Abstract
Objectives
This pilot study aimed to determine if an elemental diet could be used to treat patients with active rheumatoid arthritis and to compare its effect to that of oral prednisolone.
Methods
Thirty patients with active rheumatoid arthritis were randomly allocated to 2 weeks of treatment with an elemental diet (n = 21) or oral prednisolone 15 mg/day (n = 9). Assessments of duration of early morning stiffness (EMS), pain on a 10 cm visual analog scale (VAS), the Ritchie articular index (RAI), swollen joint score, the Stanford Health Assessment Questionnaire, global patient and physician assessment, body weight, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP) and haemoglobin, were made at 0, 2, 4 and 6 weeks.
Results
All clinical parameters improved in both groups (p<0.05) except the swollen joint score in the elemental diet group. An improvement of greater than 20% in EMS, VAS and RAI occurred in 72% of the elemental diet group and 78% of the prednisolone group. ESR, CRP and haemoglobin improved in the steroid group only (p<0.05).
Conclusions
An elemental diet for 2 weeks resulted in a clinical improvement in patients with active rheumatoid arthritis, and was as effective as a course of oral prednisolone 15 mg daily in improving subjective clinical parameters. This study supports the concept that rheumatoid arthritis may be a reaction to a food antigen(s) and that the disease process starts within the intestine.
Keywords: diet therapy, elemental diet, prednisolone, rheumatoid arthritis
The exclusion of meat, dairy products and cereals from the diet has been reported to improve the symptoms of some patients with rheumatoid arthritis.1,2,3,4,5,6 A reduction in dietary protein as part of an elimination diet or during fasting also leads to an improvement in disease activity.5,7,8
An elemental diet contains amino acids (no whole proteins), mono/di‐saccharides and medium/long‐chain triglycerides supplemented with vitamins and trace elements. It provides complete nutrition and avoids many of the problems associated with long‐term steroid use (especially growth retardation in children).9 Although its mechanism of action is not fully understood, it is a safe and effective method of treatment for some patients with active Crohn's disease.10 As the antigenic load within the bowel lumen may be important in the pathogenesis of rheumatoid arthritis,11 an elemental diet as the sole source of nutrition may be an effective treatment. Prednisolone at doses not exceeding 15 mg daily can be used to treat rheumatoid arthritis for short courses of 2 weeks.12
In designing this study we choose a non‐whole protein diet (therefore assumed to be “non‐antigenic”) that was nutritionally complete and which was likely to be well tolerated for a sufficient time to have an effect; hence an elemental diet for 2 weeks. As we did not feel it ethical to give a placebo treatment to patients with active rheumatoid arthritis seeking treatment, we chose 15 mg/day oral prednisolone for 2 weeks as a “standard” treatment for mild/moderate rheumatoid arthritis to act as a control treatment.
The primary aims of this pilot study were to determine if an elemental diet was an effective treatment for patients with active rheumatoid arthritis and to compare its effect to that of 15 mg oral prednisolone.
Methods
Patients
Thirty patients with newly diagnosed or established active rheumatoid arthritis (using the revised criteria of the American Rheumatism Association13) were recruited over 18 months to the study from outpatient clinics at Leicester General Hospital, Leicester Royal Infirmary and Edith Cavell Hospital, Peterborough. Active rheumatoid arthritis was defined by any combination of three or more of the following criteria: three or more swollen joints, six or more tender joints, morning stiffness of longer than 45 min and an erythrocyte sedimentation rate (ESR) of more than 28 mm in the first hour. Patients taking non‐steroidal anti‐inflammatory drugs (NSAIDs), disease‐modifying drugs (DMARDs) or prednisolone at a dose below 15 mg/day were eligible, providing they had been on the same dose for the previous 6 weeks. Patients with diabetes mellitus, other systemic illnesses or pregnancy were excluded. All patients gave written informed consent.
