Abstract
Background
Cryptococcal meningitis is a major cause of HIV-associated morbidity and mortality in Africa. Improved oral treatment regimens are needed, as amphotericin B is neither available nor feasible in many centers. Fluconazole 1200 mg/d is more fungicidal than 800 mg/d, but mortality remains unacceptably high. Therefore we examined the effect of adding oral flucytosine to fluconazole.
Methods
HIV-seropositive, antiretroviral-naive patients with their first episode of cryptococcal meningitis were randomized to 14 days fluconazole 1200 mg/d alone, or with flucytosine 100 mg/kg/d, followed by fluconazole 800 mg/d with 10 weeks followup. The primary endpoint was early fungicidal activity (EFA), derived from quantitative cerebrospinal fluid cultures on Days 1, 3, 7, and 14. Secondary endpoints were safety, and 2- and 10-week mortality.
Results
Forty-one patients were analyzed. Baseline mental status, cryptococcal burden, opening pressure, CD4 count, and HIV viral load were similar between groups. Combination therapy was more fungicidal than fluconazole alone: EFA −0.28 +/− 0.17 log CFU/ml/d vs. −0.11 +/− 0.09 log CFU/ml/d (p < 0.001). The combination arm had fewer deaths by 2 weeks (10% vs. 37%) and 10 weeks (43% vs. 58%). More patients had grade III or IV neutropenia with combination therapy (5 vs. 1, within first 2 weeks, p=0.2), but there was no increase in infection-related adverse events.
Conclusions
The results suggest that optimal oral treatment for cryptococcal meningitis is high dose fluconazole with flucytosine. Efforts are needed to increase availability of flucytosine in Africa.
Keywords: Cryptococcal, Meningitis, AIDS, Fluconazole, Flucytosine
Background
In areas of the world with high HIV prevalence and limited access to antiretroviral therapy (ART), Cryptococcus neoformans has become the leading cause of meningitis in adults [1–3] and a frequent AIDS-defining illness [4]. Furthermore, despite increasing availability of ART, obstacles to effective antifungal treatment have conspired to make cryptococcosis among the most common causes of HIV-related death [5–7], with a 10-week mortality of 40–60% [6, 8–12]. In developed countries where amphotericin B (AmB) and flucytosine (5FC) are readily available, 10-week mortality rates of 10 – 26% have been reported [13, 14]. However many sub-Saharan countries lack the resources to obtain and administer AmB and 5FC, relying instead on donated fluconazole.
The rate of fall of cryptococcal colony-forming units (CFU/ml) in cerebrospinal fluid (CSF) provides a rapid and inexpensive comparison of the fungicidal activity of treatment regimens and affords greater statistical power than clinical endpoints in small cohorts [15]. In addition, rate of clearance of infection is independently associated with survival at 2 and 10 weeks [16]. Previously used fluconazole doses (200–400 mg/day) are essentially fungistatic [17] but higher doses are associated with more rapid clearance of infection, and a study from Uganda showed that doses of 1200 mg/day appeared safe and provided faster cryptococcal clearance compared with 800 mg/day [12]. Limited data suggest combining 5FC with fluconazole improves clinical outcome and time to CSF sterilization [8, 18, 19]. Therefore, in a setting where standard treatment included fluconazole 800 mg/day since 2004 [20], we conducted an open-label, randomized-controlled trial to determine whether adding 5FC to high-dose fluconazole (1200 mg/d) during the initial 2 weeks of treatment of cryptococcal meningitis increased the rate of CSF infection clearance.
Methods
The UNC Project (http://id.unc.edu/Malawi/) provides patient care, laboratory facilities, training and conducts clinical research at Kamuzu Central Hospital (KCH), a tertiary referral center in Lilongwe, Malawi serving a district with approximately 230,000 HIV-infected persons. Also located on the KCH campus, the Lighthouse Trust Clinic provides free HIV care to over 5,000 patients. This study was conducted by UNC Project and sponsored by St. George’s University of London and the University of North Carolina at Chapel Hill. Approval was obtained from the Malawi National Health Sciences Research Committee (NHSRC), the University of North Carolina Institutional Review Board, and the Research Ethics Committee covering St. George’s University of London. The trial was registered, ISRCTN02725351, at http://www.controlled-trials.com. A Data Safety Monitoring Committee (DSMC) reviewed the results and adverse events after analysis of the first 41 patients.
