Abstract
Background
Isolated islet transplantation with infusions from 2-3 donor pancreata and Edmonton immunosuppression consistently achieves insulin independence in patients with type 1 diabetes. The success of this protocol has been attributed to a novel combination of immunosuppressive agents and avoidance of steroids, however, the outcome of islet transplantation may differ in kidney transplant recipients who are already immunosuppressed.
Methods
We compared the metabolic outcomes and graft survival of islet transplantation in our program where 9 patients underwent islet transplantation alone (ITA) treated with Edmonton immunosuppression and 8 patients received islet after kidney (IAK) transplants under standard kidney transplant immunosuppression often including steroids.
Results
Transplants in the IAK and ITA setting demonstrated similar islet potency (IEq/unit insulin reduction) and recipients from both groups routinely gained insulin independence, functional islet mass and duration of graft survival, however, seemed superior in the IAK group.
Conclusions
These results suggest that better islet graft function and survival may be attained using non-Edmonton rather than Edmonton immunosuppression and can include maintenance steroid therapy.
Keywords: diabetes, islet, transplantation
Introduction
During the 1990's, the success of islet transplantation in gaining insulin independence was poor though many patients demonstrated sustained graft function as assessed by C-peptide production and reduced daily insulin requirements. In 2000, investigators from Edmonton reported a novel islet transplant protocol that resulted in insulin independence in 7 consecutive patients (1). The reasons underlying the success of the Edmonton protocol remain to be defined but two factors seem most likely to be integral to their superior results. First is that the Edmonton team transplanted a large mass of high-quality islets. This was accomplished using the previously unexplored approach of re-transplanting patients with a second or third dose of islets, each procured from separate donor pancreata. Another important factor was the use of a novel immunosuppression regimen consisting of induction and maintenance therapy that was steroid-free to reduce β-cell toxicity and diabetogenicity.
The efficacy of the Edmonton approach has since been confirmed by an Immune Tolerance Network multi-center trial, and in multiple single-center trials (2-5). Unfortunately, continuing follow-up of patients transplanted using the Edmonton regimen has revealed an inexorable deterioration in islet graft function over time, with 90% of recipients returning to insulin therapy by five-years post-transplant (6). The reasons for the progressive loss of functional islet mass remain unexplained, although a number of possibilities have been suggested, including rejection, recurrent autoimmunity, site related dysfunction, marginal mass exhaustion and immunosuppression drug toxicity.
The transplantation of islets alone was an important aspect of the Edmonton protocol as it permitted flexibility in the selection of immunosuppressive agents. In contrast, when islets are transplanted to patients who have an established renal graft that was transplanted for diabetic nephropathy, the immunosuppression is often dictated by the standard of care for the kidney graft although patient might undergo immunotherapy conversion to that resembling the Edmonton protocol or other regimen (7). Nonetheless, IAK transplants have both actual and theoretical advantages: 1) the risk/benefit considerations are more favorable since the recipient is already obligated to life-long immunosuppression; and 2) the chronically immunosuppressed host may provide a more receptive milieu for islet engraftment and long-term survival. Here, we compare the metabolic outcomes and graft survival between the islet transplant alone (ITA) and islet after kidney (IAK) transplantation performed at our center.
Methods
Patients
A total of 17 patients at the University of Pennsylvania received islet transplants as 31 separate infusions between 9/21/01 and 4/29/06, including 9 islet transplant alone (between 09/2001 to 10/2002) and 8 islet after kidney transplants (between 01/2003 to 04/2006). A summary of recipient demographics for these two groups is shown in Table 1. For the IAK group, the duration of time that lapsed between the kidney transplant and the islet transplant was 6.5±4.9years with a range of 2.2 years to 14.8 years. The study protocol was approved by the institutional review board of the University of Pennsylvania, and written informed consent was obtained from all participants.
