Abstract
BACKGROUND
There is increasing discussion whether colorectal cancer (CRC) screening guidelines should be individualized by gender and race.
OBJECTIVES
To determine individualized colonoscopic screening guidelines by gender and race for the average-risk population and to compare the cost-effectiveness of this approach to that of uniform guidelines for all.
DESIGN
We used the MISCAN-Colon microsimulation model to estimate life-expectancy and lifetime CRC screening and treatment costs in a US cohort of black and white men and women at average risk for CRC. We compared the base case strategy of no screening and 3 competing colonoscopy strategies: (1) the currently recommended “uniform 10-yearly colonoscopy from age 50”, (2) with a shorter interval “uniform 8- yearly colonoscopy from age 51”, and (3) “individualized screening according to gender and race”.
RESULTS
The base case strategy of no screening was the least expensive, yet least effective. The uniform 10-yearly colonoscopy strategy was dominated. The uniform 8- yearly colonoscopy and individualized strategies both increased life-expectancy by 0.0433-0.0435 years per individual at a cost of $15,565 per life-year gained. In the individualized strategy, African Americans began screening 6 years earlier with a 1 year shorter interval compared to whites. The individualized policies were essentially the same for men and women, because the higher CRC risk in men is offset by their shorter life-expectancy. The results were robust for changes in model assumptions.
CONCLUSIONS
The improvements in costs and effects of individualizing on a population level were only marginal. Individualized guidelines, however, could contribute to decreasing disparities between African Americans and whites. The acceptability and feasibility of individualized guidelines should therefore be explored.
INTRODUCTION
For the average risk population, the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society recommend starting colorectal cancer (CRC) screening at the age of 50 years with an identical menu of screening options for men and women of all races.1, 2 There are separate guidelines for individuals at increased risk due to a family history of CRC, a genetic predisposition (e.g., FAP, HNPCC), or a personal history of colorectal cancer, adenomas, or inflammatory bowel disease. The US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society recommend that these individuals have colonoscopy screening at earlier ages and with higher frequency than the general population.1, 2 Race or gender are not used as a basis for modifying recommendations.
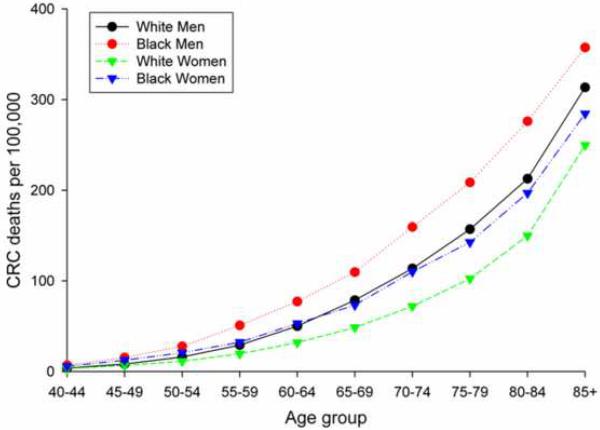
Given the differences in CRC risk by gender, race, and ethnicity, debate has arisen whether screening guidelines should be individualized accordingly.3 The American College of Gastroenterology advocates that screening should start earlier in African Americans because of the higher incidence and younger age at presentation of CRC in this population subgroup.4 During the period 1997-2001,5 African American men had the highest age-specific CRC mortality, while white women had the lowest rate in the US (Figure 1). The four curves of Figure 1 become nearly indistinguishable if the rates for African Americans are shifted five years later compared to the whites (including Hispanics) and five years earlier for women compared to men.5 The disparity seems to support individualizing age of screening initiation by gender and race.
Figure 1.
US age-specific colorectal cancer mortality rates per 100,000 white men, African American men, white women, and African American women, 1997-2001 5.
Next to mortality, other determinants should be considered for individualizing screening guidelines. Important determinants would be life-expectancy, incidence, stage distribution, survival and costs. A simulation approach can take all these aspects into account and estimate costs and life-years gained, which is a commonly used summary measure for the benefit of cancer screening,6 for different screening strategies. In this study, we used the MISCAN-Colon microsimulation model to determine individualized colonoscopy screening guidelines by gender and race for the average-risk population and compare their cost-effectiveness to uniform guidelines with the same screening ages and interval for all.
METHODS
We used the MISCAN-Colon microsimulation model to determine the most cost-effective approach for colonoscopy screening in the average-risk population. The base case strategy was no screening. This strategy was compared to two uniform and one individualized colonoscopy strategies.
MISCAN-Colon Microsimulation Model
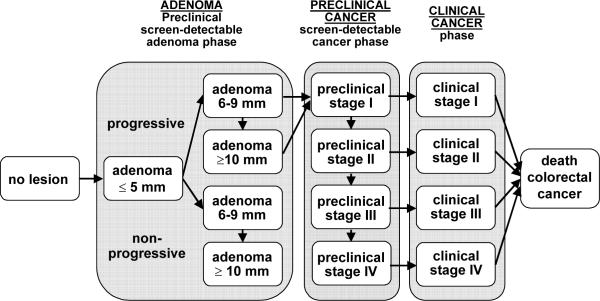
The MISCAN microsimulation model was developed at the Department of Public Health, at Erasmus MC, the Netherlands, and has been used to evaluate breast, cervical, colon, and prostate cancer screening. MISCAN-Colon, the CRC version of the MISCAN-model, was developed in collaboration with the US National Cancer Institute and experts in the field of CRC to assess the effect of different interventions on CRC. A graphical representation of the natural history in the model is given in Figure 2, and the main natural history assumptions in the model are listed in Table 1. A detailed description of the model and the data sources that informed the quantification of the model can be found in Appendix 1, in previous publications 24, 25 and also in a standardized model profiler.26 In brief, the MISCAN-Colon model simulates the relevant biographies of a large population of individuals from birth to death, first without screening and subsequently with the changes that would occur under the implementation of screening. CRC arises in this population according to the adenoma-carcinoma sequence.27, 28 More than one adenoma can occur in an individual and each adenoma can independently develop into CRC. Adenomas can progress in size from small (1-5 mm) to medium (6-9 mm) to large (10+ mm). Most adenomas will never develop into cancer (non-progressive adenomas), but some (progressive adenomas) will eventually become a clinical cancer. Diagnosis of cancer occurs on average 10 years after the manifestation of the adenoma from which it developed. This development competes with death from other causes. A preclinical cancer may progress from stage I to stage IV. In every stage there is a chance of the cancer being diagnosed because of symptoms. The cure rate and survival after diagnosis without cure depend on the stage of the cancer. The model also simulates how screening can interrupt the development of CRC and how it improves prognosis. With screening, adenomas may be detected and removed and preclinical cancers may be found, depending on sensitivity. In this way, screening may prevent CRC incidence or CRC death. The life-years gained by screening are calculated by comparing the model-predicted life-expectancy of the population with and without screening. We assumed the sensitivity of colonoscopy is 75% (CI: 70-79%) for small adenomas (1-5 mm), 85% (CI: 80-92%) for medium adenomas (6-9 mm), and 95% (CI: 92-99%) for large adenomas (10+ mm) and cancers, based on back-to-back colonoscopy studies.15 We assumed a specificity of 90% of colonoscopy. This percentage was equal to 1 minus the 10% of the population without adenomas or cancer but with hyperplastic polyps, lipomas, or other lesions that lead to polypectomy and pathology after colonoscopy. Specificity was assumed to be independent of the screening round. We assumed a cecal intubation rate of 95%.16-18 Harms associated with colonoscopy were assumed to be perforations (0.7 per 1,000 colonoscopies), serosal burns (0.3 per 1,000), bleeds requiring transfusion (0.4 per 1,000) and bleeds not requiring transfusion (1.1 per 1,000), all of which can occur with or without polypectomy.19-22 We assumed that fatal events occur at a rate of 1 per 10,000 colonoscopies. 23
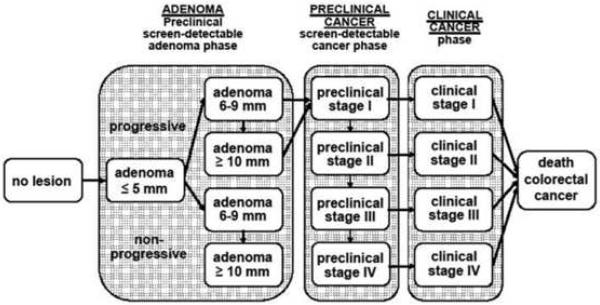
Figure 2.