Participants and procedures
Between February and December 2008 we enrolled HIV-positive adults with their first episode of cryptococcal meningitis, diagnosed by CSF India ink or cryptococcal antigen (Figure 1). We excluded those with alanine aminotransferase (ALT) concentration greater than 200 IU/ml, neutrophil count less than 500 × 103/ml, platelet count less than 50,000 × 103/ml, pregnancy, breastfeeding, or other contraindications to study drugs. Patients already taking ART were also excluded because they have lower baseline fungal burden and because of interactions between nevirapine and fluconazole [17, 21, 22]. Written informed consent was obtained from each participant, or from next of kin, prior to randomization. Participants were stratified by Glasgow Coma Score (GCS 15, and less than 15) and assigned to an intervention group by a random computer-generated list in a block size of 8.
Figure 1.
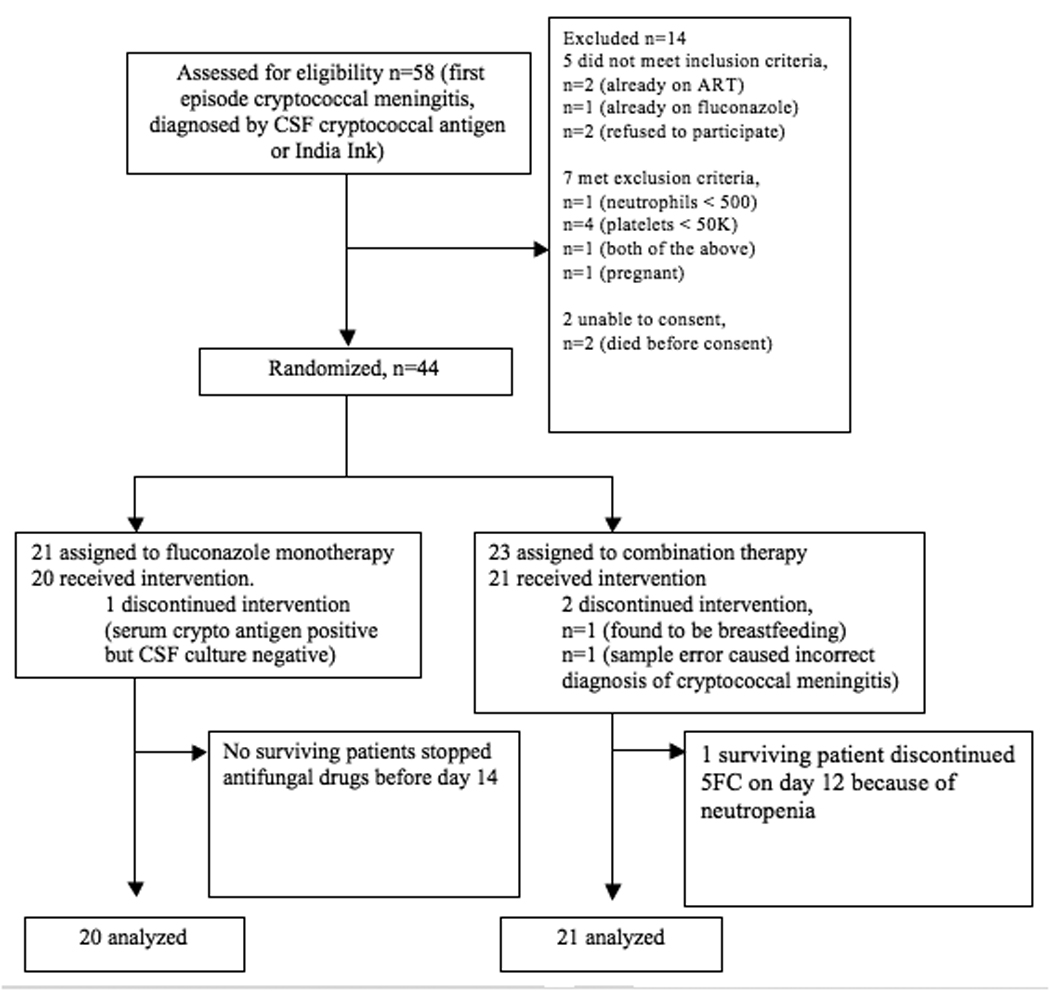
Trial Profile
5FC=flucytosine, ART=antiretroviral therapy
The two treatment groups comprised: (a) 2 weeks of fluconazole (1200 mg/day, Diflucan, Pfizer, New York, NY, USA) or (b) 2 weeks of fluconazole (1200 mg/day) in combination with 5FC (100 mg/kg/day, rounded down to the nearest gram/day, divided in 4 doses, Valeant Pharmaceuticals, California, USA). Four patients received IV fluconazole as part of their initial 2-week course (3 in the fluconazole monotherapy group, 1 in the combination group); the remainder were treated orally, via nasogastric tube if necessary. Patients otherwise received standard inpatient care including intravenous fluids when indicated. After 2 weeks all patients were given fluconazole 800 mg/day unless they were taking rifampicin, in which case fluconazole doses were increased by 50%. When necessary, fluconazole and 5FC doses were adjusted for renal function. 5FC dosage was reduced by 50% for grade III neutropenia or thrombocytopenia, and discontinued for grade IV toxicity.