Study design
Patients were randomised to 2 weeks of treatment with either an elemental diet or oral steroids. Randomisation was stratified to ensure a similar sex distribution in both groups and was 2:1 in favour of the elemental diet, using computer generated random numbers.
Diet
Eligible patients, under the supervision of a dietitian, were given liquid elemental diet E028 (86 kcal and 2.5 g protein/100 ml; Scientific Hospital Supplies, Liverpool, UK) of orange and pineapple flavour as their only source of nutrition for 2 weeks and free access to bottled water. The total energy given to the patient was calculated from the Schofield equation which estimates basal metabolic rate for each sex, age and weight then adds activity and stress factors.14
Steroids
Oral prednisolone (15 mg) was given daily for 2 weeks while the patients ate a normal diet.
After 2 weeks of treatment, the elemental diet group returned to eating a normal diet. The steroid group returned immediately to their pre‐trial dose of oral steroids. All other medications were unchanged in both groups throughout the trial and follow‐up period.
Assessments
Each patient was assessed at the same time of day on four occasions: at the initial visit and at 2, 4 and 6 weeks (trial treatment was stopped after the 2 week visit). A single assessor (TP), who was blind to the treatment, made all assessments. The following clinical parameters were assessed15: body weight, duration of morning stiffness (EMS), pain on a 10 cm visual analog scale (VAS), the Ritchie articular index (RAI) which grades joint tenderness (0–3) over 63 joints,16 swollen joint score (63 joints) and the Stanford Health Assessment Questionnaire (HAQ) modified for British patients.17 Global patient and physician assessment scores were measured using a five grade scale (much worse (−2), worse (−1), same (0), better (1), much better (2)). In addition, ESR, C‐reactive protein (CRP), haemoglobin, total white cell count, platelet count, rheumatoid factor and immunoglobulins IgA, IgG and IgM were measured at each visit.
Study end points
The primary end point for the study was an improvement of 20% in the clinical parameters of disease activity after 2 weeks of treatment.18 Secondary end points included improvements in clinical, haematological, biochemical or quality of life scores at 2 weeks.
Statistical methods
The Wilcoxon sign rank test for paired samples was used to compare differences within treatment groups and the Mann‐Whitney U test was used for differences between the groups. All reported p values are two‐sided.
Ethical approval
The study was approved by the Leicestershire and Peterborough Research Ethics Committees. All patients gave informed written consent.
Results
Thirty patients (table 1) were randomly assigned to either elemental diet (n = 21) or oral steroids (n = 9). There were no significant differences between the groups in any parameters.
Table 1 Patient details.
| Diet (range) | Steroids (range) | |
|---|---|---|
| Number (female) | 21 (16) | 9 (6) |
| Mean age, years | 47 | 58 |
| Mean duration of rheumatoid arthritis, years | 8 | 9 |
| Treatment | ||
| NSAIDs, n | 11 | 6 |
| Sulphasalazine, n | 7 | 1 |
| DMARDs, n | 8 | 6 |
| Mean prednisolone pre‐trial dose, mg | 11 (2.5–13) | 2 (7.5) |
Comparison of weeks 0 and 2
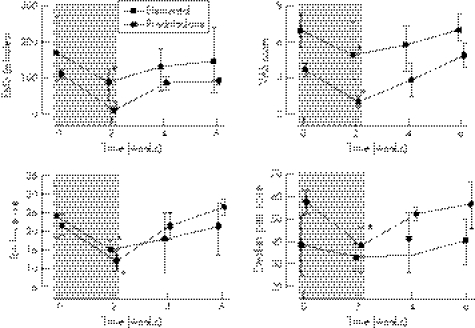
Twenty seven patients completed the 2 weeks of treatment. Changes in all parameters are shown in table 2 and the changes in EMS, pain VAS, RAI and swollen joint score are shown graphically in fig 1.