Adenoma and cancer stages in the MISCAN-Colon model. Cancer stages correspond to the American Joint Committee on Cancer / International Union Against Cancer staging system for colorectal cancer. Adenomas are categorized by size. The size-specific prevalence of adenomas as well as the proportion of adenomas that ever develop into cancer is dependent on age. It is assumed that the proportion of progressive adenomas increases from 16% at age 65 years to 37% at age 75 years, and 96% at age 100 years. It is assumed that 50% of non-progressive adenomas will remain 6-9 mm stage until death and that 50% will progress to the ≥10 mm stage. For progressive adenomas, it is assumed that 30% will develop through the sequence ≤5 mm adenoma → 6-9 mm adenoma → preclinical cancer stage I and that 70% will develop through the sequence ≤5 mm adenoma →6-9 mm adenoma → ≥10 mm adenoma → preclinical cancer stage I. The mean duration time for progressive adenoma is assumed to be 16.4 years (with an exponential distribution). The mean duration time for preclinical cancer is assumed to be 2 years (stage I), 1 year (stage II), 1.5 years (stage III), and 0.8 years (stage IV). The model is calibrated to reproduce observed CRC incidence, stage distribution and survival by gender and race (see Methods and Materials).
Table 1.
Main natural history assumptions in the MISCAN-Colon model
| Model parameter | Value | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Distribution of risk for adenomas over the general population | Gamma distributed, mean 1, variance 2 | Fit to multiplicity distribution of adenomas in autopsy studies:7 | |||||||
| Age 60: | |||||||||
| 1 or more | 20% | ||||||||
| 2 or more | 6% | ||||||||
| 3 or more | 2% | ||||||||
| Age 90: | |||||||||
| 1 or more | 37% | ||||||||
| 2 or more | 17% | ||||||||
| 3 or more | 9% | ||||||||
| Adenoma incidence per year | Age, gender and race dependent | Fit to adenoma prevalence in autopsy and colonoscopy studies of 15% in age group 50-59 to 33% in age group 70+, 7-11 and to cancer incidence per 100,000 in 1997-2001 in SEER registry: 12 | |||||||
| White | Black | White | Black | ||||||
| Age: | Men | Women | Men | Women | Age | Men | Women | Men | Women |
| 0-30 years: | 0.0% | 0.0% | 0.0% | 0.0% | 0-20: | 0.1 | 0.1 | 0.1 | 0.0 |
| 30-39 years: | 0.2% | 0.1% | 0.2% | 0.2% | 20-24: | 0.7 | 0.6 | 0.4 | 1.3 |
| 40-44 years: | 0.4% | 0.4% | 1.0% | 0.6% | 25-29: | 1.5 | 1.8 | 2.1 | 1.9 |
| 45-49 years: | 0.9% | 0.6% | 1.3% | 1.2% | 30-34: | 3.6 | 3.5 | 3.1 | 3.8 |
| 50-54 years: | 1.6% | 1.0% | 2.0% | 1.5% | 35-39: | 7.1 | 5.8 | 8.5 | 8.1 |
| 55-59 years: | 2.9% | 1.8% | 3.0% | 2.3% | 40-44: | 12.9 | 11.1 | 17.5 | 14.8 |
| 60-64 years: | 3.2% | 2.2% | 3.3% | 2.4% | 45-49: | 26.3 | 22.2 | 36.9 | 33.1 |
| 65-69 years: | 3.2% | 2.2% | 3.7% | 2.5% | 50-54: | 51.5 | 37.8 | 74.1 | 57.3 |
| 70-74 years: | 3.2% | 2.3% | 4.3% | 2.5% | 55-59: | 91.4 | 60.5 | 111.4 | 97.3 |
| 75-84 years: | 1.8% | 1.2% | 1.3% | 1.0% | 60-64: | 150.0 | 103.5 | 198.4 | 140.1 |
| 85-100 years: | 1.4% | 1.1% | 1.1% | 0.5% | 65-69: | 226.5 | 151.8 | 231.9 | 193.9 |
| 70-74: | 302.8 | 212.8 | 315.3 | 237.8 | |||||
| 75-79: | 378.1 | 279.9 | 435.7 | 309.0 | |||||
| 80-84: | 457.4 | 338.4 | 488.1 | 361.2 | |||||
| 85-100: | 500.9 | 391.6 | 469.1 | 335.6 | |||||
| Probability that a new adenoma is progressive | Dependent on age at onset: | Fit to adenoma prevalence in autopsy studies,7-11 cancer incidence in SEER registry in 1978.12 | |||||||
| 0-65 years: 14% | |||||||||
| 65-100 years: linearly increasing from 14% to 96% | |||||||||
| Regression of adenomas | No significant regression of adenomas | Expert opinion | |||||||
| Mean duration of development of progressive adenomas to clinical cancer | 20 years | Expert opinion* | |||||||
| Mean duration of preclinical cancer | 3.6 years | Estimated from cancer detection rate at first screening and background cancer incidence in FOBT trials.13,14 | |||||||
| Mean duration of adenoma | 16.4 years | 20 years - 3.6 years | |||||||
| Percent of non-progressive adenomas that stay 6-9mm | 50% | Fit to size distribution of adenomas in autopsy studies: 7-11 | |||||||
| 1-5mm: | 56% | ||||||||
| 6-9 mm: | 24% | ||||||||
| 10+ mm: | 20% | ||||||||
| Percent of non-progressive adenoma that become 10mm or larger | 50% | Fit to size distribution of adenomas in autopsy studies:7-11 | |||||||
| 1-5mm: | 56% | ||||||||
| 6-9 mm: | 24% | ||||||||
| 10+ mm: | 20% | ||||||||
| Percent of cancers that develops from 6-9mm adenoma and from 10+mm adenoma | 30% of cancer develops from 6-9 mm, 70% from 10+mm | Expert opinion | |||||||
| Localization distribution of adenomas and cancer | Dependent on gender and race: | Directly estimated from SEER 1997-2001.6 | |||||||
| White | Black | ||||||||
| Men | Women | Men | Women | ||||||
| Rectum: | 22% | 17% | 19% | 15% | |||||
| Rectosigmoid junction: | 9% | 7% | 8% | 8% | |||||
| Sigmoid colon: | 23% | 21% | 20% | 20% | |||||
| Descending colon: | 5% | 4% | 6% | 6% | |||||
| Transverse colon (incl flexures): | 14% | 15% | 16% | 16% | |||||
| Ascending colon: | 12% | 14% | 14% | 15% | |||||
| Cecum: | 16% | 21% | 18% | 21% | |||||
| 10-year survival after clinical diagnosis of CRC | Dependent on stage, gender and race: | Directly estimated from SEER 1997-2001.6 | |||||||
| White | Black | ||||||||
| Men | Women | Men | Women | ||||||
| Stage I: | 95% | 96% | 71% | 89% | |||||
| Stage II: | 76% | 80% | 73% | 68% | |||||
| Stage III: | 58% | 52% | 40% | 43% | |||||
| Stage IV: | 7% | 4% | 5% | 3% | |||||
| Sensitivity colonoscopy | Van Rijn 2007.15 | ||||||||
| Adenoma < 5 mm: | 75% | ||||||||
| Adenoma 6-9 mm: | 85% | ||||||||
| Adenoma 10+ mm: | 95% | ||||||||
| Cancer: | 95% | ||||||||
| Cecal intubation rate with colonoscopy | 95% | Aslinia 2006,16 Cotterill 2005,17 Rex 2002.18 | |||||||
| Complications with colonoscopy | Per 1,000 colonoscopies: | Levin 2006,19 Lieberman 2000,20 Pox 2007,21 | |||||||
| Perforations | 0.7 | Regula 2006,22 Jentschura 1994.23 | |||||||
| Fatal perforations | 0.1 | ||||||||
| Serosal burn | 0.3 | ||||||||
| Bleeds with transfusion | 0.4 | ||||||||
| Bleeds without transfusion | 1.1 | ||||||||
To be estimated from randomized controlled endoscopy trials, data not yet available.
The validity of the model is based on observational data before the introduction of screening, such as clinical incidence and mortality from CRC12 and the size distribution of adenomas in colonoscopy and autopsy studies.7-11 The external validity has further been tested on the results of large (randomized) screening and surveillance studies, such as the Minnesota Colon Cancer Control Study,29 the CoCap sigmoidoscopy study29 and the National Polyp Study.30 Finally, the model was able to explain observed incidence and mortality trends in the US when accounting for risk factor trends, screening practice and chemotherapy treatment.31
In this study, the model was used to simulate a US cohort born in 1967, subdivided by gender and race (African Americans and whites including Hispanics).32 For all gender and race combinations, age-specific adenoma onset, distribution of cancer localization over the colorectum, distribution of CRC stages, stage-specific CRC survival and all-cause mortality rates were adjusted to reflect observed CRC incidence and mortality and other-cause mortality in the period 1997-2001.12 Adenoma and cancer progression were assumed to be the same for all genders and races. Subsequently, the model was used to predict costs and life-expectancy for different screening strategies.