After 4 weeks of antifungal therapy, patients attended Lighthouse Clinic for ART initiation – stavudine (d4T), lamivudine (3TC), and nevirapine (NVP) – according to Malawi national guidelines [23]. Fluconazole was decreased from 800 mg/day to 400 mg/day to minimize the theoretical risk of nevirapine toxicity [21, 22]. Participants were followed for 10 weeks after enrollment, when fluconazole was changed to 200 mg/day indefinitely.
Evaluation and outcomes
In addition to the initial diagnostic lumbar puncture (LP), we performed LP with opening pressure measurement and quantitative CSF culture on treatment days 1, 3, 7, and 14. Patients with elevated opening pressure (> 30 cm water) had more frequent LPs according to guidelines [24] and quantitative cultures were also done on these samples. Quantitative cultures were plated in serial ten-fold dilution, as previously described [15], and the dilution with the least colonies, but at least 30 CFU per 200 µl, was used to calculate CFU/ml. Laboratory personnel calculating QCC results were blinded to the treatment group. Cryptococcal clearance rates were calculated using a summary statistic for each patient, defined as the decrease in log CFU/ml/day using the slope of the linear regression of log CFU/ml against time for each patient, as previously described [15]. All data points were analyzed except sterile cultures in the second week if these values lessened the slope, as sterility would have been achieved before that day’s LP and this value would therefore underestimate the true slope [15].
In addition to clinical assessment, baseline testing included hematology, aspartate aminotransferase (AST), ALT, creatinine, CD4+ cell count, and HIV viral load, performed by a certified laboratory. During the initial 2 weeks patients underwent at least 3 blood counts per week and AST, ALT, and chemistries once per week. AST and ALT were repeated again at weeks 4, 6, and 10 of study enrollment. Clinical and laboratory adverse events were graded using the NIH DAIDS Toxicity Table [25].
The primary outcome measure was mean rate of decrease in CSF cryptococcal CFU, or early fungicidal activity (EFA), for each treatment arm. Secondary outcome measures were serious adverse events, laboratory toxicities, and mortality at 2 and 10 weeks.
Statistical analysis
We compared baseline characteristics of the treatment groups using the Fisher’s exact test for categorical variables, and the Mann-Whitney U test for continuous variables. Linear regression was used to compare EFA by treatment group, adjusting where indicated for other variables, giving summary differences with 95% CI and significance levels [12, 15, 17]. CD4+ cell count, opening pressure, and CSF white cell count were categorized into equal-sized groups. Mortality was examined using Cox regression. Cox regression was also used to explore the association of rate of clearance of infection with mortality in this study, and after adding patients from this study to a previously analyzed combined cohort [16]. Statistical significance was assessed using the likelihood ratio test.
In an earlier trial, addition of 5FC to AmB was associated with a 74% increase in EFA [15]. It was originally planned to enroll 80 patients, ensuring 27 evaluable patients per arm, on the basis that this would give 90% power to detect a 60% increase in EFA with addition of 5FC to fluconazole using a 0.05 two-sided significance level. An optimal EFA of −0.25 log CFU/ml/d was assumed for fluconazole monotherapy with a standard deviation of 0.17. However the trial was stopped early after the planned DSMC analysis of the first 41 patients found a statistically significant difference (P < 0.001) between the rates of 14-day CSF fungal clearance, which was the study’s primary outcome.
Results
Forty-four patients were randomized but 3 were later found to meet exclusion criteria (1 was breastfeeding, 1 had been mistakenly diagnosed with cryptococcosis following a sample error, and 1 had negative CSF cultures despite positive serum antigen tests). Among the remaining 41 patients, baseline clinical and laboratory characteristics were similar between the groups (Table 1). At the time of enrollment, 24% of patients were on concurrent rifampicin therapy for a prior diagnosis of tuberculosis. Overall, 39% had abnormal mental status, defined as GCS less than 15. One patient in the fluconazole monotherapy arm was lost to follow up on day 1, when his family decided to take him home.
Table 1.
