Abstract
Asthma is an inflammatory disorder of the conducting airways that has strong association with allergic sensitization. The disease is characterized by a polarized Th-2 (T-helper-2)-type T-cell response, but in general targeting this component of the disease with selective therapies has been disappointing and most therapy still relies on bronchodilators and corticosteroids rather than treating underlying disease mechanisms. With the disappointing outcomes of targeting individual Th-2 cytokines or manipulating T-cells, the time has come to re-evaluate the direction of research in this disease. A case is made that asthma has its origins in the airways themselves involving defective structural and functional behaviour of the epithelium in relation to environmental insults. Specifically, a defect in barrier function and an impaired innate immune response to viral infection may provide the substrate upon which allergic sensitization takes place. Once sensitized, the repeated allergen exposure will lead to disease persistence. These mechanisms could also be used to explain airway wall remodelling and the susceptibility of the asthmatic lung to exacerbations provoked by respiratory viruses, air pollution episodes and exposure to biologically active allergens. Variable activation of this epithelial–mesenchymal trophic unit could also lead to the emergence of different asthma phenotypes and a more targeted approach to the treatment of these. It also raises the possibility of developing treatments that increase the lung's resistance to the inhaled environment rather than concentrating all efforts on trying to suppress inflammation once it has become established.
Keywords: allergen, asthma, inflammation, remodelling, T-cell, virus infection
Abbreviations: BHR, bronchial hyper-responsiveness; CT, computed tomography; DC, dendritic cell; ADC, airway DC; EBUS, endobronchial ultrasound; EMTU, epithelial–mesenchymal trophic unit; ETS, environmental tobacco smoke; IFN, interferon; IL, interleukin; IoW, Isle of Wight; LT, leukotriene; mAb, monoclonal antibody; RV, rhinovirus; TGF-β, transforming growth factor-β; Th-2, T-helper-2; TJ, tight junction; TSLP, thymic stromal lymphopoietin
INTRODUCTION
Asthma is an inflammatory disorder of the conducting airways which undergo distinct structural and functional changes, leading to non-specific BHR (bronchial hyper-responsiveness) and airflow obstruction that fluctuates over time. It is among the commonest chronic conditions in Western countries affecting 1 in 7 children and 1 in 12 adults (equivalent to 5.1 million people in the U.K.), and is responsible each year for 1500 avoidable deaths, as well as 20 million lost working days. The annual U.K. healthcare cost is estimated to be £2.5 billion. A recent assessment of asthma across Europe (Brussels Declaration) has identified substantial unmet clinical need which, in the 10% of patients with severe disease, accounts for approx. 50% of the health costs [1].
Most, but not all, asthma is associated with atopy (the inherited predisposition to generate IgE against common environmental allergens). This has led asthma to be regarded largely as an allergic disorder along with other atopic diseases. However, asthma prevention has not been achieved with allergen-reduction strategies, once established there is no cure and there are currently no medications that can alter the natural history of the disease [2].
Management is primarily directed towards suppressing airway inflammation with inhaled corticosteroids and relieving bronchoconstriction with bronchodilators. Apart from corticosteroids, the only oral medications in widespread use are cysteinyl LT (leukotriene) receptor 1 antagonists that inhibit the bronchoconstrictor and inflammatory actions of LTC4, LTD4 and LTE4 (previously known as slow-reacting substances of anaphylaxis). All of these therapies exert their effect downstream of the origins of asthma. There is an urgent need to identify the underlying basis of asthma, understand the complex genetic and environmental influences, and develop appropriate treatment strategies. Although asthma may start at any time in life, the majority begins in early childhood [3], and, although it may spontaneously remit, longitudinal studies reveal that later relapses frequently occur [4]. Severe irreversible airflow obstruction may develop despite apparently appropriate use of controller therapy, as advocated by international and national disease management guidelines [4]. That is not to say that widespread adherence to anti-inflammatory controller therapy does not influence long-term outcomes of asthma; indeed, when treatment adherence is high, as in Finland, asthma mortality and morbidity can be dramatically reduced. The problem is that, in most countries of the world, treatment adherence is low, especially when inhaled drugs are involved on account of perceived fear of systemic side effects, addiction and concerns over acquisition of drug resistance [5]. Many patients only take their anti-inflammatory treatment when they are symptomatic and stop when their symptoms abate [6]. There is some evidence that this approach works well in mild asthma [7], but not in those with more severe disease where regular inhaled corticosteroids, often in large doses and in combination with long-acting β2-bronchodilators, are required for disease control [8]. In low- and middle-income countries, inadequate diagnosis, the costs of drugs and poor education all contribute to poor disease management and overdependence on emergency interventions with the consequence of high mortality and morbidity [9].
THE EMTU (EPITHELIAL–MESENCHYMAL TROPHIC UNIT) INTEGRATES THE ASTHMATIC RESPONSE
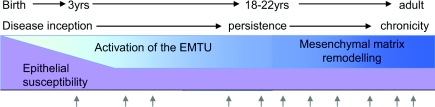
The time has arrived to establish a new research approach that will provide greater insight into the initiation of the disease and its evolution over time into different subphenotypes and lead to treatments that are upstream of airway inflammation and its consequences. To achieve this ‘high ground’, rather than viewing asthma as a series of acute events, it is important to take a life-course view initially focusing on young children and the early-life factors that drive the origins of the disease, and then to track the structural changes and functional airway responses through childhood, adolescence and into adults (Figure 1). On the basis of a large number of converging observations, it is suggested that in asthma a structurally and functionally defective lower airways epithelium underlies abnormal responses to the inhaled environment leading to enhanced signalling between the airway epithelium and underlying structural (the EMTU) and immune cells. This would promote a microenvironment that facilitates allergic sensitization, supports different types of inflammation and predisposes the airways to exacerbations leading to persistence of asthma during childhood. Activation of the EMTU might also be responsible for driving tissue remodelling that progressively leads to a loss of reversibility, reduced lung function and refractoriness to treatment in adults (Figure 2).
Figure 1. Schematic representation of asthma over the life course.
The arrows indicate exacerbations of asthma.
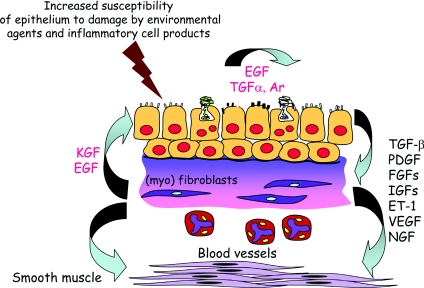
Figure 2. Chronic asthma is characterized by enhanced epithelial–mesenchymal communication with the release of a range of different growth factors linked to remodelling.