According to our model the current recommendation of colonoscopy screening every 10 years from age 50 was not optimally cost-effective, although it was close. To enable a fair and interpretable comparison between uniform and individualized guidelines, we also determined a cost-effective uniform colonoscopy strategy. To obtain this cost-effective uniform strategy, we simulated more than 1,000 colonoscopy screening policies that differed with respect to age to begin screening, screen interval and total number of screenings. Policies that were more costly and less effective than other policies were ruled out as non-efficient by simple dominance. Policies that were more costly and less effective than a combination of other strategies were ruled out as non-efficient by extended dominance. Of the remaining policies, we selected the policy that was closest to the current recommendation with respect to number of screens and the age to begin screening as the alternative uniform strategy (result: strategy 2).
To obtain individualized guidelines, we first determined the cost-effective colonoscopy policies by population subgroup as described above. For each cost-effective policy, we calculated the incremental cost-effectiveness ratio, defined as the additional cost of a specific policy divided by its additional clinical benefit compared to the closest less expensive cost-effective policy. Next, we combined cost-effective policies, one for each population subgroup, with the same threshold for the incremental cost-effectiveness ratio.33, 34 For each of the resulting individualized strategies, the costs and life-years of the four population subgroups were summed. The strategy with total costs closest to that of alternative uniform strategy was used as the individualized strategy (result: strategy 3).
Base Case
The base case for the analysis was absence of screening for CRC. All diagnoses of CRC occurred because of symptoms after which patients received treatment according to current practice.
Competing screening strategies
1. uniform 10-yearly colonoscopy at age 50
In this strategy, all individuals were offered colonoscopy screening at age 50 and every 10 years thereafter up to age 80 according to guidelines.1
2. uniform 8-yearly colonoscopy at age 51
In this strategy (resulting from the modeling analysis described before), all individuals were offered colonoscopy screening at age 51 and every 8 years thereafter up to age 75
3. Individualized screening according to gender and race
In this strategy, each population subgroup (black and white men and women) was allowed to have a different colonoscopy policy. The policies, that resulted from the modeling analysis described before, were:
white men: 4 screenings from age 53 to 74 every 7 years
black men: 5 screenings from age 47 to 75 every 7 years
white women: 4 screenings from age 53 to 77 every 8 years
black women: 5 screenings from age 47 to 75 every 7 years.
As part of all simulated screening strategies, adenoma patients (in whom adenomas had been detected and consequently removed) were kept under colonoscopy surveillance according to the guidelines of the US Multi-Society Task Force on Colorectal Cancer.35
Costs
Screening costs were based on Medicare payments of 2007 for procedures and tests associated with CRC screening and complications of screening.36 The costs of complications were based on the relevant Diagnostic Related Group (DRG) codes.36 The phase-specific costs of CRC (Table 2, footnote) were derived from comparing medical costs of CRC cases relative to matched controls in the SEER-Medicare files.37 The results were reported in 2004 dollars and subsequently updated to 2007 dollars using the medical care component of the Consumer Price Index. Appendix 2 contains a detailed overview of the derivation of the costs. The final cost inputs used in the model are summarized in Table 2.
Table 2.
Unit costs in 2007$ (confidence interval) for screening and CRC treatment, used as inputs for the MISCAN-Colon model
| Screening costs36 | CRC treatment costs37 | |||||
|---|---|---|---|---|---|---|
| Procedure | Cost* | Stage | Initial* | Continuous* | Terminal care, death CRC* | Terminal care, death other cause* |
| Colonoscopy | $662 | I | $ 28,668 ($27,905-$29,432) | $ 2,395 ($2,179-$2,612) | $ 51,935 ($49,690-$54,181) | $ 12,703 ($10,533-$14,870) |
| Colonoscopy with polypectomy | $846 | II | $ 39,700 ($38,876-$40,525) | $ 2,237 ($2,036-$2,440) | $ 51,712 ($49,989-$53,434) | $ 11,035 ($9,214-$12,856) |
| Treatment of perforation | $12,446 | III | $ 48,951 ($47,924-$49,976) | $ 3,249 ($2,966-$3,531) | $ 54,776 ($53,204-$56,348) | $ 14,708 ($11,993-$17,422) |
| Treatment of serosal burn | $5,208 | IV | $ 64,801 ($62,420-$67,181) | $ 10,419 ($9,249-$11,590) | $ 73,522 ($71,800-$75,243) | $ 39,679 ($31,826-$47,532) |
| Treatment of bleed with transfusion | $5,208 | |||||
| Treatment of bleed without transfusion | $320 | |||||
Costs for cancer care were divided into three clinically relevant phases of care - initial, continuing and terminal care. The initial phase was defined as the first 12 months following diagnosis, the terminal phase was defined as the final 12 months of life, and the continuing phase was defined as all months between the initial and last year of life phases of care. For patients surviving less than 24 months after diagnosis, the final 12 months of observation and costs of care were then allocated first to the last year of life phase, because the content of care for patients with short survival is more similar to the last year of life phase than the initial phase. The remainder of months of observation and costs were allocated to the initial phase, with no contribution to the continuing phase.
Outcomes
We projected lifetime costs and life-expectancy for a cohort of 40-year old black and white men and women in the US. Costs and future life-years were discounted at an annual rate of 3%.38
Sensitivity and uncertainty analysis
We performed a one-way sensitivity and a multivariate uncertainty analysis on influential model assumptions:
The assumption of higher incidence of adenomas versus faster progression of adenomas explaining the risk differences between population subgroups;
Duration of adenoma-carcinoma sequence;
CRC risk in African Americans;
Sensitivity, specificity and complication rate of colonoscopy;
Costs of colonoscopy, polypectomy, complications and CRC treatment.
Because the focus of the analysis was to assess the cost-effectiveness of individualized guidelines compared to uniform guidelines, we restricted the sensitivity analysis to comparing strategies 2 and 3.
In the one-way sensitivity analysis, each parameter was varied from its original value to a low and high value. For colonoscopy sensitivity and treatment costs, these values were set at the boundaries of the 95% confidence interval. Ranges reported in the literature were used for colonoscopy reach 16, 20, 39-45, specificity 20, 44, 46-48, perforation rates 48-57, and costs of colonoscopy, polypectomy and complications 46-58. The average duration between the manifestation of an adenoma and the diagnosis of CRC (base assumption 10 years) was decreased and increased by 50%, while the CRC risk in African Americans was decreased and increased by 10%.
In the multivariate uncertainty analysis, we simulated the uniform 8-yearly colonoscopy and individualized strategies 1,000 times with different sets of parameters. The test characteristic parameters (sensitivity, specificity, reach and complication rate) were drawn from a beta distribution with the mean equal to the base value. For the cost parameters and the CRC risk in African Americans, we assumed log-normal distributions with the median equal to the base value. For all parameters varied, the standard deviation was chosen such that the 95% probability mass overlapped with the low and high values used in the one-way sensitivity analysis. In 50% of runs we assumed a duration of the development of CRC of 20 years, while 10 and 30 years were used in 25% of runs each. In 75% of runs we assumed that the difference in CRC risk between population subgroups was due to different adenoma incidence, while in 25% we assumed the difference was caused by different adenoma progression rates. For each of the 1,000 simulations the difference in costs and life-expectancy between the uniform 8-yearly colonoscopy strategy and the individualized strategy were plotted in a scatterplot.59
RESULTS
The life-expectancy and lifetime costs of the no screening, uniform 8-yearly colonoscopy, uniform 10-yearly colonoscopy and individualized strategies are displayed in Table 3. The no screening strategy was the least expensive yet least effective strategy. The uniform 8-yearly colonoscopy and individualized strategies both increased life-expectancy by 0.0433-0.0435 years per individual at a cost of $15,565 per life-year gained compared with no screening. The uniform 10-yearly colonoscopy strategy was weakly dominated by both the uniform 8-yearly and the individualized strategy.
Table 3.
Results from cost-effectiveness analysis
| Strategy | CRC cases/100,000 from age 40 to age 100 | CRC deaths/100,000 from age 40 to age 100* | Life-expectancy at age 40† | Lifetime per person cost for CRC screening and treatment after age 40† | ICER† |
|---|---|---|---|---|---|
| No screening | 5712 | 2027 | 22.3929 | $ 1,663 | Base Case |
| Uniform 10-yearly cspy | 3026 | 794 | 22.4340 | $ 2,310 | Dominated |
| Uniform 8-yearly cspy | 2901 | 751 | 22.4362 | $ 2,349 | $ 15,565 |
| Individualized | 2882 | 739 | 22.4363 | $ 2,340 | $ 15,565 |
ICER: Incremental Cost-Effectiveness Ratio
Including procedural deaths from colonoscopy complications
3% discounted
Table 4 shows life-expectancy and costs for the uniform 8-yearly and individualized colonoscopy strategies by population subgroup. The increase in screening intensity in African Americans with individualization resulted in 0.0080 years longer life-expectancy in black men, while in black women the life-expectancy increased by 0.0076 years. The redistribution of resources from lower-risk whites to higher-risk African Americans, resulted in a higher starting age (2 years later) with individualization for whites and a slightly decreased life-expectancy in this group by 0.0006 years for men and 0.0016 years for women.