Baseline clinical and laboratory characteristicsa
| All patients (41) | Fluconazole monotherapy (20) |
Fluconazole/5FC combination (21) |
p value |
|
|---|---|---|---|---|
| Clinical data | ------------------------- | ------------------------- | ------------------------- | ------- |
| Male (%) | 27 (66%) | 14 (70%) | 13 (62%) | 0.41 |
| Age (years) | 36 (23 – 73) | 36.5 (27 – 71) | 36 (23 – 73) | 0.30 |
| Mean weight in kg (standard deviation) |
54.3 (12) | 53.7 (14) | 54.8 (10) | 0.52 |
| Glasgow Coma Scale < 15 |
16 (39%) | 8 (40%) | 8 (38%) | 0.57 |
| Taking tuberculosis medication |
10 (24%) | 4 (20%) | 6 (29%) | 0.39 |
| Lab data | ------------------------- | ------------------------- | ------------------------- | ------- |
| CD4 count (x 109/L) | 21 (1 – 101) | 25 (1 – 66) | 19 (3 – 101) | 0.67 |
| HIV viral load (copies/ml) |
99,097 (2,258 – 1,145,572) |
84,411 (2258 – 1,606,740) |
99,442 (4,885 – 1,145,572) | 0.96 |
| CSF data | ------------------------- | ------------------------- | ------------------------- | ------- |
| Opening pressure (cm H2O) |
34 (1 – 100) | 18 (1 – 100) | 35 (7 – 53) | 0.24 |
| CSF white cell count/ml |
11 (0 – 1307) | 15 (0 – 318) | 8 (0 – 1307) | 0.51 |
| QCC (CFU/ml CSF) | 185,000 (265 – 30,950,000) |
200,000 (265 – 30,950,000) |
165,000 (1035 – 4,300,000) | 0.97 |
median (range), unless stated
Early Fungicidal Activity
A rate of fungal clearance could not be calculated for 4 patients (3 from the fluconazole alone arm and 1 from the combination arm), who died (3) or were lost to follow up (1) before the second LP. The rate of clearance of infection was more rapid in the combination arm compared with fluconazole alone (Figure 2). Mean ± SD EFA was −0.11 ± 0.095 log CFU/ml/d for monotherapy and −0.28 ± 0.17 CFU/ml/d for fluconazole plus 5FC. The difference in EFA was 0.18 (95%CI 0.085 – 0.27) log CFU/ml/d (p=0.0005). Four patients in the combination arm and 1 in the monotherapy arm had sterile CSF cultures by day 14.
Figure 2.
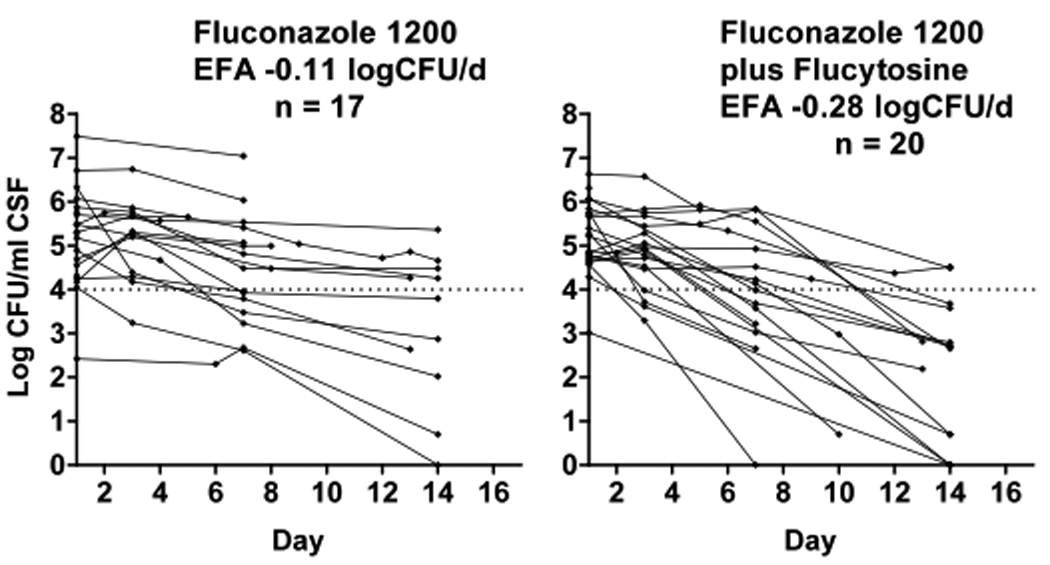
Decrease in C. neoformans CFU over time, by treatment group. Dotted lines represent a threshold of 1 × 104 CFU/mL to aid visual comparison. Each line represents results from a single patient. The mean rate of decrease of log CFU, or early fungicidal activity (EFA), was significantly greater for patients receiving combination vs. monotherapy (p=0.001).