Ar, amphiregulin; EGF, epidermal growth factor; ET-1, endothelin-1; FGF, fibroblast growth factor; IGF, insulin-like growth factor; KGF, keratinocyte growth factor; PDGF, platelet-derived growth factor; NGF, nerve growth factor; VEGF, vascular endothelial growth factor.
Although, at one time, considered to be a single disease entity, asthma subphenotypes are now recognized with differing pathology, clinical expression, response to treatment and long-term outcomes [10]. Most asthma exhibits a Th-2 (T-helper-2)-type inflammatory response with the up-regulation of cytokines of the IL (interleukin)-4 gene cluster linked to atopy; however, overinterpretation of this pathway has led to a simplistic view that asthma is purely the result of allergen exposure. Both in adults and children the indistinguishable pathological features of non-allergic and allergic asthma emphasizes that inflammation and remodelling can occur independently of atopy [11]. Atopy affects up to half of the adult population in developed countries; yet, the great majority do not progress to develop asthma [12]. Although in most patients allergen sensitization contributes to asthma, attempts to intervene using allergen-reduction strategies [13] or allergen-specific immunotherapy [14] have proved disappointing, despite its proven efficacy in allergic rhinitis [15]. In severe asthma, activated T-cells are present in abundance, but the initial promise of T-cell inhibitors [cyclosporin A, methotrexate, azathioprine, or anti-CD4- or -CD25-blocking mAbs (monoclonal antibodies)] and blockade of Th-2 cytokines (IL-4, -5, -9 and -13) and antibodies against the pleotropic cytokine TNFα (tumour necrosis factor α), have so far failed to translate into clinical use [16].
The asthma subphenotypes are illustrated well in relation to the use of an IL-5-blocking mAb directed to IL-5 (mepolizimab). Administration of this antibody at two dose levels on three occasions 1 month apart to moderate-to-severe asthmatic patients had a dramatic effect in reducing circulating eosinophils, but had no discernable effect on asthma outcomes [17] and, yet, in both children [18] and adults [19] with hypereosinophilic syndrome, this treatment is highly effective. Two recent small clinical trials of mepolizumab in severe asthmatic patients requiring high dose inhaled and oral corticosteroids with persisting sputum eosinophilia have reported efficacy especially in reducing asthma exacerbations accompanied by considerable reductions in both circulating and sputum eosinophils, but interestingly no effect on BHR [20,21]. A further exciting development related to IL-5 as a target is the development of an antibody-dependent cell cytotoxic defucosylated IgG1 monoclonal antibody (MEDI-563) directed to all cells expressing IL-5Rα (IL-5 receptor α). Engineering of mAbs by removing fucose residues from the Fc fragment leads to greatly enhanced ADCC (antigen-dependent cellular cytotoxicity) activity as compared with a highly fucosylated conventional antibody [22]. Results from a completed Phase 1 study of MEDI-563 have demonstrated the antibody is well tolerated with substantial and prolonged depletion of blood eosinophils, thereby supporting its continued development (http://clinicaltrials.gov/ct2/results?term=NCT00659659).
Beyond improvements in inhaled bronchodilators and corticosteroids to enhance their efficacy, duration of action and safety, the discovery of new anti-asthma drugs has largely been driven by animal models of allergen sensitization and challenge. However, despite almost 50 years of investment by the pharmaceutical and biotechnology industries, the only successful new treatments to emerge from this are LT modifiers such as cysteinyl LT receptor 1 antagonists (montelukast, accolate and pranlukast) and the IgG anti-human IgE mAb omalizumab, both therapeutics directed at asthma targets that were identified many years ago [23]. The view that chronic airway inflammation and remodelling in asthma is primarily caused by allergen sensitization and exposure is being challenged.
In 2000, we first suggested that allergic-type inflammation and aberrant epithelial injury/repair mechanisms were parallel phenomena leading to different asthma subtypes [24] involving activation of the EMTU, which controls the local airway tissue microenvironment [25]. We proposed that epithelial damage by environmental agents, such as viruses, air pollutants and ETS (environmental tobacco smoke), results in the production of signals that act on the underlying mesenchyme to propagate and amplify inflammatory and remodelling responses in the submucosa (Figure 2). In support of this, we and others have recently reported defective epithelial TJ (tight junction) formation both in asthmatic biopsies and in the epithelium differentiated at an air–liquid interface in vitro in association with impaired barrier function [26]. The asthmatic epithelium is also functionally abnormal in being more sensitive to oxidant injury [27] and failing to generate IFN-β (interferon-β) and IFN-λ in response to virus infection [28], both deficiencies resulting in premature cell death (Figure 3). Thus many of the chronic inflammatory and structural responses that occur in chronic asthma (including airway allergen sensitization) could follow from a defective epithelium leading to a chronic wound response to repeated environmental injury [29]. Similar mechanisms are now known to operate in other allergic diseases, such as atopic dermatitis, where loss-of-function polymorphisms in the filaggrin gene encoded in the epidermal differentiation complex on chromosome 1q21 greatly reduce skin barrier function [30], and in food allergy [31] and rhinosinusitis [32], leading to enhanced allergen sensitization.
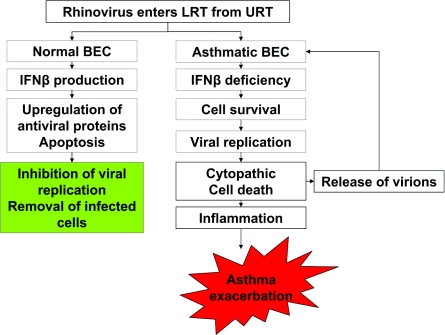
Figure 3. Defect in asthmatic epithelium to eliminate common respiratory viruses leading to cytotoxicity, mediator release and enhanced virus shedding associated with the asthma exacerbation.
BEC, bronchial endothelial cell; LRT, lower respiratory tract; URT, upper respiratory tract.
The finding that many novel asthma-susceptibility genes identified through application of hypothesis-independent approaches, such as positional cloning and genome-wide association, are expressed in the epithelium and mesenchyme adds to the evidence that places the EMTU at the centre of asthma pathogenesis [33,34]. Further evidence for a critical role of the epithelium comes from showing that the most frequent risk factors for developing, exacerbating and prolonging asthma act through the EMTU, namely enzymatically active allergens (e.g. from house mite, fungal, pollen and occupational sources), ambient air pollutants (e.g. ozone, oxides of nitrogen and particles), irritants (e.g. household and industrial chemicals), ETS, and respiratory viruses and certain bacteria (Chlamydia and Mycoplasma) [35,36]. This wide range of interactions helps make the case for a dynamic interaction between the epithelium and formed elements of the airways in the development of different asthma subphenotypes [5].