Table 1. Recipient Characteristics for Two Groups of Islet Recipients.
| ITA | IAK | |
|---|---|---|
| Male/Female | 5/4 | 4/4 |
| Age (year) | 41.4±8.2 | 44.1±7.0 |
| Body Weight (kg) | 67.9±9.1 | 64.9±14.9 |
| BMI | 24.2±2.7 | 22.4±3.5 |
| Diabetes duration (y) | 28.1±10.2 | 27.9±5.8 |
| C-peptide (ng/ml)* | All <0.05 | All <0.05 |
| HgA1c (%) | 7.1±1.2 | 7.9±1.4 |
| Insulin Unit/day | 36.7±9.8 | 42.3±14.9 |
| Insulin Unit/kg/day | 0.56±0.22 | 0.64±0.25 |
Abbreviations: ITA, Islet Transplant Alone; IAK, Islet After Kidney; BMI, Body Mass Index.
C-peptide negative defined as the stimulated C-peptide <0.05ng/ml after OGTT.
Immunosuppression
All patients received induction therapy with daclizumab IV 2.0 mg/kg at the time of islet transplant and 1.0 mg/kg every 14 days for a total of five doses. ITA patients received Edmonton immunosuppression: tacrolimus 1mg orally prior to transplant then 1 mg PO BID, adjusted to maintain a trough level of 3-5 ng/ml and sirolimus 0.2mg/kg PO immediately pre-transplant and then 0.1 mg/kg daily in order to maintain target trough levels of 12-15 ng/ml as measured by HPLC. In IAK patients, the subsequent immunosuppression was dictated by the optimal management of the kidney graft. In the IAK group immunosuppression consisted of: tacrolimus-sirolimus (one patient), tacrolimus-MMF (one patient), cyclosporine-MMF-prednisone (one patient), and tacrolimus-MMF-prednisone (five patients). Of note, 6 out of the 8 patients in this group received 5mg of prednisone daily as a part of their regimen, and 4 had received T-cell depletion therapy at the time of their kidney transplant (thymoglobulin n=3, OKT3 n=1).
Islet Isolation
Pancreatic islets were isolated using a modification of the automated Ricordi method (7) by the same isolation team. Collagenase enzyme (Liberase 0.5g, Roche) in Hanks' solution was infused into the main pancreatic duct using a hand-held syringe and a Webster cannula (Sigma, St. Louis, MO). The Ricordi digestion chamber was agitated with a mechanical shaker to facilitate the digestion process. Islets were re-suspended in WU solution and separated from non-endocrine tissues using the COBE 2991 (Gambro, Lakewood, CO) (not refrigerated) and a top-loaded continuous Ficoll gradient (density range 1.055– 1.120). The purified islet cells were transplanted to patients either promptly (without culture) or after a short-term culture. Isolated islets were assessed routinely for their viability and function, using both in vitro and in vivo assays. All preparations were evaluated by gram-stain and culture on completion of the isolation procedure. None of the preparations developed positive culture results. The release criteria for transplant were with minimal islet mass ≥4,000IEq/kgof recipient body weight; viability ≥70%; purity ≥30%; and endotoxin ≤5.0EU/kg of recipient body weight. Regarding to the quality of islet preparations (based on the release criteria), there were no differences between the ITA and the IAK groups.
Pre-Transplant Islet Culture
In the IAK group, 36.8% of the islet preparations were cultured in vitro for a period of 6-72 hours prior to transplant, whereas none of the islet preparations were cultured pre-transplant in the ITA group. In the cultured preparations, approximately 30,000 islets were placed in 175 ml flasks after being suspended in supplemented CMRL with 5% human albumin. Culture flasks were kept at 22°C in incubator with 5% CO2 concentration and culture media was changed every 24-48 hours. Cultured vs. non-cultured preparations were also compared in terms of islet potency (number of islet equivalents required for one unit decrease in the daily insulin requirement of the recipient) and amount of islet mass lost as a result of long-term culturing was calculated.