Of the variables examined in this dataset (age, gender, weight, altered mental status, concomitant rifampicin, CD4 cell count, HIV viral load, baseline CFU/ml, opening pressure, CSF white cell count) the only other variable that was associated with EFA was concomitant antituberculous therapy; patients taking rifampicin had slower clearance of infection. Adjusting for rifampicin or variables associated with rate of clearance of infection in other, larger datasets (CD4 cell count, baseline organism load [15, 16]) did not make any difference to the strength of the association between EFA and treatment arm: in a model including treatment arm and concomitant rifampicin, the difference in EFA between treatment arms was 0.17 (95%CI 0.09 – 0.25) log CFU/ml/d (p<0.001). The difference in EFA between patients who were or were not taking rifampicin was 0.17 (95%CI 0.08 – 0.26) log CFU/ml/d (p=0.001).
Mortality
The combination arm had fewer deaths at 2 weeks (2/21, 10% vs. 7/19, 37%) and at 10 weeks (9/21, 43% vs. 11/19, 58%). Figure 3 shows the Kaplan-Meier survival curves over the 10-week period. The hazard ratios for death by 2 and 10 weeks in the combination arm were 0.24 (0.05–1.16, p=0.05) and 0.59 (0.25–1.44, p=0.25), respectively. If the patient lost to follow up on day 1 was considered to have died, rather than being censored, these ratios were 0.21 (0.05–1.0, p=0.03) and 0.55 (0.23–1.31, p=0.17), respectively. Diagnostic limitations made it impossible to determine causes of death with certainty, but most deaths were presumed to be related to cryptococcosis or other infection (Table 2).
Figure 3.
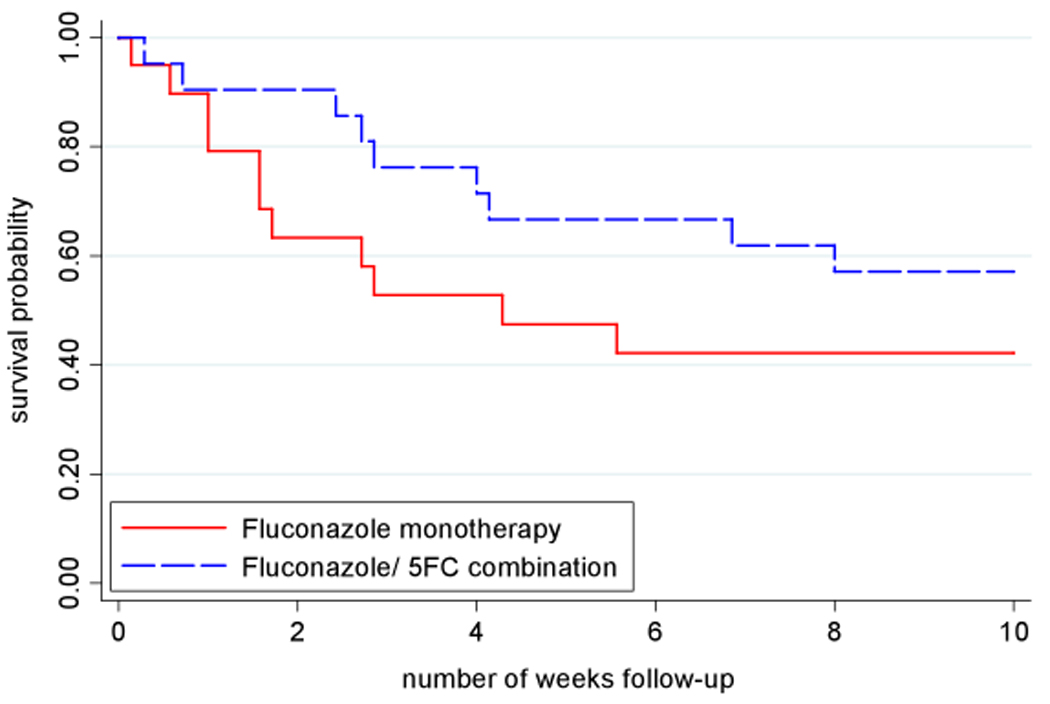
Survival curves, by treatment group. One patient lost to follow up was censored. p=0.05 at 2 weeks and p=0.25 at 10 weeks by Cox regression.
Table 2.