ONSET OF ASTHMA IN EARLY LIFE
Although asthma may begin at any time in life, most frequently it first expresses itself early in the first few years of life, with suboptimal foetal growth, maternal micronutrient deficiencies (e.g. vitamins E and D) and maternal smoking all being associated with impaired infant lung function and later development of asthma [37,38]. Both BHR and asthma also have a strong genetic basis independent of atopy. For example, polymorphism of the asthma-susceptibility gene ADAM33 (a disintegrin and metalloproteinase 33) is associated with reduced lung function in infants and the later development of BHR [39]. Birth cohort studies have also revealed that severe asthma is predicted by impaired infant lung function and BHR [40–42]; however, the central role of allergy itself as the initiator of asthma is also being questioned. Thus, in children who develop asthma, atopy has little influence on disease expression until 5 years of age, after which it predicts disease persistence [43,44] with those destined for severe disease acquiring IgE-sensitization earlier (3–4 years of age) [45]. In the case of food allergy (e.g. peanut, milk and egg), high exposure in early life induces immunological tolerance [46]. In contrast with exposure via the gastrointestinal tract, continued allergen exposure via the airways or skin facilitates persistence of sensitization [47,48].
However, although allergen exposure is important as a driver of ongoing asthma in children, its role as an initiating factor is undermined by showing that prolonged suppression of inflammation by inhaled corticosteroids at the onset of disease in infants or later in childhood has no influence over its natural history, despite effective control of symptoms [49,50]. Other environmental factors are now emerging as being important in initiating asthma. A recent important discovery is that repeated infections with RV (rhinovirus) during the first 3 years of life increased the risk of developing asthma by age 6 years 26-fold compared with 3-fold for allergen sensitization [51]. In a U.K. IoW (Isle of Wight) cohort study, the adjusted risk of asthma at age 10 years was 4-fold in children who had recurrent chest infection before 2 years of age [52,53]. The key role of early-life virus infection also extends into adult asthma in the European Community Respiratory Health Survey [54]. In a U.S.A. 95000 infant cohort study, the timing of birth in relationship to the winter virus season conferred a 30% increased risk of developing asthma by 6 years of age [55], whereas in a Perth cohort respiratory virus infection [RV: 70%, and RSV (respiratory syncytial virus): 16%] positively interacts with atopy to promote later asthma at 5 years of age [56]. Importantly, the target for viruses and environmental stimuli, such as ETS and other pollutants, is the airway epithelium. Understanding why the airway epithelium of these children is so susceptible to these stimuli and how it affects allergic sensitization could provide a potential novel route to prevent asthma.
ROLE OF THE AIRWAY EPITHELIUM IN THE INCEPTION OF ASTHMA IN EARLY CHILDHOOD
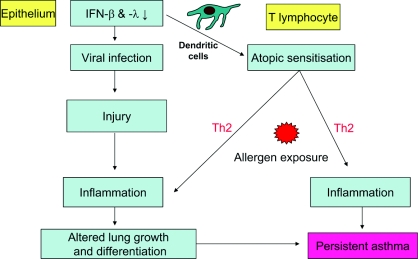
Several studies have highlighted the potential association of early respiratory virus infections and the subsequent risk of asthma. However, many children who wheeze with viral infections during infancy will not progress to asthma. Early-life exposure to pollutants such as ETS and atopy [53,54] are also risk factors for persistent asthma, suggesting a requirement for multiple environmental interactions with locally expressed susceptibility genes. Antigen-presenting cells, especially ADCs [airway DCs (dendritic cells)], play a critical role in initiating and regulating early inflammatory events at epithelial surfaces. While in the first year of life, infants do not typically exhibit ADCs in the absence of inflammation, severe respiratory infection is associated with the appearance in infant airways of mature ADCs [57]. Although the proteolytically active allergen Der p1 can disrupt epithelial TJs to facilitate transport of allergen across the epithelium [58], it has been suggested that ADCs need a ‘danger signal’ to activate T-cells sufficiently and thus avoid tolerance [59]. We suggest that respiratory virus infection of the pre-asthmatic epithelium causes ADC maturation with a preferential bias towards a Th-2 response to the penetrating allergen. Thus a structurally and functionally defective airway epithelium could underlie the abnormally destructive responses to respiratory viruses and other components of the inhaled environment. It follows that these events could promote a microenvironment which would facilitate allergic sensitization, support different types of inflammation and predispose the airways to the development of asthma during childhood (Figure 4).
Figure 4. Two or multiple ‘hit’ theory of the induction of new asthma through an interaction between virus infection and allergic sensitization.
We have found that airway epithelial cells from adult asthmatics have a deficient innate immune response to RV infection [28,60], providing an explanation for the tendency of asthmatic subjects to have lingering and more severe lower respiratory tract problems as a consequence of RV infection. House dust mite allergens can act synergistically with either RV or short-term tobacco smoke exposure to induce mediator release from airway epithelial cells [61,62]. When compared with airway epithelial cells isolated from the conducting airways of non-smoking adults, we have found that those from smokers are much more susceptible to RV infection, allowing more viral replication and triggering marked cytopathic cell death, indicating that smoke exposure powerfully suppresses the innate immune response. In view of the importance of respiratory viruses and other environmental agents on the early-life origins of asthma, it is likely that multiple triggers that include pollutants, allergens and ETS attenuate the innate antiviral responses of paediatric airway epithelial cells to RV, allowing viral persistence and augmenting pro-inflammatory responses, initiation and persistence of asthma.
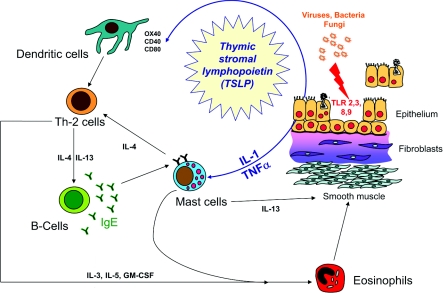
Both at baseline in asthma [63], and to a greater extent, after allergen [58], ETS [64] and RV infection [65,66], epithelial TJs are disrupted. On the basis of the observation of elevated TSLP (thymic stromal lymphopoietin) production by asthmatic airway epithelial cells, we postulate that viral infection not only facilitates allergen penetration, but also biases DC responses towards allergen sensitization in asthma [67]. TSLP is released in response to activation of Toll-like receptors in the epithelium and directs T-cell differentiation towards a Th-2 phenotype by up-regulating the co-stimulatory molecule OX40 (CD134) on adjacent DCs [68] (Figure 5).
Figure 5. Potential role of TSLP in connecting epithelial activation to Th-2-type inflammation.