Post-Transplant Testing
Metabolic outcomes were evaluated by assessment of islet potency defined as the number of IEs required to reduce the daily insulin requirement by 1 Unit (calculated for first infusions only), by islet function in metabolic studies, and by duration of islet graft survival. Metabolic studies were performed at defined intervals, and graft survival was assessed by insulin independence and C-peptide positivity (>0.5 ng/ml). Metabolic tests of glycemic control and islet graft function consisted of measuring HbA1c and performance of liquid meal (Boost® 6 ml/kg up to 360 ml) tolerance tests (MTT) to obtain pre- and 90min post-stimulus levels of serum glucose and C-peptide. In the comparison of the metabolic test results between the two groups, all the data acquired at 3, 6, and 9-month time points were combined. A second analysis included seven patients who were insulin-independent at the time of study. Since C-peptide clearance is dependent on kidney function, we also compared C-peptide levels in ITA and IAK groups corrected for kidney function by dividing the serum creatinine. To assess functionally engrafted β-cell mass a subgroup of four patients from each group underwent arginine stimulation testing (AST) a median 3 months following their second islet infusion where β-cell secretory capacity was estimated from the incremental acute insulin response to arginine (AIRarg) as previously described (8). Log-rank (Mantel-Haenszel) tests were used to compare rates of graft survival, and two-tailed unpaired Student's t-tests were used to compare means between the two groups. Means across the two transplant subgroups and the control group for the AST were compared by one-way ANOVA, and when significant between group means were compared by least significant difference post hoc tests. Significance was defined at a p≤0.05.
Results
Fourteen patients completed the planned therapy. Thirteen of them gained insulin independence after their first or second islet infusions. Three patients in the ITA group became insulin independent even after the first infusion of islets from a single donor. However, one patient in the ITA group who had an extremely high titer of anti-insulin autoantibody (77.4 U/ml; normal < 1.0) failed to reach insulin independence perhaps due to insulin resistance. Another patient in the ITA group withdrew from the study before receiving a second islet infusion because of recalcitrant mouth ulceration related to sirolimus therapy. One patient in the IAK group received only a single islet infusion because a left portal branch vein thrombosis was detected in preparation for a second infusion.
The ITA and IAK groups were similar in terms of demographic data including weight, BMI and pre-transplant insulin requirements (Table 1). Follow-up was longer in the ITA group since the groups were transplanted sequentially. In the ITA group, 9 patients received a total of 16 infusions obtained from 19 donors whereas in the IAK group 8 patients received a total of 15 infusions obtained from 17 donors. Patients received an average of 13,838 islet equivalents (IEq)/kg in the ITA group and 13,411 IEq/kg in the IAK group (p=ns; Table 2). Islet potency as measured by the number of IEqs required for one unit reduction in insulin requirement was calculated for the first islet dose in each patient; and based on the maximal insulin reduction (thus at the different time points) after the first infusion for all patients, the islet potency was not significantly different between the two groups (27,722±13,864 vs. 19,823 ±11,034 p= ns; Table 2).
Table 2. Donor and Graft Characteristics for Two Groups of Islet Recipients.
| ITA | IAK | |
|---|---|---|
| Number of donors | 19 | 17 |
| Donor Age (year) | 48.8±9.6 | 49.6±10.5 |
| Donor BMI | 34.2±9.8 | 34.1±13.7 |
| CIT (min) | 372±79m (6h12m±1h19m) | 405±95m (6h45m±1h35m) |
| Pancreas weight (g) | 115.3±29.8 | 118.5±27.8 |
| Islet yield (IEq) | 495,259±160,926 | 451,891±94,938 |
| Proportion cultured (%) | 0 | 36.8* |
| IEq/kg | 13,838±3,121 | 13,411±5,903 |
| IEq/Ins | 27,722±13,864 | 19,823±11,034** |
Abbreviations: ITA, Islet Transplant Alone; IAK, Islet After Kidney; BMI, Body Mass Index; CIT, Cold Ischemia Time; IEq, Islet Equivalents; and IEq/kg, number of islets in islet equivalent per kg of recipient body weight; IEq/Ins, number of islets in islet equivalent required for reduction of one unit of insulin per day.