Presumed causes of death
| Fluconazole monotherapy | Fluconazole + Flucytosine | |||
|---|---|---|---|---|
| <2 weeks | 2–10 weeks | <2 weeks | 2–10 weeks | |
| Cryptococcus meningitis-related | 5 | 2 | 2 | 2 |
| Other Infection | 2 | 2 | 0 | 3 |
| Pulmonary KS | 0 | 0 | 0 | 1 |
| Unknown | 0 | 0 | 0 | 1 |
Safety
There were more neutropenia episodes in the combination arm (5 vs. 1, grade III and IV within the first 2 weeks, Table 3). Specifically, 1 patient in the combination arm developed Grade III neutropenia on day 14, which was the last day of 5FC as per protocol. For 2 other patients who developed neutropenia on 5FC, the drug was discontinued during the second week of therapy. One of these patients had a normal neutrophil count on repeat testing, had 5FC restarted, and completed the 14-day course having missed only 3 doses. The other developed a peripheral IV phlebitis while neutropenic, which resolved with antibiotics and removal of the catheter. This patient’s neutrophil count recovered by day 19 and the patient was doing well at 10 weeks. The remaining 2 neutropenia events in patients on combination therapy and the 1 on fluconazole monotherapy resolved spontaneously on a follow up blood draw without intervention.
Table 3.
Laboratory adverse events in the first 2 weeks
| Fluconazole monotherapy | Fluconazole + 5FC | |||
|---|---|---|---|---|
| Type/Grade of eventa | Grade III | Grade IV | Grade III | Grade IV |
| Thrombocytopenia | 1 | 0 | 1 | 0 |
| Neutropeniab | 1 | 0 | 2 | 3 |
| Anemia | 1 | 0 | 1 | 0 |
| Transaminase elevation | 0 | 0 | 0 | 0 |
| Hyponatremia | 1 | 1 | 1 | 1 |
| Renal failure | 0 | 2 | 1 | 0 |
| Total | 4 | 4 | 6 | 4 |
Cutoffs used to define DAIDS Grade III and Grade IV lab abnormalities: platelets (III 25,000 – 49,999/mm3; IV <35,000/mm3), absolute neutrophil count (III 500 – 749/mm3; IV < 500/mm3), hemoglobin (III 6.50 – 7.4 g/dl; IV < 6.5 g/dl), ALT/SGPT (III >175 – 350 IU/L; IV > 350 IU/L), AST/SGOT (III >190 – 380 IU/L IV > 380 IU/L), sodium (III 121 – 124 mEq/L; IV ≤ 120 mEq/L), creatinine (III male >2.2 – 4.1 mg/dl, female >2.3 – 4.4 mg/dl; IV male >4.1 mg/dl, female >4.4 mg/dl).
p value for neutropenia = 0.2
One additional patient with severe meningitis (CSF opening pressure 35 cm H20 on admission, rising to 60 cm H20 on day 14 despite repeated LPs) and GCS 11 throughout hospitalization had normal neutrophil counts, with no reduction in counts, during the 2 weeks of 5FC. On day 16 the patient developed a urinary tract infection with new Grade IV neutropenia, and died on day 17 despite appropriate antibiotics. This death was reported as possibly related to 5FC. There was no increase in infection-related serious adverse events in the combination group (7, versus 8 in the fluconazole arm), nor differences in rates of any other laboratory (Table 3) or clinical adverse events. There were no liver function test abnormalities related to fluconazole.
Association of rate of infection clearance and mortality in this study, and in a combined cohort including patients from this study
In this study, rate of clearance of infection was associated with 2-week mortality in univariate analysis (HR for each decrease in rate of clearance quartile 3.0, 95%CI 1.1–8.5, p=0.01). In a recently published combined cohort, rate of clearance of infection was associated with 2- and 10-week mortality, independent of altered mental status and baseline fungal burden, the other major prognostic factors [16]. When data from this trial were added to the combined cohort (now totaling 303 patients), and rate of clearance was fit onto a continuum scale, the hazard ratio (95% CI) for death after adjusting for altered mental status and baseline CFU count was 1.47 (1.19, 1.82; p=0.0002) at 2 weeks and 1.20 (1.07, 1.35, p=0.008) at 10 weeks for each 0.1 log unit decrease in rate of fall of CFU.
Discussion
The combination of fluconazole 1200 mg/d and flucytosine 100 mg/kg/d was associated with a markedly more rapid rate of clearance of infection compared to fluconazole alone. Indeed, accepting the limitations of comparisons between trials, the EFA of this combination (−0.28 log CFU/ml/d) is the closest an oral antifungal regimen has come to the fungicidal activity of AmB (−0.31 log CFU/ml/d for AmB 0.7 mg/kg monotherapy in Thailand [15]). This study was not powered for clinical endpoints, however there was a trend toward decreased early mortality in favor of the combination arm. Although this study was too small to conclude a clinical benefit, given the association of EFA with survival in this and in prior analyses [16] the improvement in fungicidal activity with combination therapy also suggests a survival advantage.
