allergan, Amgen, Astellas Pharma, Bristol-Myers Squibb, The Duke Endowment, Genentech, Inspire Pharmaceuticals, Johnson & Johnson, Kellogg Foundation, Kureha Corporation, LifeMasters Supported SelfCare, Medtronic, Merck & Co, Nabi Biopharmaceuticals, National Patient Advocate Foundation, North Carolina Biotechnology Center, NovaCardia, Novartis, OSI Eyetech, Pfizer, Sanofi-Aventis, Scios, Tengion, Theravance, Thomson Healthcare, and Vertex Pharmaceuticals; receiving personal income for consulting from McKinsey & Company and the National Pharmaceutical Council; having equity in Alnylam Pharmaceuticals; having equity in and serving on the board of directors of Cancer Consultants, Inc; and having equity in and serving on the executive board of Faculty Connection, LLC. Dr Schulman has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp). No other disclosures were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
References
- 1.Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–7. doi: 10.1056/NEJM199605303342206. [DOI] [PubMed] [Google Scholar]
- 2.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20(9):1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(Suppl 2):S125–37. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 6.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart. 2006;92(1):62–67. doi: 10.1136/hrt.2004.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eurich DT, Johnson JA, Reid KJ, Spertus JA. Assessing responsiveness of generic and specific health related quality of life measures in heart failure. Health Qual Life Outcomes. 2006;4:89. doi: 10.1186/1477-7525-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstam MA, Neaton JD, Poole-Wilson PA, Pitt B, Segal R, Sharma D, et al. Comparison of losartan and captopril on heart failure-related outcomes and symptoms from the losartan heart failure survival study (ELITE II) Am Heart J. 2005;150(1):123–31. doi: 10.1016/j.ahj.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, et al. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150(4):707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JR. Exercise and the failing heart. Cardiol Clin. 1987;5(2):171–81. [PubMed] [Google Scholar]
- 13.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993;270(14):1702–7. [PubMed] [Google Scholar]
- 14.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th. Boston, MA: Little, Brown & Co; 1994. [Google Scholar]
- 15.Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- 16.Wilson IIB, Cleary PPD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 17.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12(6):439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Masoudi FA, Rumsfeld JS, Havranek EP, House JA, Peterson ED, Krumholz HM, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10(5):368–373. doi: 10.1016/j.cardfail.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–756. doi: 10.1016/j.jacc.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Luther SA, McCullough PA, Havranek EP, Rumsfeld JS, Jones PG, Heidenreich PA, et al. The relationship between B-type natriuretic peptide and health status in patients with heart failure. J Card Fail. 2005;11(6):414–421. doi: 10.1016/j.cardfail.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 22.Savage PD, Toth MJ, Ades PA. A re-examination of the metabolic equivalent concept in individuals with coronary heart disease. J Cardiopulm Rehabil Prev. 2007;27(3):143–8. doi: 10.1097/01.HCR.0000270693.16882.d9. [DOI] [PubMed] [Google Scholar]
- 23.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 24.Cook J, Glick H, Kinosian B. An assesment of the “ladder of life” score as a value measure for health status in congestive heart failure. Medical Decision Making. 1993;13(4):386. [Google Scholar]
- 25.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–81. doi: 10.1161/CIRCULATIONAHA.106.670901. [DOI] [PubMed] [Google Scholar]