Table 2 Median (range) results at 0 and 2 weeks.
| Elemental diet (n = 18) | Prednisolone (n = 9) | |||
|---|---|---|---|---|
| Week 0 | Week 2 | Week 0 | Week 2 | |
| Weight (kg) | 64.0 (38.3–97.2) | 64.2 (38.0–96.9) | 74.9 (54.4–96.5) | 73.9 (55.8–96.5) |
| Duration of morning stiffness (min) | 150 (30–1440) | 105 (5–1440)* | 120 (30–480) | 9 (0–75)* |
| Pain on VAS (cm) | 6.6 (4.6–9.8) | 4.9 (1.7–8.7)* | 4.3 (1.3–7.5) | 2.6 (0.0–5.1)* |
| RAI | 23 (8–72) | 16 (4–68)* | 24 (8–63) | 13 (4–41)* |
| Swollen joint score | 39 (2–88) | 33 (4–94) | 58 (24–118) | 43 (13–81)* |
| HAQ | 2.0 (0.5–3.0) | 1.8 (0.13–3.0)* | 1.9 (1.3–2.5) | 1.4 (0.0–2.4)* |
| Global patient assessment | – | 1 ((−1)–2) | – | 2 (0–2) |
| Global physician assessment | – | 1 (0–2) | – | 2 (0–2) |
| ESR (mm/h) | 63 (3–131) | 59 (6–123) | 60 (20–95) | 15 (5–65)* |
| CRP (mg/dl) | 3.3 (0.5–15.0) | 3.6 (0.5–17.7) | 4.3 (2.1–7.0) | 0.5 (0.5–3.3)* |
| Haemoglobin (g/dl) | 11.6 (8.9–14.5) | 11.3 (9.3–14.4) | 11.7 (11.0–14.9) | 12.1 (10.9–15.2)* |
| White cell count (×109/l) | 9.3 (5.1–13.8) | 8.8 (5.4–16.7) | 7.5 (4.7–9.6) | 10.8 (6.6–16.8)* |
| Platelets (×109/l) | 387 (195–573) | 395 (206–698) | 295 (172–604) | 297 (147–511) |
| Rheumatoid factor (titre) | 0.001955 (0–0.25) | 0.002345 (0–0.125) | 0.00156 (0–0.25) | 0.00313 (0–0.125) |
| IgA (g/dl) | 3.5 (2–9) | 3.5 (2–5) | 3 (1–8) | 3 (1–9) |
| IgG (g/dl) | 14 (5–23) | 14 (9–26) | 12 (10–16) | 11 (8–14) |
| IgM (g/dl) | 1.5 (1–6) | 1.5 (1–7) | 1 (0–2) | 1 (1–2) |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; RAI, the Ritchie articular index; VAS, visual analog scale.
*p<0.05.
Figure 1 Graphs of median and interquartile ranges for early morning stiffness (EMS), visual analog pain score (top right), the Ritchie articular index (bottom left) and swollen joint score (bottom right) with the 2 week period of treatment shaded. *p<0.05.
Diet group
Compared to the start of the study, there were significant improvements in EMS by a median of 48 min (p<0.001), VAS by 2.1 cm (p = 0.003), RAI by 7 (p<0.001) and the HAQ score by 0.3 (p = 0.03). There was a greater than 20% improvement in EMS, VAS and RAI in 72% of patients. The patient and physician global assessments improved in 13 (72%) and 16 (89%) patients, respectively. The median reduction of 5.5 in swollen joint score did not achieve significance (p = 0.13).
Steroid group
There were significant improvements in EMS by a median of 120 min (p = 0.004), VAS by 2.1 cm (p = 0.01), RAI by 13 (p = 0.01), swollen joint score by 18 (p = 0.008) and HAQ score by 0.75 (p = 0.02). There was a greater than 20% improvement in EMS, VAS, RAI, swollen joint score and ESR in 78% of patients, HAQ in 67% and CRP in 89%. The patient and physician global assessments improved in eight (89%) patients. There was a reduction in ESR of 35 mm/1 h (p = 0.02) and in CRP of 3.7 mg/dl (p = 0.004). There was a significant median rise in haemoglobin of 0.5 g/dl (p = 0.03) and of total white cell count of 1.9×109/l.