Flucytosine provided an additive effect when combined with fluconazole in murine models of cryptococcal infection [26–28] although not in a study in rabbits [29]. One mouse study evaluated a range of fluconazole and 5FC doses with AmB and determined the optimal regimen combined high doses of fluconazole with lower doses of 5FC [28]. A clinical trial combining fluconazole 400 mg/day with high dose 5FC (150 mg/kg/day) for 10 weeks produced a relatively short median time to CSF sterilization of 23 days, but there was no control arm, and side effects by 10 weeks were frequent [18]. Two subsequent trials suggested an additive effect when fluconazole was combined with 5FC. In one, the dose of fluconazole was low (200 mg/day) [8]. In the second, 5FC 100 mg/kg/d was given for 4 weeks with increasing doses of fluconazole and did have an additive benefit (higher percent of patients alive with CSF culture negative at 10 weeks) that was most pronounced with fluconazole 800 – 1200 mg/day [19]. As suggested by this latter trial and the earlier mouse data, we used high dose fluconazole and historically low dose 5FC to unequivocally demonstrate the microbiological efficacy of combining 5FC with fluconazole.
Neutropenia, although more frequent in the combination arm compared to fluconazole alone, was not a clinically significant problem in most instances and rarely limited treatment in this study, suggesting 5FC could be used with benefit in resource limited settings. However, our study alone was too small to adequately address toxicity issues. An earlier study using 5FC 100 mg/kg/d (but for 4 weeks instead of 2) found grade IV neutropenia occurring in 18% of patients, without evidence of increased infection [19]. In an ongoing study in Cape Town using 2 weeks of 5FC with AmB, 4.5% of 66 patients developed grade IV neutropenia (authors’ unpublished data). In Thailand, the same 5FC regimen was not associated with significant neutropenia, and no patients discontinued 5FC before 2 weeks. In that setting, bioavailability of oral 5FC was 50% that of intravenous 5FC, and the serum levels with oral 5FC were well below those associated with bone marrow toxicity [30]. Analysis of 5FC and 5-fluorouracil (5FU) levels in this trial will determine whether bioavailability or gut conversion of 5FC to 5FU [31] is different for patients in Africa. It will be important to continue to monitor the tolerability of 5FC in Africa, as more data will determine the optimal hematological monitoring needed for safe expanded use of 5FC in this setting. Any potential for toxicity must be balanced against the advantage of 5FC in antifungal efficacy and possibly survival.
Analysis of serum and CSF fluconazole levels in this trial will clarify the effect of rifampicin on the bioavailability of fluconazole. The association of concomitant rifampicin with reduced rates of fungal clearance implies a clinically significant reduction in fluconazole levels and suggests that, even at relatively high doses, fluconazole should be increased when given with rifampicin.
Donation of fluconazole has allowed countries like Malawi to increase the fluconazole dose recommended in cryptococcal treatment guidelines to 800 mg/day. However, the rate of clearance of infection is unacceptably slow and mortality unacceptably high at this dose [12]. Even with fluconazole 1200 mg/day – both in this study and in Uganda [12]– the rate of fungal clearance, although more rapid, was well below that achieved with AmB. Although it is impossible to accurately compare the EFA slopes between trials in different patient populations, the combination of fluconazole 1200 mg/day and 5FC studied in this trial is the most rapidly fungicidal oral regimen to date, and therefore may be the optimal regimen in the absence of AmB.
In many parts of the developing world, limited resources, personnel, and clinical and laboratory facilities prevent use of the standard 2-week induction course of AmB [32]. Until these problems are resolved, our results argue that wider access to flucytosine should be a priority in resource limited settings. Flucytosine is a simple molecule that is off-patent and was once registered in South Africa. Further studies are needed to evaluate regimens that incorporate, in addition, a short course of AmB, which should require minimal additional monitoring, and be easier to implement compared with standard 2 week AmB courses.
Acknowledgments
Financial support: Medical Research Council (United Kingdom) and the UNC Center for AIDS Research (NIAID P30-AI50410).
Footnotes
Summary: Improved oral regimens for cryptococcosis are needed where available resources limit use of amphotericin B. Adding flucytosine to high-dose fluconazole improved the rate of fungal clearance from cerebrospinal fluid and was associated with a strong trend towards improved early survival.