GM-CSF, granulocyte/macrophage colony-stimulating factor; TLR, Toll-like receptor.
Asthma progression or remission in children and young adults
Bronchial biopsies obtained from very young children with early-life virus-associated wheezing reveal little abnormal pathology, but, by the time they reach 3 years of age, epithelial injury and thickening of the lamina reticularis is evident, either in the absence or presence of Th-2-type inflammation [11,70–72]. Although thickening of the lamina reticularis is almost diagnostic of asthma in children and adults, there is doubt over its significance to airway remodelling since it does not relate to asthma duration [73], although it may increase with severity [74]. On the basis of its unique presence in asthma and also its occurrence following lung transplantation [75], the deposition of new matrix in the lamina reticularis would seem to be indicative of a special type of chronic epithelial injury. In adult and childhood asthma, epithelial overexpression of EGFR (epidermal growth factor receptor), reduced markers of cell proliferation [Ki67 and PCNA (proliferating cell nuclear antigen)] and increased nuclear translocation of the cell-cycle inhibitor p21waf are consistent with impaired epithelial repair responses [76,77]. Most recently, airway epithelial cells cultured from atopic asthmatic compared with atopic normal children impaired wound repair responses following injury [78].
Growing out of asthma compared with persistent asthma
Most asthma in children undergoes repeated remission and relapse [79,80] with a high proportion ‘outgrowing’ their disease [43,45]. Factors predicting persistence of asthma include early-onset persistent wheezing, disease severity, reduced lung function and BHR, sensitization to multiple allergens and allergic co-morbidity [81]. During adolescence, major changes occur in the natural history of asthma, including gender reversal with female preponderance [82] and remission in approx. 50%, although, in a proportion asthma, it returns later in those presumed to be disease-free [4]. In many asymptomatic teenagers, there is pathological evidence of subclinical asthma, persistent BHR, airway inflammation and thickening of the lamina reticularis [83–85]. Very little is known about the factors that lead to persistent or recurrent disease during adolescence, especially any benefit of lung growth against deleterious effects of airway remodelling. In persistent asthma, it is not known whether the chronic wound response in the epithelium and lamina reticularis is propagated into the deeper layers of the submucosa which we propose is crucial for disease progression, reduced lung function, loss of reversibility and refractoriness to treatment. By understanding the factors underlying recurrent and persistent disease, we believe new targets will be uncovered for modulating the natural history of asthma.
Although the natural history of asthma is one of remission and relapse, it is important to consider the disease over the life-course (Figure 1). From early infancy it is likely that, when subjected to appropriate environmental exposures, epithelial injury results in remodelling of a genetically susceptible mesenchyme to contribute to the persistence of asthma during childhood exacerbated by allergen sensitization and exposure. This concept is supported by longitudinal studies showing that early onset of severe disease and reduced lung function/BHR in early childhood and infection/allergen sensitization are both risk factors for persistent asthma [86]. Findings from our IoW birth cohort suggest that those subjects have ‘outgrown’ asthma at 18 years of age have a significantly greater increase in FEV1 (forced expiratory volume at 1 s) from 10 to 18 years compared with new-onset or persistent asthma [53]. Since lung growth continues into early adulthood, tissue plasticity during the growth period may counteract the negative impact of remodelling until maximum lung function is achieved, providing an explanation for disease remission in some children. Thereafter, remodelling is unchecked leading to accelerated decline in lung function in adulthood and recurrence of symptoms in those who remit in adolescence. Thus, during the teenage years, subjects with structurally and functionally defective epithelium and earlier exposure to allergens, infections and pollutants most probably have evidence of persistent activation of EMTU with evidence of remodelling, leading to persistent asthma.
Chronic persistent asthma in adults
Transient, persistent and late-onset wheezing in early childhood have been shown to track into different asthma phenotypes in adults, with early-onset and persistent wheezing predicting severe asthma [87]. On the basis of a range of measures, multiple asthma subtypes with different clinical, physiological, inflammatory and treatment responses are now being identified [88]. BHR is a fundamental abnormality in asthma which increases in proportion to disease severity and is functionally antagonized by β2-adrenoceptor agonists. The mechanisms underlying BHR are still not known for certain, but an increase in airway smooth muscle [89], alterations to its physicochemical properties [90] and mast cell infiltration [91,92] are considered important. Cross-sectional studies reveal that many adult patients with asthma have some evidence of persistent irreversible airflow obstruction with an accelerated decline in lung function over time and both are linked to airway wall remodelling [93–95]. Moreover, the nature of the matrix that surrounds the smooth muscle bundles does influence the behaviour of the muscle [96]. The contractile scope of airway smooth muscle cultured in the presence of different matrix proteins was least when the cell was adherent upon collagen V, followed by collagen IV, laminin and collagen I, and greatest for fibronectin. Although the early introduction and maintained use of inhaled corticosteroid therapy may exert some modifying effects on inflammation that is linked to matrix deposition [97], chronic aspects of asthma arise and progress despite continuous use of corticosteroids, suggesting that, in part, they occur independently of inflammation, possibly as a consequence of repeated cycles of bronchoconstriction [98,99].
To better understand structural changes of asthma in vivo, we have used a radial EBUS (endobronchial ultrasound) probe inserted into the airways at fibre-optic bronchoscopy [100]. Even in non-corticosteroid-treated asthma, the proximal airway wall is thicker than in non-asthmatic healthy controls; the thickness/diameter ratio being inversely related to BHR (rs=0.71, P=0.002). Studies using high-resolution CT (computed tomography) have reported similar findings in asthma in adult [101,102] and children [103], but the discriminatory resolution of CT is limited to the outer airway wall, whereas EBUS allows measurement of the inner wall that is altered most in asthma [104]. Mechanical deformation of the airways from repeated bronchoconstriction is a powerful stimulus for growth factor release from epithelial and mesenchymal cells to drive remodelling [105,106]. These growth factors include TGF-β (transforming growth factor-β) which not only drives differentiation of fibroblasts to myofibroblsts, but also is capable of initiating epithelial–mesenchymal transition [107]. Thus, what initially may be serving as a protective mechanism to provide resistive load to reduce bronchoconstriction associated with BHR, over time may subsequently progress to limit bronchodilation. From a mechanistic standpoint, TGF-β or asthmatic BAL (broncho-alveolar lavage) fluid has been shown to direct asthmatic fibroblasts more to adopting a contractile and synthetic phenotype when compared with the responses of fibroblasts cultured from normal airways [106]. A detailed understanding of the factors that drive progressive deposition of interstitial collagen in the inner airway wall and their relationship with a decline in lung function and poor response to standard therapy may enable earlier or alternative interventions to be developed.