Comparison between the groups
Improvements in swollen joint score (p = 0.03), ESR (p = 0.006), CRP (p<0.001) and haemoglobin (p = 0.015) were greater in the steroid treated group compared to the elemental diet group. A significant rise in white cell count was noted in the steroid group (p = 0.008). However, there were no significant differences in all other clinical and laboratory parameters between the groups.
Comparison of weeks 2 and 6
Improvement in all clinical, haematological and immunological measurements slowed, stopped or reversed in both treatment groups during the 4 weeks after stopping the treatment (fig 1).
Problems with treatments
During the first 2 weeks, three patients in the elemental diet group withdrew from the study; two found the elemental diet unpalatable and one had a deterioration of symptoms (this patient did not subsequently respond to oral steroid therapy). One patient receiving the elemental diet complained of constipation but none had diarrhoea. No steroid treated patient withdrew during the first 2 weeks.
Discussion
This randomised pilot study shows that an elemental diet given for 2 weeks improves the subjective clinical disease activity parameters in rheumatoid arthritis and in this respect may be as effective as 15 mg/day of oral prednisolone. An elemental diet, like oral prednisolone, resulted in significant improvements in all clinical measurements except the swollen joint score. Due to the large placebo/natural remission component in studies of rheumatoid arthritis,19 the American College of Rheumatology has recommended that an improvement of greater than 20% in a clinical parameter is needed before a result is considered significant in a clinical trial as this makes a purely placebo effect unlikely.18 Of the patients on the elemental diet, 72% had a greater than 20% improvement in EMS, VAS and RAI. The patient and physician global assessments showed an improvement in 72% and 89% of patients, respectively. Unlike oral prednisolone, the elemental diet did not result in a significant improvement in laboratory tests (ESR, CRP and haemoglobin), which may be partly due to the relatively short time of treatment. The elemental diet was found to be unpalatable by two patients, but it did not cause any to complain of diarrhoea. This study also showed that symptoms returned when treatment with an elemental diet or oral steroids was stopped.
There was no placebo group in this study as we felt it would be unethical to withhold treatment from patients who had presented or were seeking treatment for active rheumatoid arthritis. The study was not double blind, as the patient inevitably knew which treatment they were receiving (tablets or drinks) since we felt it would be unethical to give a placebo nutrient drink (with no calories) to a patient, who may be already at risk of the consequences of undernutrition, for 2 weeks.
Previous studies using an elemental diet in rheumatoid arthritis have compared it to a blended soup diet (3 weeks)20 or given it as a supplement to a defined diet (4 weeks),21 and, respectively, have shown significant improvements in the number of tender joints20 and RAI.21 A peptide diet (4 weeks) was not successful in treating active rheumatoid arthritis.22
The gut may have a primary role in the inflammatory process in rheumatoid arthritis. In 1986 localisation of indium labelled white blood cells to the terminal ileum and right colon was observed in 46% of patients with active rheumatoid arthritis.23 Lymphocytes from gut mucosal associated lymphoid tissue (MALT) may migrate to the synovium.24 Serum IgA rheumatoid factor, derived from MALT, is present in patients with more severe rheumatoid arthritis and its titre may be an indicator of disease severity.25,26 Rheumatoid arthritis has been treated successfully with sulphazalazine and a non‐absorbable steroid (budesonide),27,28 both of which may have their primary action on the MALT. There are other parallels with inflammatory bowel disease as the same immunosuppressive drugs have been found to be effective in both inflammatory bowel disease and rheumatoid arthritis (eg azathioprine, mercaptopurine and methotrexate).