p<0.05, and
p>0.05
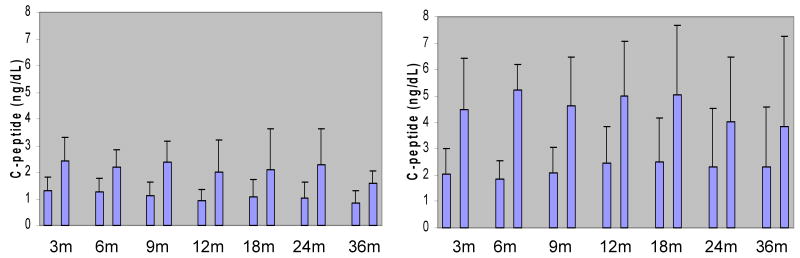
In the comparison of the metabolic data during the first 3-9 months post-transplantation, HbA1c and pre- and post-Boost® serum glucose levels were significantly lower in the IAK group compared to the ITA group (Table 3a). The same analysis was repeated including only the 7 patients (4 ITA and 3 IAK) who were insulin independent at 3-6 months post-transplant, and no differences in HbA1c or serum glucose levels were found (Table 3b). In both analyses the IAK patients had much higher pre- and post-Boost® C-peptide levels compared to ITA patients (p<0.01), a difference that continued up to 3 years post-transplantation (Fig. 2, p values <0.05). When analyzing C-peptide levels corrected for kidney function, the IAK patients also exhibited significantly higher C-peptide/Cr levels both at basal levels and after the liquid meal challenge (with a mean of 1.65 to 3.91 ng/ml per mg/dl vs. 1.54 to 2.94 ng/ml per mg/dl in the ITA group, p<0.05).
Table 3. Assessment of Islet Graft Function - metabolic studies.
| 3,6,9 MONTHS POST-TRANSPLANT (All pts) | |||
|---|---|---|---|
| ITA (n = 9) | IAK (n = 8) | ||
| HbA1c | 6.5 ± 0.6 | 5.9 ±0.6* | |
| C-peptide (pre-MTT) (ng/ml) | 1.2 ± 0.5 | 2.4 ±0.9** | |
| Glucose (pre-MTT) (mg/dl) | 119 ±26 | 101 ±14* | |
| Glucose (post-MTT) (mg/dl) | 190±64 | 139±56* | |
| 3,6 MONTHS POST-TRANSPLANT (7 insulin free pts) | |||
| ITA (n = 4) | IAK (n = 3) | ||
| HbA1c | 6.2±0.5 | 6.3±0.4 | |
| C-peptide (pre-MTT) (ng/ml) | 1.4±0.5 | 2.3±1.2** | |
| C-peptide (post-MTT) (ng/ml) | 2.7±1.2 | 4.0±1.6** | |
| Glucose (pre-MTT) (mg/dl) | 107±23 | 104±14 | |
| Glucose (post-MTT) (mg/dl) | 151±24 | 137±49 | |
Abbreviations: MTT, Mixed-Meal Tolerance Test.
p<0.05;
p<0.01
Figure 2.
Comparison of Islet Graft Function. Metabolic control in patients of both ITA and IAK groups was assessed by measurement of the basal and the stimulated C-peptide levels 90min after the MTT at the different time points post-transplantation.
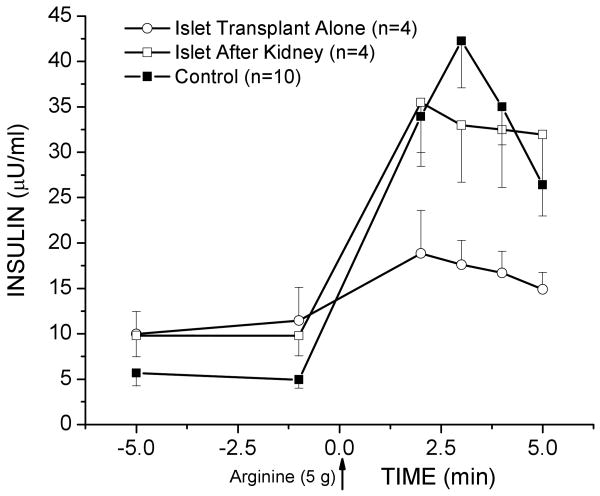
Functional β-cell mass was estimated from arginine stimulation testing (AST) in a subgroup of four patients from each group at a median 3 months following their second islet infusion. The acute incremental insulin response to arginine (AIRarg) was significantly lower in the ITA group (6.3±4.3 μU/ml) compared to the IAK (23.4±3.6; p<0.05) and control (29.1±3.5; p<0.01) groups (Fig. 1), suggesting the presence of a greater initially engrafted islet mass in the IAK group.