Although asthma is classically defined as reversible airflow obstruction, in the long-term airflow obstruction may become increasingly difficult to reverse despite optimal pharmacological therapy. On the basis of findings that a thickened airway wall in asthma is associated with limited bronchoconstrictor and bronchodilator responses [108,109], we propose that the mechanical strain, caused by repeated smooth muscle contraction, leads to progressive extracellular matrix deposition both in the large and small airways, which would serve to increase ‘stiffness’ of the tissue to limit narrowing. However, over time, excessive matrix deposition would lead to progressive fixed airflow obstruction, a feature characteristic of chronic steroid refractory asthma. Using a Flexercell® Tension Plus system, cyclical stretch of a lung fibroblast cell line induces expression of α-SMA (smooth muscle α-actin), suggesting a switch to a myofibroblast phenotype, as reported in other systems, and also promotes smooth muscle and myofibroblast differentiation [110]. This mechanism, as well as an aberrant response to epithelial injury and pro-fibrotic growth factor release from inflammatory cells, could provide the basis for airway allergen remodelling in chronic asthma, only a proportion of which is corticosteroid-sensitive.
CONCLUSIONS
Asthma can no longer be considered simply in terms as a single cellular and mediator response to inhaled allergens, but a complex interaction between the inhaled environment and the formed elements of the airways. Of importance is the concept that the state of the airway epithelium and underlying mesenchyme (EMTU) may be crucial in translating the atopic phenotype into the lower airways, and this may vary over time and between patients giving rise to different subphenotypes with differing responses to treatment and natural histories. Connecting life-course studies with mechanistic research in well-phenotyped patients should provide much needed new insight into disease mechanisms and, hopefully, will lead to the identification of new therapeutic targets for disease prevention and treatment.
FUNDING
We wish to acknowledge the Medical Research Council (MRC), Asthma UK, Asthma, Allergy Inflammation and Repair (AAIR), and the British Lung Foundation for supporting research that underpins this review. S.T.H. is an MRC Clinical Professor.
References
- 1.Holgate S., Bisgaard H., Bjermer L., Haahtela T., Haughney J., Horne R., McIvor A., Palkonen S., Price D. B., Thomas M., et al. The Brussels Declaration: the need for change in asthma management. Eur. Respir. J. 2008;32:1433–1442. doi: 10.1183/09031936.00053108. [DOI] [PubMed] [Google Scholar]
- 2.Editorial. Still more questions than answers. Lancet. 2008;372:1009. doi: 10.1016/S0140-6736(08)61414-2. [DOI] [PubMed] [Google Scholar]
- 3.Sly P. D., Boner A. L., Björksten B., Bush A., Custovic A., Eigenmann P. A., Gern J. E., Gerritsen J., Hamelmann E., Helms P. J., et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sears M. R., Greene J. M., Willan A. R., Wiecek E. M., Taylor D. R., Flannery E. M., Cowan J. O., Herbison G. P., Silva P. A., Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N. Engl. J. Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 5.Horne R., Price D., Cleland J., Costa R., Covey D., Gruffydd-Jones K., Haughney J., Henrichsen S. H., Kaplan A., Langhammer A., et al. Can asthma control be improved by understanding the patient's perspective? BMC Pulm. Med. 2007;7:8. doi: 10.1186/1471-2466-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haughney J., Price D., Kaplan A., Chrystyn H., Horne R., May N., Moffat M., Versnel J., Shanahan E. R., Hillyer E. V., et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir. Med. 2008;102:1681–1693. doi: 10.1016/j.rmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Boushey H. A., Sorkness C. A., King T. S., Sullivan S. D., Fahy J. V., Lazarus S. C., Chinchilli V. M., Craig T. J., Dimango E. A., Deykin A., et al. Daily versus as-needed corticosteroids for mild persistent asthma. N. Engl. J. Med. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen S. E., Bateman E. D., Bousquet J., Busse W. W., Yoxall S., Clark T. J. Determinants of response to fluticasone propionate and salmeterol/fluticasone propionate combination in the Gaining Optimal Asthma controL study. J. Allergy Clin. Immunol. 2007;120:1036–1042. doi: 10.1016/j.jaci.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Mendis S., Fukino K., Cameron A., Laing R., Filipe A., Jr, Khatib O., Leowski J., Ewen M. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull. World Health Organ. 2007;85:279–288. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson G. P. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 11.Turato G., Barbato A., Baraldo S., Zanin M. E., Bazzan E., Lokar-Oliani K., Calabrese F., Panizzolo C., Snijders D., Maestrelli P., et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am. J. Respir. Crit. Care Med. 2008;178:476–482. doi: 10.1164/rccm.200712-1818OC. [DOI] [PubMed] [Google Scholar]
- 12.Pearce N., Pekkanen J., Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodcock A., Forster L., Matthews E., Martin J., Letley L., Vickers M., Britton J., Strachan D., Howarth P., Altmann D., et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N. Engl. J. Med. 17; 2003;349:225–236. doi: 10.1056/NEJMoa023175. [DOI] [PubMed] [Google Scholar]
- 14.Barnes P. J. Is there a role for immunotherapy in the treatment of asthma? No. Am. J. Respir. Crit. Care Med. 1996;154:1227–1228. doi: 10.1164/ajrccm.154.5.8912730. [DOI] [PubMed] [Google Scholar]
- 15.Scadding G. K., Durham S. R., Mirakian R., Jones N. S., Leech S. C., Farooque S., Ryan D., Walker S. M., Clark A. T., Dixon T. A., et al. British Society for Allergy and Clinical Immunology guidelines for the management of allergic and non-allergic rhinitis. Clin. Exp. Allergy. 2008;38:19–42. doi: 10.1111/j.1365-2222.2007.02888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holgate S. T. Novel targets of therapy in asthma. Curr. Opin. Pulm. Med. 2009;15:63–71. doi: 10.1097/MCP.0b013e32831da867. [DOI] [PubMed] [Google Scholar]