Dietary therapy for both inflammatory bowel disease and rheumatoid arthritis involves either excluding (eliminating) something (eg whole protein or long chain fatty acids) or supplementing the diet with a compound (eg fish oils or anti‐oxidants). There are many mechanisms by which an elemental diet may be effective in treating rheumatoid arthritis. These include a reduction of the bowel intraluminal antigenic load, alteration of the intestinal flora, reducing intestinal permeability and suppressing the MALT, therefore all ultimately reducing the migration of lymphocytes from the intestinal lymphoid tissue to the synovium.29
This preliminary study has shown that an elemental diet can rapidly improve subjective clinical parameters in active rheumatoid arthritis. Presumably this is because the stimulus starting the inflammatory process has been eliminated. However, while the symptoms improve within 2 weeks, the biochemical markers take longer. Although it is unlikely to replace conventional treatment with steroids, an elemental diet may be a useful alternative for treating children, patients who are unwilling to take steroids or patients who are unresponsive to steroids/immunosuppressive drugs. Further larger studies comparing an elemental diet with steroids and/or DMARDs and using the elemental diet for a longer time and with a food reintroduction diet are needed. This study may encourage more research into the role and mechanisms of dietary factors in the causation of rheumatological diseases. It also supports the hypothesis that a primary abnormality in rheumatoid arthritis may be a reaction to a food antigen(s) and that the disease process starts within the intestine.
Acknowledgements
We thank Scientific Hospital Supplies Ltd for providing us with a grant and supplying the E028, Liz Moore, BSc and Carol Wright, BSc for nutritional assessment of patients and dietary advice, Graham Scott, PhD and Nick Taub, PhD for statistical help and Dr Frank Nichol for helping identify suitable patients.
Abbreviations
CRP - C‐reactive protein
EMS - early morning stiffness
ESR - erythrocyte sedimentation rate
DMARDs - disease‐modifying drugs
HAQ - Health Assessment Questionnaire
MALT - mucosal associated lymphoid tissue
NSAIDs - non‐steroidal anti‐inflammatory drugs
RAI - Ritchie articular index
VAS - visual analog scale
Footnotes
Competing interests: None declared.
References
- 1.Sköldstam L, Larsson L, Lindström F D. Effects of fasting and lactovegetarian diet on rheumatoid arthritis. Scand J Rheumatol 19798249–255. [DOI] [PubMed] [Google Scholar]
- 2.Panush R S, Carter R L, Katz P.et al Diet therapy for rheumatoid arthritis. Arthritis Rheum 198326462–471. [DOI] [PubMed] [Google Scholar]
- 3.Darlington I G, Ramsey N W, Mansfield J R. Placebo‐controlled, blind study of dietary manipulation therapy in rheumatoid arthritis. Lancet 1986i236–238. [DOI] [PubMed] [Google Scholar]
- 4.Beri D, Malaviya A N, Shandilaya R.et al Effect of dietary restrictions on disease activity in rheumatoid arthritis. Ann Rheum Dis 19884769–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kjeldsen‐Kragh J, Haugen M, Borchgrevink C F.et al Controlled trial of fasting and one‐year vegetarian diet in rheumatoid arthritis. Lancet 1991338899–902. [DOI] [PubMed] [Google Scholar]
- 6.Hafstrom I, Ringertz B, Spangberg A.et al A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001401175–1179. [DOI] [PubMed] [Google Scholar]
- 7.Sundqvist T, Lindstrom F, Magnusson K.et al Influence of fasting on intestinal permeability and disease activity in patients with rheumatoid arthritis. Scand J Rheumatol 19821133–38. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulou A, Rawashdeh M O, Brown G A.et al Remission following an elemental diet or prednisolone in Crohn's disease. Acta Paediatr 19958479–83. [DOI] [PubMed] [Google Scholar]
- 9.Kroker G F, Stroud R M, Marshall R.et al Fasting and rheumatoid arthritis: a multicentre study. Clin Ecol 19842137–144. [Google Scholar]