Table 4.
Comparison of 3% discounted costs and life-expectancy between the uniform 8-yearly colonoscopy and individualized strategies, by gender and race and for the total population.
| Population subgroup | Uniform 8-yearly colonoscopy strategy | Individualized strategy | Difference in life-expectancy | ||||
|---|---|---|---|---|---|---|---|
| Uniform strategy | Costs ($)* | LE-40 | Individualized strategy | Costs ($)* | LE-40 | ||
| White Men | 4 screens, every 8 years, age: 51-75 | 2,408 | 21.9418 | 4 screens, every 7 years, age: 53-74 | 2,361 | 21.9412 | -0.0006 |
| Black Men | 4 screens, every 8 years, age: 51-75 | 2,240 | 19.8791 | 5 screens, every 7 years, age: 47-75 | 2,582 | 19.8871 | +0.0080 |
| White Women | 4 screens, every 8 years, age: 51-75 | 2,314 | 23.4309 | 4 screens, every 8 years, age: 53-77 | 2,221 | 23.4293 | -0.0016 |
| Black Women | 4 screens, every 8 years, age: 51-75 | 2,299 | 21.9359 | 5 screens, every 7 years, age: 47-75 | 2,671 | 21.9435 | +0.0076 |
| Total Population† | 2,349 | 22.4362 | 2,340 | 22.4363 | +0.0002 | ||
LE-40: Life-expectancy at age 40
Lifetime per person cost for CRC screening and treatment after age 40
Average of results for population subgroups, weighted by size of population subgroup
Sensitivity and uncertainty analysis
In the one-way sensitivity analysis we assessed the influence of model assumptions on the differences between uniform 8-yearly colonoscopy and individualized screening. Table 5 shows that in all analyses both strategies were equivalent in costs and effects. The difference in costs never exceeded $12, and the maximum difference in life-years gained was 0.0005 years. With a longer duration of the adenoma-carcinoma sequence of 30 years, the uniform 8-yearly strategy became most effective, nullifying the already very small advantage of individualized screening. Other influential model assumptions on effectiveness were the disparity in CRC risk, colonoscopy sensitivity and reach and whether disparities in incidence are caused by difference in adenoma onset versus faster progression. Costs were mostly influenced by colonoscopy costs and the duration of the adenoma-carcinoma sequence.
Table 5.
Results one-way sensitivity analysis: Comparison between 3% discounted costs and life-expectancy in the total population between the uniform 8-yearly colonoscopy and individualized strategies, for different model assumptions
| Model parameter | Differences of the individualized strategy compared to the uniform 8-yearly colonoscopy strategy | |||||
|---|---|---|---|---|---|---|
| Low value | High value | |||||
| Gain in LE | Costs ($)* | ICER | Gain in LE | Costs ($)* | ICER | |
| Base assumptions | +0.0002 | -9.09 | Dominant | +0.0002 | -9.09 | Dominant |
| Fast progression of adenomas† | +0.0001 | -7.89 | Dominant | |||
| Duration adenoma carcinoma sequence** | +0.0005 | -10.11 | Dominant | -0.0000 | -6.66 | $ 546,213 |
| Risk African Americans** | +0.0001 | -6.67 | Dominant | +0.0003 | -9.40 | Dominant |
| Reach colonoscopy** | +0.0003 | -8.01 | Dominant | +0.0002 | -8.09 | Dominant |
| Sensitivity colonoscopy** | +0.0002 | -8.58 | Dominant | +0.0001 | -7.82 | Dominant |
| Specificity colonoscopy** | +0.0002 | -8.05 | Dominant | +0.0002 | -7.97 | Dominant |
| Cost colonoscopy** | +0.0002 | -4.18 | Dominant | +0.0002 | -11.56 | Dominant |
| Cost polypectomy** | +0.0002 | -8.03 | Dominant | +0.0002 | -7.65 | Dominant |
| Complication rate of colonoscopy** | +0.0002 | -7.93 | Dominant | +0.0002 | -8.09 | Dominant |
| Costs treatment complications** | +0.0002 | -7.99 | Dominant | +0.0002 | -8.51 | Dominant |
| Cost treatment** | +0.0002 | -8.05 | Dominant | +0.0002 | -7.96 | Dominant |
LE: Life-expectancy
ICER: Incremental Cost-effectiveness ratio. Italic numbers represent the incremental cost per life-year gained of the uniform 8-yearly colonoscopy strategy compared to the individualized strategy. “Dominant” means that the individualized strategy was both more effective and less costly than the uniform strategy.
Lifetime per person cost for CRC screening and treatment after age 40
For this assumption there is no high or low value. It was just varied from the base assumption where differences in CRC risk were caused by differences in adenoma incidence. In this sensitivity analysis it was assumed that differences were caused by differences in progression rates of adenomas
Duration: low value = 10 years, high value = 30 years
Risk African Americans: low value = 10% lower risk, high value = 10% higher risk than values in Table 1
Reach colonoscopy: low value = 80% reach cecum, high value = 99% reach cecum
Sensitivity colonoscopy: low and high values set at confidence intervals (see MISCAN-Colon microsimulation model in Methods)
Specificity colonoscopy: low value = 0.78, high value = 1
Cost colonoscopy: low value = $285, high value = $1012
Cost polypectomy: low value = $159, high value = $507
Complication rate colonoscopy: low value = 1 per 1,000, high value = 4 per 1,000
Cost complications: low value = $4,360, high value = $26,000
Cost treatment: low and high values set at confidence intervals (see Table 2)
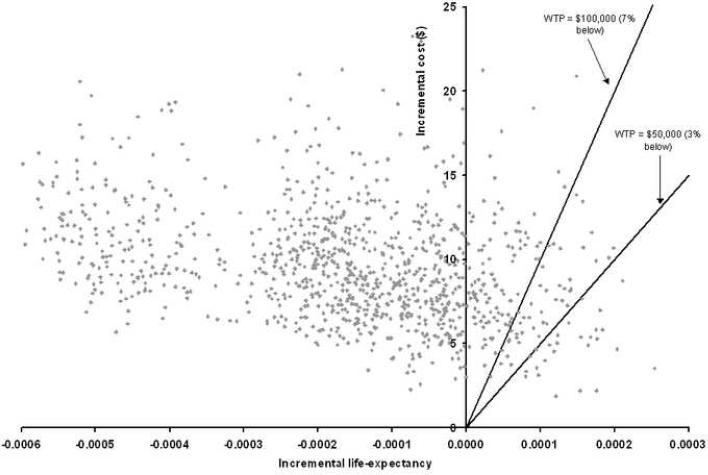
For the multivariate uncertainty analysis, the results from 1,000 simulations comparing the uniform 8-yearly colonoscopy and individualized strategies are shown in Figure 3. In all simulations, the uniform 8-yearly colonoscopy and individualized strategies remained equivalent in costs and effects. The median difference in life-years gained was 0.0002 life-years. The 25% and 75% percentiles of the increase in life-years gained from the individualized strategy compared to the uniform strategy was 0.0001 and 0.0002 life-years respectively, while the 25% and 75% percentiles for the decrease in cost were $7.20 to $11.50. For African Americans the 25% and 75% percentiles of additional life-years gained were 0.0063 and 0.0079 years and of additional costs $260 to $465. In 83% of the simulations, the individualized strategy was more effective and less costly than the uniform strategy. In 3% of simulations was uniform screening more effective at an incremental cost per life-year gained of $50,000 or less, and in 7% at costs of $100,000 or less.
Figure 3.
Multivariate uncertainty analysis using 1,000 simulations. This analysis simultaneously varies all parameters over the full range of possible values. Each point represents the incremental costs and life-years gained of uniform 8-yearly colonoscopy screening over individualized screening generated in one simulation. 83% of points fall in the upper left corner of the graph, meaning that uniform screening is both less effective and more costly than individualized screening. The solid lines represent the willingness to pay (WTP) thresholds of $50,000 and $100,000. Points to the right and under this line represent simulations in which uniform screening was more effective than individualized screening at incremental costs of $50,000 or less/$100,000 or less respectively.
DISCUSSION
The present analysis suggests that 8-yearly uniform and individualized colonoscopy recommendations by gender and race on a total population level are comparable in costs and effects: the overall (total population) benefit of individualization is limited (0.0002 additional life-years gained, $9.09 lower costs per person). This is explained by the fact that the African American population constitutes no more than approximately 20% of the population. For African Americans, the increase in life-years gained was more substantial (0.0078 life-years, approximately 14% of total life-years saved with screening), decreasing the disparity in incidence and mortality compared to whites. Our results were robust for changes in model assumptions. In 1,000 simulations with different model parameter values, the 8-yearly uniform and individualized strategies remained equivalent in costs and effects. We found that with individualizing screening, African Americans are screened with a one-year shorter interval than whites and start screening 6 years earlier, whereas the recommended screening ages and frequency for men and women remain similar.