- 17.Flood-Page P., Swenson C., Faiferman I., Matthews J., Williams M., Brannick L., Robinson D., Wenzel S., Busse W., Hansel T. T., et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am. J. Respir. Crit. Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg M. E., Klion A. D., Roufosse F. E., Kahn J. E., Weller P. F., Simon H. U., Schwartz L. B., Rosenwasser L. J., Ring J., Griffin E. F., et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 2008;358:1215–1228. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 19.Mehr S., Rego S., Kakakios A., Kilham H., Kemp A. Treatment of a case of pediatric hypereosinophilic syndrome with anti-interleukin-5. J. Pediatr. 2009;155:289–291. doi: 10.1016/j.jpeds.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 20.Nair P., Pizzichini M. M., Kjarsgaard M., Inman M. D., Efthimiadis A., Pizzichini E., Hargreave F. E., O'Byrne P. M. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 21.Haldar P., Brightling C. E., Hargadon B., Gupta S., Monteiro W., Sousa A., Marshall R. P., Bradding P., Green R. H., Wardlaw A. J., Pavord I. D. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamane-Ohnuki N., Kinoshita S., Inoue-Urakubo M., Kusunoki M., Iida S., Nakano R., Wakitani M., Niwa R., Sakurada M., Uchida K., et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 23.Austen K. F. Biologic implications of the structural and functional characteristics of the chemical mediators of immediate-type hypersensitivity. Harvey Lect. 1979;73:93–161. [PubMed] [Google Scholar]
- 24.Holgate S. T., Davies D. E., Lackie P. M., Wilson S. J., Puddicombe S. M., Lordan J. L. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2000;105:193–204. doi: 10.1016/s0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 25.Holgate S. T. Asthma: more than an inflammatory disease. Curr. Opin. Allergy Clin. Immunol. 2002;2:27–29. doi: 10.1097/00130832-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Holgate S. T. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Bucchieri F., Puddicombe S. M., Lordan J. L., Richter A., Buchanan D., Wilson S. J., Ward J., Zummo G., Howarth P. H., Djukanovic R., et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am. J. Respir. Cell. Mol. Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 28.Wark P. A., Johnston S. L., Bucchieri F., Powell R., Puddicombe S., Laza-Stanca V., Holgate S. T., Davies D. E. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swindle E. J., Collins J. E., Davies D. E. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J. Allergy Clin. Immunol. 2009;124:23–34. doi: 10.1016/j.jaci.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 30.O'Regan G. M., Sandilands A., McLean W. H., Irvine A. D. Filaggrin in atopic dermatitis. J. Allergy Clin. Immunol. 2008;122:689–693. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Groschwitz K. R., Hogan S. P. Intestinal barrier function: molecular regulation and disease pathogenesis J. Allergy Clin. Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tieu D. D., Kern R. C., Schleimer R. P. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cookson W. The immunogenetics of asthma and eczema: a new focus on the epithelium. Nat. Rev. Immunol. 2004;4:978–988. doi: 10.1038/nri1500. [DOI] [PubMed] [Google Scholar]
- 34.Holgate S. T., Davies D. E., Powell R. M., Howarth P. H., Haitchi H. M., Holloway J. W. Local genetic and environmental factors in asthma disease pathogenesis: chronicity and persistence mechanisms. Eur. Respir. J. 2007;29:793–803. doi: 10.1183/09031936.00087506. [DOI] [PubMed] [Google Scholar]
- 35.Eder W., Ege M. J., von Mutius E. The asthma epidemic. N. Engl. J. Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 36.Nadeem A., Masood A., Siddiqui N. Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther. Adv. Respir. Dis. 2008;2:215–235. doi: 10.1177/1753465808094971. [DOI] [PubMed] [Google Scholar]
- 37.Sears M. R. Epidemiology of asthma exacerbations. J. Allergy Clin. Immunol. 2008;122:662–668. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Martinez F. D. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109(Suppl. 2):362–367. [PubMed] [Google Scholar]
- 39.Simpson A., Maniatis N., Jury F., Cakebread J. A., Lowe L. A., Holgate S. T., Woodcock A., Ollier W. E., Collins A., Custovic A., et al. Polymorphisms in a disintegrin and metalloprotease 33 (ADAM33) predict impaired early-life lung function. Am. J. Respir. Crit. Care Med. 2005;172:55–60. doi: 10.1164/rccm.200412-1708OC. [DOI] [PubMed] [Google Scholar]
- 40.Turner S. W., Devereux G. Early life influences on the development of allergy and asthma - how early is early? Clin. Exp. Allergy. 2007;37:163–165. doi: 10.1111/j.1365-2222.2007.02661.x. [DOI] [PubMed] [Google Scholar]
- 41.Lucas J. S., Inskip H. M., Godfrey K. M., Foreman C. T., Warner J. O., Gregson R. K., Clough J. B. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am. J. Respir. Crit. Care Med. 2004;170:534–540. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- 42.Håland G., Carlsen K. C., Sandvik L., Devulapalli C. S., Munthe-Kaas M. C., Pettersen M., Carlsen K. H. Reduced lung function at birth and the risk of asthma at 10 years of age. N. Engl. J. Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 43.Illi S., von Mutius E., Lau S., Niggemann B., Grüber C., Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 44.Kurukulaaratchy R. J., Matthews S., Waterhouse L., Arshad S. H. Factors influencing symptom expression in children with bronchial hyperresponsiveness at 10 years of age. J. Allergy Clin. Immunol. 2003;112:311–316. doi: 10.1067/mai.2003.1623. [DOI] [PubMed] [Google Scholar]
- 45.Matricardi P. M., Illi S., Grüber C., Keil T., Nickel R., Wahn U., Lau S. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur. Respir. J. 2008;32:585–592. doi: 10.1183/09031936.00066307. [DOI] [PubMed] [Google Scholar]
- 46.Wennergren G. What if it is the other way around?. Early introduction of peanut and fish seems to be better than avoidance. Acta Paediatr. 2009;98:1085–1087. doi: 10.1111/j.1651-2227.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 47.Sporik R., Holgate S. T., Platts-Mills T. A., Cogswell J. J. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N. Engl. J. Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 48.Haselden B. M., Larché M., Meng Q., Shirley K., Dworski R., Kaplan A. P., Bates C., Robinson D. S., Ying S., Kay A. B. Late asthmatic reactions provoked by intradermal injection of T-cell peptide epitopes are not associated with bronchial mucosal infiltration of eosinophils or TH2-type cells or with elevated concentrations of histamine or eicosanoids in bronchoalveolar fluid. J. Allergy Clin. Immunol. 2001;108:394–401. doi: 10.1067/mai.2001.117460. [DOI] [PubMed] [Google Scholar]
- 49.Guilbert T. W., Morgan W. J., Zeiger R. S., Mauger D. T., Boehmer S. J., Szefler S. J., Bacharier L. B., Lemanske R. F., Jr, Strunk R. C., et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 50.Murray C. S., Woodcock A., Langley S. J., Morris J., Custovic A. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006;368:754–762. doi: 10.1016/S0140-6736(06)69285-4. [DOI] [PubMed] [Google Scholar]
- 51.Jackson D. J., Gangnon R. E., Evans M. D., Roberg K. A., Anderson E. L., Pappas T. E., Printz M. C., Lee W. M., Shult P. A., Reisdorf E., et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raza A., Kurukulaaratchy R. J., Arshad S. H. Predicting risk of asthma in wheezing infants. Thorax. 2008;63:842. [PubMed] [Google Scholar]