Figure 1.
Assessment of the Functional Islet Mass. Plasma insulin responses to the intravenous administration of 5g arginine at t=0 min. The incremental acute insulin response (AIRarg) was significantly lower in the ITA group compared to the IAK (p<0.05) and control (p<0.01) groups.
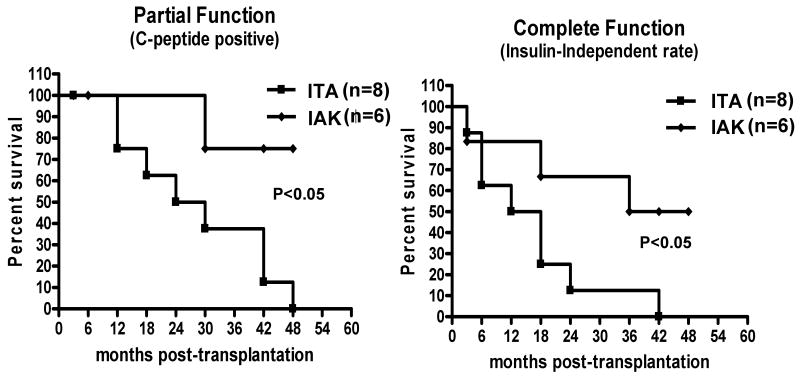
Graft survival as defined by insulin independence was similar in both groups for the first 6 months following transplantation, but the function of islet grafts in the ITA group deteriorated progressively over time, whereas more grafts in the IAK group showed a sustained and stable function (Fig.3b, p<0.05). When we evaluated graft survival as determined by C-peptide positivity, overall survival (ITA and IAK together) was 100% at one year; when survival analysis was done separately for each group however, the IAK group had much better long-term survival compared to the ITA group in which no graft functioned for more than 4 years (Fig. 3a, p<0.05).
Figure 3.
Comparison of Islet Graft Survival. Islet graft survival in patients achieving insulin independence in the study groups was assessed as partial function by persistence of measurable levels of basal C-peptide (>0.5 ng/mL) and complete function with independence from exogenous insulin after islet transplantation.
Additional analysis on the impact of islet culture on islet number and islet potency showed that average pre-culture IEq per isolation was 894,741±414,356 while it dropped significantly down to 583,166±153,149 post-culture (p=0.05). There was no difference in the comparison of the non-cultured vs. cultured islets in terms of potency (25,575±12,408 IEqs/U vs. 21,766±12,971 IEqs/U) (p>0.05). Overall as a result of long-term culturing there was a trend toward gain in islet potency (15%) but a concomitant trend toward a reduction in islet mass (35%) (p NS).
Discussion
Insulin independence was consistently achieved in both ITA and IAK patients in this study; unexpectedly however, the IAK group exhibited superior function by a variety of measures. First, metabolic testing by MTT revealed superior responses to a liquid meal challenge as assessed by basal and stimulated C-peptide levels as well as fasting and 90 minute glucose tolerance. In addition, arginine stimulation testing indicated a significantly greater initially engrafted islet mass in IAK versus ITA patients. HbA1c levels in IAK patients were corrected to more normal levels following IAK than in ITA patients. Finally, both graft function as defined by C-peptide positivity and insulin independence was markedly more durable in the IAK group with 4 of 7 patients maintaining insulin independent graft function for more than 4-5 years. In addition, a fifth patient in this group, despite being unable to receive a second infusion due to left portal branch vein thrombosis, has maintained a minimal and stable insulin requirement indicative of a stable islet mass for almost 4 years.
There are a number of possible explanations for the better initial and sustained function in the IAK group. First, IAK patients are already immunosuppressed at the time of islet transplantation. This could both make the host more receptive to islet infusion with better engraftment and less initial islet loss, and also may promote superior long-term function through enhanced avoidance of allogeneic rejection and/or autoimmune recurrence. Of note is that half of the IAK group had received a T-cell depleting agent at the time of their kidney transplant; prior exposure to the T-cell depleting polyclonal antibody thymoglobulin has previously been correlated with improved islet graft engraftment and survival (9).