Potential conflicts of interest: All authors: no conflicts
References
- 1.Gordon SB, Walsh AL, Chaponda M, et al. Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clinical Infectious Diseases. 2000;31:53–57. doi: 10.1086/313910. [DOI] [PubMed] [Google Scholar]
- 2.Hakim JG, Gangaidzo IT, Heyderman RS, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–1407. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 3.Scarborough M, Gordon SB, Whitty CJM, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007;357:2441–2450. doi: 10.1056/NEJMoa065711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis. 2003;36:652–662. doi: 10.1086/367655. [DOI] [PubMed] [Google Scholar]
- 5.Corbett EL, Churchyard GJ, Charalambos S, et al. Morbidity and mortality in South African gold miners: impact of untreated disease due to human immunodeficiency virus. Clin Infect Dis. 2002;34:1251–1258. doi: 10.1086/339540. [DOI] [PubMed] [Google Scholar]
- 6.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 7.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 8.Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998;26:1362–1366. doi: 10.1086/516372. [DOI] [PubMed] [Google Scholar]
- 9.Mwaba P, Mwansa J, Chintu C, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–773. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaars CF, Meintjes GA, Morroni C, Post FA, Maartens G. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect Dis. 2006;6:118. doi: 10.1186/1471-2334-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of HAART. Clin Infect Dis. 2008;46:1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longley N, Muzoora C, Taseera K, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47:1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 13.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 14.Robinson PA, Bauer M, Leal MA, et al. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:82–92. doi: 10.1086/515074. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 16.Bicanic T, Muzoora C, Brouwer A, et al. Independent association between rate of clearance of infection and outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009;49:702–709. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-na�ve or -experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 18.Larsen RA, Bozzette SA, Jones BE, et al. Fluconazole combined with flucytosine for treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1994;19:741–745. doi: 10.1093/clinids/19.4.741. [DOI] [PubMed] [Google Scholar]
- 19.Milefchik E, Leal MA, Haubrich R, et al. Fluconazole alone or combined with flucytosine for the treatment of AIDS associated cryptococcal meningitis. Medical Mycology. 2008;46:393–395. doi: 10.1080/13693780701851695. [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Health (MOH) Management of HIV-related diseases. 1st ed. Lilongwe, Malawi: MOH; 2004. Malawi; pp. 36–38. [Google Scholar]
- 21.Geel J, Pitt J, Orrell CJ, Van Dyk M, Wood R. Effect of fluconazole on nevirapine pharmacokinetics. International Conference on AIDS [abstract TuPeB4606]; Meeting abstracts of the 15th International Conference on AIDS (Bangkok): International AIDS Society; 2004. p. 369. [Google Scholar]
- 22.Manosuthi W, Athichathanabadi C, Uttayamakul S, Phoorisri T, Sungkanuparph S. Plasma nevirapine levels, adverse events and efficacy of antiretroviral therapy among HIV-infected patients concurrently receiving nevirapine-based antiretroviral therapy and fluconazole. BMC Infectious Diseases. 2007;7:14. doi: 10.1186/1471-2334-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National AIDS Commission (NAC) and Malawi Ministry of Health and Population. Treatment of AIDS: Guidelines For The Use of Antiretroviral therapy in Malawi. 2nd ed. Lilongwe, Malawi: Ministry of Health; 2008. Apr, [Accessed 20 May 2009]. Also available at http://www.hivunitmohmw.org/Main/AntiretroviralTherapy. [Google Scholar]
- 24.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 25.DAIDS Toxicity Tables. [Accessed 20 May 2009];Tables for grading severity of adult and pediatric adverse events, Division of AIDS, NIAID, NIH. Available at: http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/Safety.htm.
- 26.Allendoerfer R, Marquis AJ, Rinaldi MG, Graybill JR. Combined therapy with fluconazole and flucytosine in murine cryptococcal meningitis. Antimicrob Agents Chemother. 1991;35:726–729. doi: 10.1128/aac.35.4.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding JC, Bauer M, Diamond DM, et al. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–1593. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diamond DM, Bauer M, Daniel BE, et al. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob Agents Chemother. 1998;42:528–533. doi: 10.1128/aac.42.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kartalija M, Kaye K, Tureen JH, et al. Treatment of experimental cryptococcal meningitis with fluconazole: impact of dose and addition of flucytosine on mycologic and pathophysiologic outcome. J Infect Dis. 1996;173:1216–1221. doi: 10.1093/infdis/173.5.1216. [DOI] [PubMed] [Google Scholar]
- 30.Brouwer AE, van Kan HJ, Johnson E, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2007;51:1038–1042. doi: 10.1128/AAC.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermes A, Kuijper EJ, Guchelaar HJ, Dankert J. An in vitro study on the active conversion of flucytosine to fluorouracil by microorganisms in the human intestinal microflora. Chemotherapy. 2003;49:17–23. doi: 10.1159/000069784. [DOI] [PubMed] [Google Scholar]
- 32.Muula AS, Chipeta J, Siziya S, Rudatsikira E, Mataya RH, Kataika E. Human resources requirements for highly active antiretroviral therapy scale-up in Malawi. BMC Health Services Research. 2007;7:208. doi: 10.1186/1472-6963-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]