- 53.Arshad S. H., Kurukulaaratchy R. J., Fenn M., Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127:502–508. doi: 10.1378/chest.127.2.502. [DOI] [PubMed] [Google Scholar]
- 54.Dharmage S. C., Erbas B., Jarvis D., Wjst M., Raherison C., Norbäck D., Heinrich J., Sunyer J., Svanes C. Do childhood respiratory infections continue to influence adult respiratory morbidity? Eur. Respir. J. 2009;33:237–244. doi: 10.1183/09031936.00062907. [DOI] [PubMed] [Google Scholar]
- 55.Wu P., Dupont W. D., Griffin M. R., Carroll K. N., Mitchel E. F., Gebretsadik T., Hartert T. V. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am. J. Respir. Crit. Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turato G., Barbato A., Baraldo S., Zanin M. E., Bazzan E., Lokar-Oliani K., Calabrese F., Panizzolo C., Snijders D., Maestrelli P., et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma. Am. J. Respir. Crit. Care Med. 2008;178:476–482. doi: 10.1164/rccm.200712-1818OC. [DOI] [PubMed] [Google Scholar]
- 57.Tschernig T., Debertin A. S., Paulsen F., Kleemann W. J., Pabst R. Dendritic cells in the mucosa of the human trachea are not regularly found in the first year of life. Thorax. 2001;56:427–431. doi: 10.1136/thorax.56.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan H., Winton H. L., Soeller C., Tovey E. R., Gruenert D. C., Thompson P. J., Stewart G. A., Taylor G. W., Garrod D. R., Cannell M. B., Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Invest. 1999;104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat. Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 60.Contoli M., Message S. D., Laza-Stanca V., Edwards M. R., Wark P. A., Bartlett N. W., Kebadze T., Mallia P., Stanciu L. A., Parker H. L., et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat. Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 61.Bossios A., Gourgiotis D., Skevaki C. L., Saxoni-Papageorgiou P., Lötvall J., Psarras S., Karpathios T., Constandopoulos A. G., Johnston S. L., Papadopoulos N. G. Rhinovirus infection and house dust mite exposure synergize in inducing bronchial epithelial cell interleukin-8 release. Clin. Exp. Allergy. 2008;38:1615–1626. doi: 10.1111/j.1365-2222.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 62.de Boer W. I., Sharma H. S., Baelemans S. M., Hoogsteden H. C., Lambrecht B. N., Braunstahl G. J. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can. J. Physiol. Pharmacol. 2008;86:105–112. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]
- 63.Rusznak C., Sapsford R. J., Devalia J. L., Shah S. S., Hewitt E. L., Lamont A. G., Davies R. J., Lozewicz S. Interaction of cigarette smoke and house dust mite allergens on inflammatory mediator release from primary cultures of human bronchial epithelial cells. Clin. Exp. Allergy. 2001;31:226–238. doi: 10.1046/j.1365-2222.2001.01000.x. [DOI] [PubMed] [Google Scholar]
- 64.Rothen-Rutishauser B. M., Kiama S. G., Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am. J. Respir. Cell. Mol. Biol. 2005;32:281–289. doi: 10.1165/rcmb.2004-0187OC. [DOI] [PubMed] [Google Scholar]
- 65.Sajjan U., Wang Q., Zhao Y., Gruenert D. C., Hershenson M. B. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holgate S. T. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 67.Rochman Y., Leonard W. J. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr. Opin. Pharmacol. 2008;8:249–254. doi: 10.1016/j.coph.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y. J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J. Allergy Clin. Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 69. Reference deleted.
- 70.Saglani S., Malmström K., Pelkonen A. S., Malmberg L. P., Lindahl H., Kajosaari M., Turpeinen M., Rogers A. V., Payne D. N., Bush A., et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am. J. Respir. Crit. Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 71.Barbato A., Turato G., Baraldo S., Bazzan E., Calabrese F., Panizzolo C., Zanin M. E., Zuin R., Maestrelli P., Fabbri L. M., Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. Am. J. Respir. Crit. Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 72.Saglani S., Payne D. N., Zhu J., Wang Z., Nicholson A. G., Bush A., Jeffery P. K. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am. J. Respir. Crit. Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 73.Kim E. S., Kim S. H., Kim K. W., Park J. W., Kim Y. S., Sohn M. H., Kim K. E. Basement membrane thickening and clinical features of children with asthma. Allergy. 2007;62:635–640. doi: 10.1111/j.1398-9995.2007.01375.x. [DOI] [PubMed] [Google Scholar]
- 74.Bourdin A., Neveu D., Vachier I., Paganin F., Godard P., Chanez P. Specificity of basement membrane thickening in severe asthma. J. Allergy Clin. Immunol. 2007;119:1367–1374. doi: 10.1016/j.jaci.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 75.Law L., Zheng L., Orsida B., Levvey B., Oto T., Kotsimbos A. T., Snell G. I., Williams T. J. Early changes in basement membrane thickness in airway walls post-lung transplantation. J. Heart Lung Transplant. 2005;24:1571–1576. doi: 10.1016/j.healun.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Puddicombe S. M., Torres-Lozano C., Richter A., Bucchieri F., Lordan J. L., Howarth P. H., Vrugt B., Albers R., Djukanovic R., Holgate S. T., et al. Increased expression of p21waf cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 2003;28:61–68. doi: 10.1165/rcmb.4715. [DOI] [PubMed] [Google Scholar]
- 77.Fedorov I. A., Wilson S. J., Davies D. E., Holgate S. T. Epithelial stress and structural remodelling in childhood asthma. Thorax. 2005;60:389–394. doi: 10.1136/thx.2004.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens P. T., Kicic A., Sutanto E. N., Knight D. A., Stick S. M. Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin. Exp. Allergy. 2008;38:1901–1910. doi: 10.1111/j.1365-2222.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- 79.To T., Gershon A., Wang C., Dell S., Cicutto L. Persistence and remission in childhood asthma: a population-based asthma birth cohort study. Arch. Pediatr. Adolesc. Med. 2007;161:1197–1204. doi: 10.1001/archpedi.161.12.1197. [DOI] [PubMed] [Google Scholar]
- 80.Bjerg-Bäcklund A., Perzanowski M. S., Platts-Mills T., Sandström T., Lundbäck B., Rönmark E. Asthma during the primary school ages: prevalence, remission and the impact of allergic sensitization. Allergy. 2006;61:549–555. doi: 10.1111/j.1398-9995.2006.01027.x. [DOI] [PubMed] [Google Scholar]