Our findings support the recommendation of the American College of Gastroenterology to begin screening 5 years earlier in African Americans than whites. Starting screening at an earlier age without increasing the number of screenings, results in saving 0.0052 additional life-years in African Americans (data not shown). Also increasing the number of screens as recommended from our study, significantly further increases the additional life-years gained to 0.0078. Individualization can therefore play a significant role in reducing disparities between African Americans and whites. Our results are in line with other studies that have shown that the average cost-effectiveness of CRC screening is better in African American men than in other population subgroups.60, 61 Based on these results, the authors advocate earlier screening in African Americans. However, basing individualized guidelines on average cost-effectiveness does not necessarily lead to efficient use of resources. In the present analysis, we determined individualized guidelines based on incremental cost-effectiveness and hence ensuring efficient use of resources.33, 34
Besides the current recommendation of four screenings every 10 years from age 50 to age 80, we also used another uniform colonoscopy strategy as a comparator to enable a fair comparison between uniform and individualized screening. We could not use the exact recommendation for that purpose, because it was not optimally cost-effective, although it was close. The current guidelines were not based on a formal decision analysis, but on studies on colonoscopic efficiency1 and on simplicity and clarity. Individualized guidelines are more complex than uniform ones, and one could therefore argue that recommendations should not be individualized unless benefits are substantial. Individualized screening guidelines may confuse providers and consumers to the point of decreasing adherence. A decrease in adherence will easily offset the gains from individualization. Currently, 40% of African American men and 32% of African American women aged 50 years and older reported having had either a Fecal Occult Blood Test (FOBT) within the past year or a colorectal endoscopy within the past 5 years.62 Based on these figures much can be gained from increased adherence to screening guidelines. On the other hand, individualization of screening guidelines must be considered in the context of a general trend towards personalized medical care.63, 64 As a result, screening adherence might improve because individuals appreciate that the recommendation is based on their personal risk profile. In any case, in a situation where individualization of medical care and especially of screening, becomes the standard, it would be only natural to account also for race and gender differences, given the expected benefit and regardless of its size. To avoid too much complexity, one could recommend not changing the guidelines for whites but changing screening for African Americans to every 9 years from age 45 years onward (a similar change as the results of this study). Compared with the current screening guidelines, this recommendation would result in 0.0076 more life-years gained for blacks, comparable with the 0.0078 found in this study.
In this analysis, we assumed that all disparities in cancer incidence are caused by differences in adenoma incidence. This assumption is supported by results from the Clinical Outcomes Research Initiative (CORI), showing a higher percentage of adenoma patients with polyps of size > 9mm in African Americans than in whites.65 Furthermore, observational studies show that CRC risk factors have a similar effect on adenoma prevalence as on CRC incidence.66-71 Theoretically, higher CRC incidence could also be caused by more rapid adenoma and cancer progression. In this case, development of adenomas into CRC would have a shorter duration in blacks than in whites. When we assumed faster progression for blacks, with a strongly reduced average preclinical disease duration, the benefit of individualization slightly reduced.
We assumed that differences in observed CRC incidence and stage distribution between African Americans and whites reflect true differences in risk and are not due to differences in screening utilization. However, considering that screening rates are lower for African Americans than for whites,62 the risk difference between African Americans and whites may be smaller. The sensitivity analysis shows that with a lower CRC risk in African Americans (i.e. smaller difference with whites), the benefit of individualization was reduced. Furthermore, we only considered life-years gained and not quality adjusted life-years. The reason for this is that the effect of CRC screening on quality of life has hardly been studied. There has been one study estimating quality of life 30 days before and after colonoscopy, which found that mental health and vitality domains of quality of life significantly improved after colonoscopy.72 However, quality of life at the moment of colonoscopy was not assessed. In population screening large numbers of individuals undergo colonoscopy and even a minor effect of colonoscopy on quality of life will have a large impact on quality adjusted life-years gained. Our results are only influenced by adjusting for quality of life when this differs between population subgroups. Crimmens has shown that African Americans and whites not only differ in life-expectancy (for which we accounted in the present analysis) but also in the proportion of healthy life-years,73 due to the fact that African Americans have more comorbidities at older ages. Therefore, intensive colonoscopy screening at older ages may be less feasible in African Americans and also less beneficial in terms of quality adjusted life years gained, reducing the potential benefit of individualization.
Age-specific CRC incidence and mortality in men reaches levels of risk comparable to women four to eight years later in life.74 Also, more women than men need to be screened for the detection of one advanced neoplasia.22, 65, 75 Therefore, one may have expected that men need earlier and more intensive screening than women. However, our results show that the cost-effective individualized policies for men and women are comparable. This is due to longer life-expectancy of women. Although women have fewer advanced adenomas than men, more of those adenomas can evolve into CRC during the longer lifetime. This means that the number needed to screen to detect one advanced adenoma in women may be higher than in men, but that the number of detected adenomas needed to prevent one case of CRC is lower. This makes the number needed to screen to prevent one CRC case similar for men and women. Our finding of similar screening strategies is supported by the fact that the absolute number of CRC cases in men and women is comparable.76
This study aimed to explore the cost-effectiveness of individualization of screening guidelines. We restricted ourselves to colonoscopy, the preferred method of screening according to the American College of Gastroenterology.77 Fortunately, the results can be generalized to other screening modalities. The costs per life-year gained will be different for other screening modalities, but the conclusion that individualization is cost-effective will remain, as well as the result that it is more cost-effective for African Americans to be screened over a wider age range and with greater frequency than whites. We focused the analysis on African American and white (including Hispanics) population subgroups. In a more extensive study Hispanics and non-Hispanics could be considered separately and Asians, Pacific Islanders, American Indians and Alaskan Natives could be included to explore further benefit of individualization. However for these groups, incidence and mortality data will be based on small numbers. CRC incidence and mortality tend to be lower in Hispanics, Asians, Pacific Islanders, American Indians and Alaskan Natives than in whites.78 When these data are confirmed, a less intensive screening schedule for these groups could be considered.
In conclusion, our study suggests that 8-yearly uniform and individualized colonoscopy screening are comparable in costs and effects in the total population. However, individualized guidelines could contribute to decreasing disparities between African Americans and whites. The acceptability and feasibility of individualized guidelines should therefore be explored.
ACKNOWLEDGEMENT
We acknowledge Martin Brown, Ph.D. and Robin Yabroff, Ph.D. of NCI for their assistance with obtaining cancer treatment costs using SEER-Medicare data; John Allen, M.D. and Joel Brill, M.D. for their assistance in estimating costs of screening and complications; and William Larson, Marjorie Baldo, and Marilu Hu of CMS for providing cost data from the Centers for Medicaid and Medicare Services (CMS).
STUDY SUPPORT The National Cancer Institute U01 CA97426 and U01 CA115935 supported this work for the Cancer Intervention and Surveillance Modeling Network. The study sponsor has played no role in the study design, collection, analysis and interpretation of the data, nor in the writing of the report.
List of Acronyms
- CRC
Colorectal Cancer
- FOBT
Fecal Occult Blood Test
- FAP
Familial Adenomatous Polyposis
- HNPCC
Hereditary Non-Polyposis Colorectal Cancer
- WTP
Willingness to pay
Appendix 1: The MISCAN-Colon microsimulation model
MODEL OVERVIEW
The MISCAN-Colon model is a semi-Markov microsimulation model. The population is simulated individual by individual, and each person can evolve through discrete disease states. However, instead of modeling yearly transitions with associated transition probabilities, the MISCAN-Colon model generates durations in states. This improves model performance. With the assumption of exponential distribution of the duration in each state, this way of simulating leads to the same results as a Markov model with yearly transition probabilities. The advantage of the MISCAN approach is that durations in a certain state need not necessarily be a discrete value but can be continuous. MISCAN uses the Monte Carlo method to simulate all events in the program. Possible events are birth and death of a person, adenoma incidence and transitions from one state of disease to another.
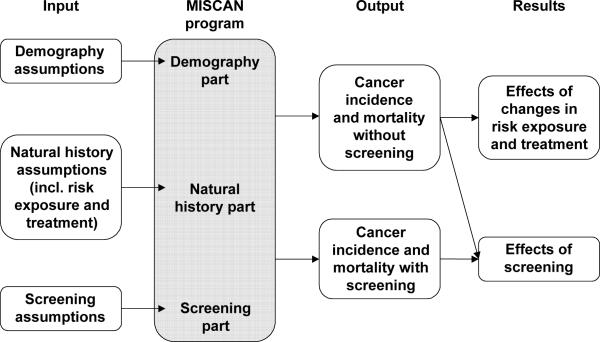
The basic structure of MISCAN-Colon is illustrated in figure A1-1. Figure A1-1 clearly demonstrates that MISCAN-Colon consists of three parts:
demography part
natural history part
screening part
Figure A1-1.