A second important difference between the ITA and IAK groups in our study was that the groups were conducted sequentially. An important change in our practice during this period was adoption of pre-transplant in vitro culture of many islet preparations. The fact that one third of IAK but no ITA preparations were maintained in vitro prior to transplant could affect our attempt to assess potency if culture is associated with a loss of damaged islets that would function less well or not at all post-transplant.
Finally, perhaps the most significant difference between the ITA and IAK groups is the differences in maintenance immunosuppression. Whereas all ITA patients received the standard Edmonton regimen with chronic administration of tacrolimus and sirolimus, this regimen was used in only 1 of 8 IAK patients. In the other 7 IAK patients, sirolimus was completely avoided and the regimen was CNI based (6 tacrolimus, 1 cyclosporine) with 6 of 8 patients received daily low-dose steroids (prednisone 5 mg).
The avoidance of the mTOR inhibitor sirolimus (or the combination of tacrolimus-sirolimus) in the islet transplant setting may be beneficial given its well documented anti-angiogenic and anti-proliferative properties. Recent studies by Dor et al using a transgenic mouse model to induce β-cell death and incite β-cell proliferation revealed that combined sirolimus-tacrolimus administration during the recovery phase dramatically impaired β-cell regeneration (10). In fact, this could explain at least in part why no apparent survival advantage was observed in two recently reported series of IAK transplants in which original immumosuppression therapy was switched to the sirolimus-tacrolimus based Edmonton-like protocol (11,12).
The possibility of a non-immunological loss of β-cells is supported by three autopsy cases reported in the literature of islet transplant recipients who died from unrelated causes (13-15). In all three cases, pathological examination revealed a small islet mass that was only a fraction (10-15%) of that originally infused and an absence of inflammation or infiltration of the residual islets with immune cells. Also, gradual loss of syngeneic islets has been observed in primate experiments and in humans undergoing autologous islet transplantation following total pancreatectomy for chronic pancreatitis (16, 17). In these cases, CNI and mTOR inhibitors were not involved so other factors such as marginal islet mass exhaustion may be occurring. The inability for self-renewal induced by anti-proliferative agents would be expected to act synergistically to exacerbate dysfunction of a marginal islet mass.
Other recently reported studies have also documented improved long-term outcome compared with the Edmonton experience (18, 19). In both series, potent induction therapy with a T-cell-depleting agent was employed as was a later switch from tacrolimus-sirolimus to alternative immunosuppression. Also in our study the administration of steroids in 6 out of 8 patients in the IAK group was associated with better function and survival of islets compared to the ITA group in which the steroid-free Edmonton protocol was followed. In fact, better results obtained in our IAK group may be due to the inclusion of steroids in the regimen as well as avoiding the potentially more islet-toxic combination of tacrolimus-sirolimus compared to other conventional protocols (10). Longer follow-up with more patients is needed to draw definite conclusions.
Long-term culturing of islets before transplant is now preferred by many centers based on the belief that the fraction of islets which are unlikely to survive in the recipient die in culture and so only the fittest are transplanted thus eliminating the infusion of unnecessary tissue into the portal vein (18, 19). This culturing period also provides valuable time for quality and sterility assessment of the final product, calling the recipient in and making arrangements for either surgical or radiological access to the portal vein. There are also some authors who claim that culturing is detrimental for islet function and survival (20). In our experience, by long-term culturing there was a statistical trend of 15% gain in islet potency but 35% loss in islet mass. What remains unclear is whether this loss of physical mass also reduces functional mass or whether those islets lost would not have survived in the recipient.
Conclusion
Both ITA and IAK transplants consistently gained insulin independence. Islet potency (as measured by reduction in insulin/islet mass) was similar in ITA and IAK recipients, but islet graft function and survival seemed superior in IAK patients receiving a standard kidney transplant maintenance immunosuppressive regimen. The reasons for the superior function in IAK recipients in this study remain to be defined but could be a result of differences in immunosuppression, including previous exposure to T-cell-depleting agents, the use of low-dose steroids, and the avoidance of sirolimus in combination with a CNI agent. Given these results, we conclude that sufficiently potent immunosuppression (irrespective of the type) coupled with sufficient islet implant mass is key to functional success of islet transplantation.