- 81.Stern D. A., Morgan W. J., Halonen M., Wright A. L., Martinez F. D. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Postma D. S. Gender differences in asthma development and progression. Gend. Med. 2007;4(Suppl. B):S133–S146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 83.van den Toorn L. M., Overbeek S. E., de Jongste J. C., Leman K., Hoogsteden H. C., Prins J. B. Airway inflammation is present during clinical remission of atopic asthma. Am. J. Respir. Crit. Care Med. 2001;164:2107–2113. doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
- 84.Obase Y., Shimoda T., Kawano T., Saeki S., Tomari S., Izaki K., Fukushima C., Matsuse H., Kohno S. Bronchial hyperresponsiveness and airway inflammation in adolescents with asymptomatic childhood asthma. Allergy. 2003;58:213–220. doi: 10.1034/j.1398-9995.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 85.Hara J., Fujimura M., Myou S., Kita T., Abo M., Katayama N., Furusho S., Nobata K., Oribe Y., Kimura H., et al. Sputum eosinophilia, airway hyperresponsiveness and airway narrowing in young adults with former asthma. Allergol. Int. 2008;57:211–217. doi: 10.2332/allergolint.O-06-461. [DOI] [PubMed] [Google Scholar]
- 86.Morgan W. J., Stern D. A., Sherrill D. L., Guerra S., Holberg C. J., Guilbert T. W., Taussig L. M., Wright A. L., Martinez F. D. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am. J. Respir. Crit. Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panettieri R. A., Jr, Covar R., Grant E., Hillyer E. V., Bacharier L. Natural history of asthma: persistence versus progression: does the beginning predict the end? J. Allergy Clin. Immunol. 2008;121:607–613. doi: 10.1016/j.jaci.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Haldar P., Pavord I. D., Shaw D. E., Berry M. A., Thomas M., Brightling C. E., Wardlaw A. J., Green R. H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.James A. L., Bai T. R., Mauad T., Abramson M. J., Dolhnikoff M., McKay K. O., Maxwell P. S., Elliot J. G., Green F. H. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur. Respir. J. 2009;34:1040–1045. doi: 10.1183/09031936.00181608. [DOI] [PubMed] [Google Scholar]
- 90.An S. S., Fredberg J. J. Biophysical basis for airway hyperresponsiveness. Can. J. Physiol. Pharmacol. 2007;85:700–714. doi: 10.1139/Y07-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brightling C. E., Bradding P., Symon F. A., Holgate S. T, Wardlaw A. J., Pavord I. D. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 92.Begueret H., Berger P., Vernejoux J. M., Dubuisson L., Marthan R., Tunon-de-Lara J. M. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62:8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siddiqui S., Mistry V., Doe C., Roach K., Morgan A., Wardlaw A., Pavord I., Bradding P., Brightling C. Airway hyperresponsiveness is dissociated from airway wall structural remodeling. J. Allergy Clin. Immunol. 2008;122:335–341. doi: 10.1016/j.jaci.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8:63–69. doi: 10.1097/ACI.0b013e3282f3b5b5. [DOI] [PubMed] [Google Scholar]
- 95.Lange P., Parner J., Vestbo J., Schnohr P., Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N. Engl. J. Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 96.An S. S., Kim J., Ahn K., Trepat X., Drake K. J., Kumar S., Ling G., Purington C., Rangasamy T., Kensler T. W., et al. Cell stiffness, contractile stress and the role of extracellular matrix. Biochem. Biophys. Res. Commun. 2009;382:697–703. doi: 10.1016/j.bbrc.2009.03.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dijkstra A., Vonk J. M., Jongepier H., Koppelman G. H., Schouten J. P., ten Hacken N. H., Timens W., Postma D. S. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006;61:105–110. doi: 10.1136/thx.2004.039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tschumperlin D. J., Shively J. D., Kikuchi T., Drazen J. M. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 2003;28:142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 99.Choe M. M., Sporn P. H., Swartz M. A. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am. J. Respir. Cell. Mol. Biol. 2006;35:306–313. doi: 10.1165/rcmb.2005-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaw T. J., Wakely S. L., Peebles C. R., Mehta R. L., Turner J. M., Wilson S. J., Howarth P. H. Endobronchial ultrasound to assess airway wall thickening: validation in vitro and in vivo. Eur. Respir. J. 2004;23:813–817. doi: 10.1183/09031936.04.00119904. [DOI] [PubMed] [Google Scholar]
- 101.Wenzel S. E., Busse W. W. Severe asthma: lessons from the Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007;119:14–21. doi: 10.1016/j.jaci.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 102.Ueda T., Niimi A., Matsumoto H., Takemura M., Hirai T., Yamaguchi M., Matsuoka H., Jinnai M., Muro S., Chin K., Mishima M. Role of small airways in asthma: investigation using high-resolution computed tomography. J. Allergy Clin. Immunol. 2006;118:1019–1025. doi: 10.1016/j.jaci.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 103.de Blic J., Tillie-Leblond I., Emond S., Mahut B., Dang Duy T. L., Scheinmann P. High-resolution computed tomography scan and airway remodeling in children with severe asthma. J. Allergy Clin. Immunol. 2005;116:750–754. doi: 10.1016/j.jaci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 104.James A. L., Paré P. D., Hogg J. C. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 105.Saunders R., Siddiqui S., Kaur D., Doe C., Sutcliffe A., Hollins F., Bradding P., Wardlaw A., Brightling C. E. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J. Allergy Clin. Immunol. 2009;123:376–384. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wicks J., Haitchi H. M., Holgate S. T., Davies D. E., Powell R. M. Enhanced upregulation of smooth muscle related transcripts by TGFβ2 in asthmatic (myo)fibroblasts. Thorax. 2006;61:313–319. doi: 10.1136/thx.2005.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hackett T. L., Warner S. M., Stefanowicz D., Shaheen F., Pechkovsky D. V., Murray L. A., Argentieri R., Kicic A., Stick S. M., Bai T. R., Knight D. A. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-β1. Am. J. Respir. Crit. Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 108.Tschumperlin D. J., Shively J. D., Kikuchi T., Drazen J. M. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am. J. Respir. Cell. Mol. Biol. 2003;28:142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 109.Malavia N. K., Raub C. B., Mahon S. B., Brenner M., Panettieri R. A., Jr, George S. C. Airway epithelium stimulates smooth muscle proliferation. Am. J. Respir. Cell. Mol. Biol. 2009;41:297–304. doi: 10.1165/rcmb.2008-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J., Fan J., Laschinger C., Arora P. D., Kapus A., Seth A., McCulloch C. A. Smooth muscle actin determines mechanical force-induced p38 activation. J. Biol. Chem. 2005;280:7273–7284. doi: 10.1074/jbc.M410819200. [DOI] [PubMed] [Google Scholar]