Structure of MISCAN-Colon
These parts are not physically separated in the program, but it is useful to consider them separately.
DEMOGRAPHY PART
The demography part of the model simulates individual life histories without colorectal cancer to form a population. For each person, a date of birth and a date of death of other causes than colorectal cancer are simulated. The distribution of births and deaths can be adjusted to represent the population simulated. For example, a population of Caucasian females will have higher death ages than a population of African American males.
NATURAL HISTORY PART
The Natural History part of MISCAN-Colon simulates the development of colorectal cancer in the population. We assume all colorectal cancers develop according to the adenoma-carcinoma sequence of Morson1 and Vogelstein2 (Figure A1-2). For each individual in the simulated population a personal risk index is generated. Subsequently, adenomas are generated in the population according to this personal risk index and an age specific incidence rate of adenomas. This results in no adenomas for most persons and one or more adenomas for others. The distribution of adenomas over the colorectum is simulated according to the observed distribution of colorectal cancer incidence. Each of the adenomas can independently develop into colorectal cancer. Adenomas can progress in size from small (1-5 mm) to medium (6-9 mm) to large (10+ mm). Most adenomas will never develop into cancer (non-progressive adenomas), but some (progressive adenomas) may eventually become malignant, transforming to a stage I cancer. The cancer may then progress from stage I to stage IV. In every stage there is a chance of the cancer being diagnosed because of symptoms. The survival after clinical diagnosis depends on the stage of the cancer.
Figure A1-2.
Adenoma and cancer stages in the MISCAN-Colon model. Cancer stages correspond to the American Joint Committee on Cancer / International Union Against Cancer staging system for colorectal cancer. Adenomas are categorized by size. The size-specific prevalence of adenomas as well as the proportion of adenomas that ever develop into cancer is dependent on age.
SCREENING PART
Screening interrupts the development of CRC. With screening, adenomas may be detected and removed and cancers may be found, usually in an earlier stage than with clinical diagnosis. In this way screening prevents CRC incidence or CRC death. The life-years gained by screening are calculated by comparing the model-predicted life-years lived in the population with and without screening. The effects of different screening policies can be compared by applying them to identical natural histories.
INTEGRATION OF THE THREE MODEL COMPONENTS
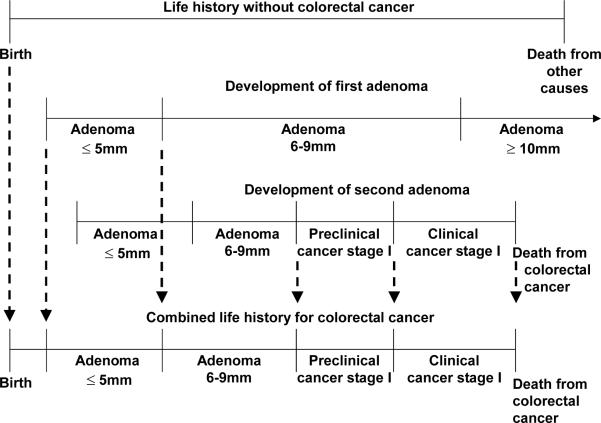
For each individual, the demography part of the model simulates a time of birth and a time of death of other causes than colorectal cancer, creating a life history without colorectal cancer (top line in figure A1-3a). Subsequently adenomas are simulated for that individual. For most individuals no adenomas are generated, for other multiple. In the example in figure A1-3, the person gets two adenomas (2nd and 3rd line in figure A1-3a). The first adenoma arises at a certain age, grows into 6-9 mm and eventually becomes larger than 10 mm. However, this adenoma does not become cancer before the death of the person. The second adenoma is a progressive adenoma. After having grown to 6-9 mm, the adenoma transforms into a malignant carcinoma, causing symptoms and diagnosis and eventually resulting in an earlier death from CRC. The life history without CRC and the development of the two adenomas in figure A1-3 together lead to the combined life history with CRC depicted in the bottom line. Because this person dies from colorectal cancer before he dies from other causes, his death age is adjusted accordingly.
Figure A1-3a.
Modeling natural history into life history
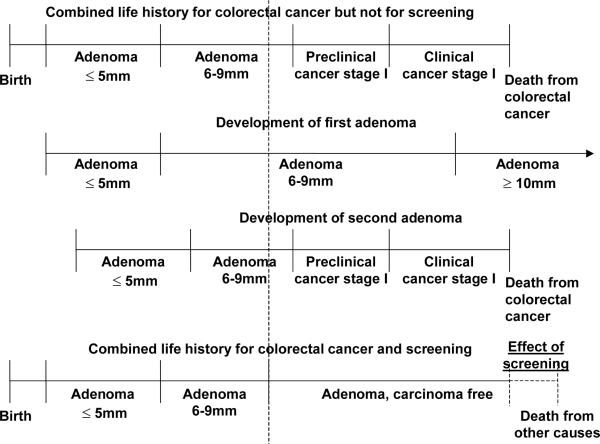
After the life history of a person is adjusted for colorectal cancer, the history will now be adjusted for the effects of screening. The effect of screening on life history is explained in figure A1-3b.The top line in this figure is the combined life history for colorectal cancer from figure A1-3a. The development of the separate adenomas is repeated in the second and third line. In this picture there is one screening intervention. During the screening both prevalent adenomas are detected and removed. This results in a combined life history for colorectal cancer and screening (bottom line). From the moment of screening the adenomas are removed and this individual becomes adenoma and carcinoma free. He does not develop cancer because the precursor lesion has been removed. Therefore the person dies at the moment of death from other causes and the effect of screening is the difference in life-years in the situation without screening and the situation with screening. Of course many other possibilities could have occurred: a person could have developed new adenomas after the screening moment, or an adenoma could have been missed by the screening test, but in this case this individual really benefited from the screening intervention.
Figure A1-3b.
Modeling screening into life history
MODEL QUANTIFICATION
For this analysis we developed four different models, for white men, white women, African American men and African American women. For each group we simulated a cohort born in 1967.
DEMOGRAPHY PARAMETERS
There are two types of demography parameters: birth tables and life tables. In this case all individuals were born in 1967. The life tables were derived from the 2000 US Life Table published by the National Center for Health Statistics (http://www.cdc.gov/nchs/products/pubs/pubd/lftbls/life/1966.htm). These life tables include colorectal cancer mortality and the demography part simulates mortality from other causes than colorectal cancer. However, we decided not to adjust the life tables because the percentage of colorectal cancer mortality in overall mortality is small and the data on colorectal cancers deaths by age, gender and race are sparse.
NATURAL HISTORY PARAMETERS
The parameters for natural history model that could not be directly estimated from data or fit to reference data, were established based on expert opinion. At two expert meetings at the NCI on June 5-7, 1996, and May 12-13, 1997, a model structure was devised in agreement with the currently accepted model of the adenoma-carcinoma sequence. It was assumed that all cancers are preceded by adenomas.
The expert panel agreed on an estimate of the average sojourn time (i.e., the duration between onset of a progressive adenoma and the clinical diagnosis of subsequent cancer) of 20 years. However, some adenomas do not make it to cancer in that time period, because people die of other causes before the cancer could actually manifest. These are mainly the slower-developing adenomas with a longer duration than the average. The result is that the average duration of the adenomas that actually make it to diagnosed cancer is shorter, on average 10 years. The average duration of cancer in preclinical stages I-IV was 2 years, 1 year, 1.5 years, and 0.8 year, respectively, which resulted in a total average duration of 3.6 years because not every cancer reaches stage IV before clinical diagnosis. These sojourn times were based on the ratio between the stage-specific detection rate at first screening in fecal occult blood test trials and the background incidence, accounting for a 60% sensitivity of fecal occult blood test for all cancer stages.3, 4 All durations were governed by an exponential probability distribution. Durations in each of the invasive cancer stages as well as durations in the stages of the noninvasive adenomas were assumed to be 100% associated with each other, but the durations in invasive stages as a whole were independent of durations in noninvasive adenoma stages that precede cancer. These assumptions resulted in an exponential distribution of the total duration of progressive noninvasive adenomas and of the total duration of preclinical cancer, which has also been used in other cancer screening models.3, 5
It was assumed that 30% of the cancers arise from adenomas of 6-9 mm and that 70% arise from larger adenomas. Initially, the preclinical incidence of progressive adenomas was chosen to reproduce the colorectal cancer incidence by age, stage, and localization in the United States in 1978.6 During this period, almost no screening was performed. The size distribution of adenomas over all ages was assumed to be 56% for stages less than or equal to 5 mm, 24% for stages 6-9 mm, and 20% for stages greater than or equal to 10 mm.7-11 The preclinical incidence of non-progressive adenomas that will never grow into cancer was varied until the simulated prevalence of all adenomas was about 15% in age group 50-59 years, 27% in age group 60-69 years, and 33% in age group 70 or more years, in agreement with data from the Kaiser study in Northern California12 and with data from autopsy and colonoscopy studies.7-11
For this analysis, the 1978 total population model was adjusted to obtain 1997-2001 models for white and black men and women. We assumed that all difference in CRC incidence between the 1978 general population model and the 1997-2001 race- and gender specific models was caused by differences in adenoma incidence. We therefore adjusted the age-specific incidence of both progressive and non-progressive adenomas so that the colorectal cancer incidence by gender, race, age, stage and location from 1997-2001 was reproduced. The anatomic site distribution of both progressive and non-progressive adenomas and thus of preclinical and clinical cancers is assumed to be equal to the site distribution of colorectal cancers in the United States in 1997-2001.6 The stage-specific survival after the clinical diagnosis of colorectal cancer is taken from the Surveillance, Epidemiology, and End Results registry data from 1987 through 2001.6
Table A1-2.