- 10.Zachos M, Tondeur M, Griffiths A M. Enteral nutritional therapy for inducing remission of Crohn's disease. Cochrane Database Syst Rev 2001(3)CD000542. [DOI] [PubMed]
- 11.Sartor R B. Importance of intestinal mucosal immunity and luminal bacterial cell wall polymers in the aetiology of inflammatory joint diseases. Baillieres Clin Rheumatol 19893223–245. [DOI] [PubMed] [Google Scholar]
- 12.Gotzsche P C, Johansen H K. Short‐term low‐dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev 2004(3)CD000189. [DOI] [PMC free article] [PubMed]
- 13.Arnett F C, Edworthy S M, Bloch D A.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 14.Schofield W N, Schofield C, James W P T. Basal metabolic rate—review and prediction together with an annotated bibliography of source material. Hum Nutr Clin Nutr 198539C(Suppl 1)5–96. [PubMed] [Google Scholar]
- 15.Boers M. OMERACT: conclusions of an international conference on outcome measures in rheumatoid arthritis clinical trials in Maastrich. Arthritis Rheum 199235(Suppl)S202 [Google Scholar]
- 16.Ritchie D M, Boyle J A, McInnes J M.et al Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med 196837393–406. [PubMed] [Google Scholar]
- 17.Kirwan J R, Reeback J S. Stanford Health Assessment Questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheumatol 198625206–209. [DOI] [PubMed] [Google Scholar]
- 18.Felson D T, Anderson J J, Boers M.et al American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 199538727–735. [DOI] [PubMed] [Google Scholar]
- 19.Paulus H E, Egger M J, Ward J R.et al Analysis of improvement in individual rheumatoid arthritis patients treated with disease‐modifying antirheumatic drugs, based on the findings in patients treated with placebo. The Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis Rheum 199033477–484. [DOI] [PubMed] [Google Scholar]
- 20.Haugen M, Kjeldsen‐Kragh J, Førre Ø. A pilot study of the effect of an elemental diet in the management of rheumatoid arthritis. Clin Exp Rheumatol 199412275–279. [PubMed] [Google Scholar]
- 21.Kavanagh R, Workman E, Nash P.et al The effects of elemental diet and subsequent food reintroduction on rheumatoid arthritis. Br J Rheumatol 199534270–273. [DOI] [PubMed] [Google Scholar]
- 22.Holst‐Jensen S E, Pfeiffer‐Jensen M, Monsrud M.et al Treatment of rheumatoid arthritis with a peptide diet. Scand J Rheumatol 199827329–336. [DOI] [PubMed] [Google Scholar]
- 23.Segal A W, Isenberg D A, Hajirousou V.et al Preliminary evidence for gut involvement in the pathogenesis of rheumatoid arthritis? Br J Rheumatol 198625162–166. [DOI] [PubMed] [Google Scholar]
- 24.Kadioglu A, Sheldon P. Adhesion of rheumatoid lymphocytes to mucosal endothelium: the gut revisited. Br J Rheumatol 199635218–225. [DOI] [PubMed] [Google Scholar]
- 25.Teitsson I, Withrington R H, Seifert M H.et al Prospective study of early rheumatoid arthritis. I. Prognostic value of IgA rheumatoid factor. Ann Rheum Dis 198443673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Withrington R H, Teitsson I, Valdimarsson H.et al Prospective study of early rheumatoid arthritis. II. Association of rheumatoid factor isotopes with fluctuations in disease activity. Ann Rheum Dis 198443679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldon P. Ileum‐targeted steroid therapy in rheumatoid arthritis: double blind, placebo‐controlled trial of controlled‐release budesonide, Rheumatol Int 200323154–158. [DOI] [PubMed] [Google Scholar]
- 28.Kirwan J R, Hallgren R, Mielants H.et al A randomised placebo controlled 12 week trial of budesonide and prednisolone in rheumatoid arthritis. Ann Rheum Dis 200463688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deitch E A, Xu D, Qi L.et al Elemental diet‐induced immune suppression is caused by both bacterial and dietary factors. J Parenter Enteral Nutr 199317332–336. [DOI] [PubMed] [Google Scholar]