Acknowledgments
Supported by the Juvenile Diabetes Research Foundation and Public Health Services Research Grants U42-RR-016600 (Penn Islet Cell Resource Center), UL1-RR-024134 (Penn Clinical & Translational Research Center), and P30-DK-19525 (Penn Diabetes Endocrinology Research Center) from the National Institutes of Health.
Footnotes
NIH and JDRF Grants (AN)
Author Contributions: Research design (SD, JFM, CFB, AN), patient management (JFM, MR, HY, EM, MP, CFB, AN), research lab work (SD, JIK, MML, YG), data analysis (SD, JFM, MR EM, MP), and paper writing (SD, JFM, MR, CFB, AN).
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. New England Journal of Medicine. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Markmann JF, Deng S, Huang X, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237:741. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. American Journal of Transplantation. 2005;5:2037. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 5.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 6.Ryan EA, Paty BW, Senior PA, Bigam D, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman DB, Hering B. Islet Transplantation. In: Stuart F, Abecassis M, Kaufman DB, Bioscience Landes, editors. Organ Transplantation. 2nd. 2003. pp. 183–204. Chapter 8. [Google Scholar]
- 8.Rickels MR, Naji A, Teff KL. Acute insulin responses to glucose and arginine as predictors of beta-cell secretory capacity in human islet transplantation. Transplantation. 2007;84:1357. doi: 10.1097/01.tp.0000287595.16442.a7. [DOI] [PubMed] [Google Scholar]
- 9.van de Linde P, Vd Boog PJ, Tysma OM, Elliott JF, et al. Selective unresponsiveness to beta cell autoantigens after induction immunosuppression in pancreas transplantation with anti-interleukin-2 receptor antibody versus anti-thymocyte globulin. Clinical & Experimental Immunology. 2007;149:56. doi: 10.1111/j.1365-2249.2007.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. Journal of Clinical Investigation. 2007;117:2553. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toso C, Baertschiger R, Morel P, Bosco D, et al. Sequential kidney/islet transplantation: efficacy and safety assessment of a steroid-free immunosuppression protocol. American Journal of Transplantation. 2006;6:1049. doi: 10.1111/j.1600-6143.2006.01303.x. [DOI] [PubMed] [Google Scholar]
- 12.Cure P, Pileggi A, Froud T, Messinger S, et al. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85:801. doi: 10.1097/TP.0b013e318166a27b. [DOI] [PubMed] [Google Scholar]
- 13.Davalli AM, Maffi P, Socci C, Sanvito F, Freschi M, Bertuzzi F, Falqui L, Di Carlo V, Pozza G, Secchi A. Insights from a successful case of intrahepatic islet transplantation into a type 1 diabetic patient. Journal of Clinical Endocrinology & Metabolism. 2000;85:3847. doi: 10.1210/jcem.85.10.6877. [DOI] [PubMed] [Google Scholar]
- 14.Smith RN, Kent SC, Nagle J, Selig M, et al. Pathology of an islet transplant 2 years after transplantation: evidence for a nonimmunological loss. Transplantation. 2008;86:54. doi: 10.1097/TP.0b013e318173a5da. [DOI] [PubMed] [Google Scholar]
- 15.Westermark GT, Westermakrk P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreastic islets. N Engl J Med. 2008;359:977. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 16.Ranuncoli A, Cautero N, Ricordi C, Masetti M, et al. Islet cell transplantation: in vivo and in vitro functional assessment of nonhuman primate pancreatic islets. Cell Transplantation. 2000;9:409. [PubMed] [Google Scholar]
- 17.Blondet JJ, Carlson AM, Kobayashi T, Jie T, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surgical Clinics of North America. 2007;87:1477. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 19.Huurman VA, Hilbrands R, Pinkse GG, Gillard P, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson R, Carlsson PO. Better vascular engraftment and function in pancreatic islets transplanted without prior culture. Diabetologia. 2005;48:469. doi: 10.1007/s00125-004-1650-x. [DOI] [PubMed] [Google Scholar]