Main natural history assumptions in the MISCAN-Colon model
| Model parameter | Value | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Distribution of risk for adenomas over the general population | Gamma distributed, mean 1, variance 2 | Fit to multiplicity distribution of adenomas in autopsy studies:9 | |||||||
| Age 60: | |||||||||
| 1 or more | 20% | ||||||||
| 2 or more | 6% | ||||||||
| 3 or more | 2% | ||||||||
| Age 90: | |||||||||
| 1 or more | 37% | ||||||||
| 2 or more | 17% | ||||||||
| 3 or more | 9% | ||||||||
| Adenoma incidence per year | Age, gender and race dependent | Fit to adenoma prevalence in autopsy and colonoscopy studies of 15% in age group 50-59 to 33% in age group 70+,7-11 and to cancer incidence per 100,000 in 1997-2001 in SEER registry:6 | |||||||
| White | Black | White | Black | ||||||
| Age: | Men | Women | Men | Women | Age | Men | Women | Men | Women |
| 0-30 years: | 0.0% | 0.0% | 0.0% | 0.0% | 0-20: | 0.1 | 0.1 | 0.1 | 0.0 |
| 30-39 years: | 0.2% | 0.1% | 0.2% | 0.2% | 20-24: | 0.7 | 0.6 | 0.4 | 1.3 |
| 40-44 years: | 0.4% | 0.4% | 1.0% | 0.6% | 25-29: | 1.5 | 1.8 | 2.1 | 1.9 |
| 45-49 years: | 0.9% | 0.6% | 1.3% | 1.2% | 30-34: | 3.6 | 3.5 | 3.1 | 3.8 |
| 50-54 years: | 1.6% | 1.0% | 2.0% | 1.5% | 35-39: | 7.1 | 5.8 | 8.5 | 8.1 |
| 55-59 years: | 2.9% | 1.8% | 3.0% | 2.3% | 40-44: | 12.9 | 11.1 | 17.5 | 14.8 |
| 60-64 years: | 3.2% | 2.2% | 3.3% | 2.4% | 45-49: | 26.3 | 22.2 | 36.9 | 33.1 |
| 65-69 years: | 3.2% | 2.2% | 3.7% | 2.5% | 50-54: | 51.5 | 37.8 | 74.1 | 57.3 |
| 70-74 years: | 3.2% | 2.3% | 4.3% | 2.5% | 55-59: | 91.4 | 60.5 | 111.4 | 97.3 |
| 75-84 years: | 1.8% | 1.2% | 1.3% | 1.0% | 60-64: | 150.0 | 103.5 | 198.4 | 140.1 |
| 85-100 years: | 1.4% | 1.1% | 1.1% | 0.5% | 65-69: | 226.5 | 151.8 | 231.9 | 193.9 |
| 70-74: | 302.8 | 212.8 | 315.3 | 237.8 | |||||
| 75-79: | 378.1 | 279.9 | 435.7 | 309.0 | |||||
| 80-84: | 457.4 | 338.4 | 488.1 | 361.2 | |||||
| 85-100: | 500.9 | 391.6 | 469.1 | 335.6 | |||||
| Probability that a new adenoma is progressive | Dependent on age at onset: | Fit to adenoma prevalence in autopsy studies,7-11 cancer incidence in SEER registry in 1978.6 | |||||||
| 0-65 years: 14% | |||||||||
| 65-100 years: linearly increasing from 14% to 96% | |||||||||
| Regression of adenomas | No significant regression of adenomas | Expert opinion | |||||||
| Mean duration of development of progressive adenomas to clinical cancer | 20 years | Expert opinion* | |||||||
| Mean duration of preclinical cancer | 3.6 years | Estimated from cancer detection rate at first screening and background cancer incidence in FOBT trials.3,4 | |||||||
| Mean duration of adenoma | 16.4 years | 20 years - 3.6 years | |||||||
| Percent of non-progressive adenomas that stay 6-9mm | 50% | Fit to size distribution of adenomas in autopsy studies:7-11 | |||||||
| 1-5mm: | 56% | ||||||||
| 6-9 mm: | 24% | ||||||||
| 10+ mm: | 20% | ||||||||
| Percent of non-progressive adenoma that become 10mm or larger | 50% | Fit to size distribution of adenomas in autopsy studies:7,11 | |||||||
| 1-5mm: | 56% | ||||||||
| 6-9 mm: | 24% | ||||||||
| 10+ mm: | 20% | ||||||||
| Percent of cancers that develops from 6-9mm adenoma and from 10+mm adenoma | 30% of cancer develops from 6-9 mm, 70% from 10+mm | Expert opinion | |||||||
| Localization distribution of adenomas and cancer | Dependent on gender and race: | Directly estimated from SEER 1997-2001.6 | |||||||
| White | Black | ||||||||
| Men | Women | Men | Women | ||||||
| Rectum: | 22% | 17% | 19% | 15% | |||||
| Rectosigmoid junction: | 9% | 7% | 8% | 8% | |||||
| Sigmoid colon: | 23% | 21% | 20% | 20% | |||||
| Descending colon: | 5% | 4% | 6% | 6% | |||||
| Transverse colon (incl flexures): | 14% | 15% | 16% | 16% | |||||
| Ascending colon: | 12% | 14% | 14% | 15% | |||||
| Cecum: | 16% | 21% | 18% | 21% | |||||
| 10-year survival after clinical diagnosis of CRC | Dependent on stage, gender and race: | Directly estimated from SEER 1997-2001.6 | |||||||
| White | Black | ||||||||
| Men | Women | Men | Women | ||||||
| Stage I: | 95% | 96% | 71% | 89% | |||||
| Stage II: | 76% | 80% | 73% | 68% | |||||
| Stage III: | 58% | 52% | 40% | 43% | |||||
| Stage IV: | 7% | 4% | 5% | 3% | |||||
To be estimated from randomized controlled endoscopy trials, data not yet available.
SCREEN PARAMETERS
We assumed a cecal intubation rate of 95%.13-15 The sensitivity of colonoscopy for each lesion within realized reach was based on back-to-back colonoscopy studies: 75% in adenomas less than or equal to 5 mm, 85% in adenomas 6-9 mm, and 95% in adenomas greater than or equal to 10 mm and cancers.16 At detection, lesions are removed immediately. The percentage of the population without adenomas or cancer but with hyperplastic polyps, lipomas, or other lesions that lead to polypectomy and pathology after colonoscopy has been estimated from Kaiser data:17 10%. This percentage was assumed to be independent of the screening round.
The stage-specific survival of patients with screen-detected cancer is assumed to be the same as the survival of patients with cancers clinically diagnosed in the same stage.18 Removal of an adenoma always prevents development of any subsequent cancer that may have arisen from this adenoma. Risks of complications reported in organized screening programs19-21 are lower than those reported for general practice colonoscopies.22, 23 The major complications of colonoscopy are perforations (which can occur with or without polypectomy), serosal burns, bleeds requiring transfusion and bleeds not requiring transfusion.19-23 We estimated a rate of death among persons of 0.1 per 1,000 colonoscopies.24
Table A1-3.
| Parameter | Value | Source |
|---|---|---|
| Sensitivity colonoscopy | Dependent on stage of disease | Back-to-back colonoscopy studies 16 |
| Adenoma 1-5mm: 75% | ||
| Adenoma 6-9mm: 85% | ||
| Adenoma 10+ mm: 95% | ||
| Preclinical cancer: 95% | ||
| Cecal intubation rate | 95% | General practice 13, 14 and guidelines 15 |
| Complication rate with colonoscopy | 2.4 per 1,000 colonoscopies | Organized screening programs19-21 and general practice 22, 23 |
| Perforation | 0.7 per 1,000 | |
| Serosal burn | 0.3 per 1,000 | |
| Bleed with transfusion | 0.4 per 1,000 | |
| Bleed without transfusion | 1.1 per 1,000 | |
| Fatal complication rate with colonoscopy | 0.1 per 1,000 colonoscopies | Prospective endoscopy study 24 |
| Probability to develop cancer from removed adenoma | 0% | Expert opinion |
| Survival after screen detection of cancer | As after clinical diagnosis in the same stage | FOBT trial18 |
MODEL OUTPUTS
The model generates the following output, both undiscounted and discounted:
Demography
Life-years lived in the population by calendar year and age
Deaths from other causes than colorectal cancer by calendar year and age
Natural history
Colorectal cancer cases by calendar year, stage and age
Colorectal cancer deaths by calendar year and age
Life-years lived with colorectal cancer by calendar year, stage and age
Total number of life years with surveillance for adenoma patients
Total number of life years with initial therapy after screen-detected or clinical invasive cancer by stage
Total number of life years with continuing therapy after screen-detected or clinical invasive cancer by stage
Total number of life years with terminal care before death from other causes by stage
Total number of life years with terminal care before death from colorectal cancer by stage
Screening
Number of invitations for screen-tests, screen-tests, diagnostic tests, surveillance and opportunistic screen tests by calendar year
Number of positive and negative test results per preclinical state and per year
Total number of life years lived, life years lost due to cancer, number of specific deaths and non specific deaths
Number of screenings that prevented cancer by year of screening
Number of screenings that detected cancer early by year of screening
Number of surveillance tests that prevented cancer by year of surveillance
Number of surveillance tests that detected cancer early by year of surveillance
Number of life years gained due to screening by year of screening
Appendix 2: Derivation of costs
GENERAL
Screening costs were based on information provided by CMS on Medicare payments of 2007 for procedures and tests associated with CRC screening and complications of screening.25 Net costs of the management of invasive CRC treatment were obtained from an analysis of SEER-Medicare data.26 Only direct medical costs were considered, including the co-pays from the beneficiaries.
SCREENING COSTS
Costs for colonoscopy were based on the set of CPT (costs for procedure and treatment) codes relevant to CRC screening in conjunction with the points of service for the procedure. The CPT codes for screening are those stated by the National Coverage Decision (http://www.medicare.gov/health/coloncancer.asp) for the CRC screening benefit as well as those for associated colonic biopsy or polypectomy (personal communication, John Allen, M.D., and Joel Brill, M.D.). We used the national unadjusted payment amounts under the physician fee schedule for these analyses. Using the national unadjusted payment means that the costs do not adjust for payment for the geographic location.
Points of service considered for screening were the outpatient prospective payment system (OPPS) and the ambulatory surgery center payment system (ASC) with the associated facility charge, and the physician fee schedule (PFS) office system. We did not include any CPT codes of screening associated with in-patient procedures as registered in the inpatient prospective payment system (IPPS). Complication costs were based primarily on in-patient DRG level reimbursement costs.
Screening procedure costs were based on a weighted average of procedures per setting. The cost values per setting and CPT code are given in tables A2-1 to A2-3. The costs for the ASC setting include the ASC payment rates and the PFS facility charge (Table A2-1). The costs for the OPPS setting included the OPPS payment rates and the PFS facility charge (Table A2-2). For the PFS office setting we used the office payment rates (Table A2-3). The costs for colonoscopy without polypectomy were based on CPT codes 45378 (diagnostic colonoscopy), G0105 (colon screen in high risk individuals) and G0121 (colon cancer screening for non high risk individual). Costs for colonoscopy with polypectomy or biopsy were composed of codes 45380 (colonoscopy and biopsy), 45381 (colonoscopy, submucous injection), 45382 (colonoscopy/control bleeding), 45383 (lesion removal colonoscopy - fulguration), 45384 (lesion removal colonoscopy-hot biopsy) and 45385 (lesion removal colonoscopy-snare polypectomy).
Table A2-1.
Ambulatory surgery center (ASC) payment rates
| CPT Code | ASC Payment, $ |
PFS*- Facility, $ |
Total ASC (ASC payment + PFS), $ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Medicare (M) |
Beneficiary (B) |
Total (M+B) |
Medicare (M) |
Beneficiary (B) |
Total (M+B) |
Beneficiary (B) |
Medicare (M) |
Total (M+B) |
|
| Colonoscopy without polypectomy | |||||||||
| 45378 | 357 | 89 | 446 | 158 | 39 | 197 | 129 | 514 | 643 |
| G0105 | 335 | 111 | 446 | 158 | 39 | 197 | 151 | 492 | 643 |
| G0121 | 335 | 111 | 446 | 158 | 39 | 197 | 151 | 492 | 643 |
| Colonoscopy with polypectomy | |||||||||
| 45380 | 357 | 89 | 446 | 188 | 47 | 235 | 136 | 545 | 681 |
| 45381 | 357 | 89 | 446 | 178 | 44 | 222 | 134 | 534 | 668 |
| 45382 | 357 | 89 | 446 | 239 | 60 | 299 | 149 | 596 | 745 |
| 45383 | 357 | 89 | 446 | 246 | 61 | 307 | 151 | 602 | 753 |
| 45384 | 357 | 89 | 446 | 198 | 49 | 247 | 139 | 554 | 693 |
| 45385 | 357 | 89 | 446 | 223 | 56 | 279 | 145 | 580 | 725 |
| Pathology | |||||||||
| 88305 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Physician fee schedule
Table A2-3.
Office payment rates
| CPT Code | PFS- Office Medicare (M), $ | PFS- Office Beneficiary (B), $ | PFS- Office Total (M+B), $ |
|---|---|---|---|
| Colonoscopy without polypectomy | |||
| 45378 | 298 | 74 | 372 |
| G0105 | 298 | 74 | 372 |
| G0121 | 298 | 74 | 372 |
| Colonoscopy with polypectomy | |||
| 45380 | 354 | 88 | 442 |
| 45381 | 343 | 86 | 429 |
| 45382 | 472 | 118 | 590 |
| 45383 | 419 | 105 | 524 |
| 45384 | 349 | 87 | 436 |
| 45385 | 398 | 100 | 498 |
| Pathology | |||
| 88305 | 82 | 21 | 103 |
Table A2-2.
Outpatient prospective payment system (OPPS) payment rates
| CPT Code | OPPS Payment, $ |
PFS- Facility, $ |
Total OPPS (OPPS payment + PFS), $ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Medicare (M) |
Beneficiary (B) |
Total (M+B) |
Medicare (M) |
Beneficiary (B) |
Total (M+B) |
Beneficiary (B) |
Medicare (M) |
Total Cost (M+B) |
|
| Colonoscopy without polypectomy | |||||||||
| 45378 | 353 | 186 | 539 | 158 | 39 | 197 | 225 | 511 | 736 |
| G0105 | 335 | 111 | 446 | 158 | 39 | 197 | 151 | 492 | 643 |
| G0121 | 335 | 111 | 446 | 158 | 39 | 197 | 151 | 492 | 643 |
| Colonoscopy with polypectomy | |||||||||
| 45380 | 353 | 186 | 539 | 188 | 47 | 235 | 233 | 541 | 774 |
| 45381 | 353 | 186 | 539 | 178 | 44 | 222 | 230 | 531 | 761 |
| 45382 | 353 | 186 | 539 | 239 | 60 | 299 | 246 | 592 | 838 |
| 45383 | 353 | 186 | 539 | 246 | 61 | 307 | 247 | 599 | 846 |
| 45384 | 353 | 186 | 539 | 198 | 49 | 247 | 235 | 551 | 786 |
| 45385 | 353 | 186 | 539 | 223 | 56 | 279 | 242 | 576 | 818 |
| Pathology | |||||||||
| 88305 | 21 | 11 | 32 | 30 | 8 | 38 | 18 | 52 | 70 |
Polyp removal and pathology review
For the procedures with polypectomy or biopsy we included a pathology charge (CPT code 88305). The Medicare payment rates per jar were $82.40 for the PFS office and ASC setting, and $51.59 for the OPPS setting. All biopsies or polyps are reviewed by pathology. We assumed a separate jar is submitted to pathology for each of 4 colon segments. The intention of a separate jar for separate segments is that the resection area could be identified should the patient require surgery. We used the simplified assumption of four segments; right colon (cecum and ascending), transverse (hepatic flexure and transverse colon), left colon (splenic flexure, descending colon, and sigmoid), and rectum. Data from the National Colonoscopy Study with screening colonoscopy for individuals aged 40-69 were used to provide the estimate of 1.38 as the average number of jars per patient with polyps (hyperplastic, other polyps, and adenomas) where there is one jar for polyps in each of the 4 sections (personal communication, Ann Zauber, Ph.D.).
Consequently, we used the pathology fee times 1.38 as the pathology cost associated with colonoscopy with polypectomy. Total costs per setting and CPT code are given with and without pathology charge (Table A2-4)
Table A2-4.
ASC, OPPS, and office payment rates with the addition of pathology costs (when applicable)
| Total ASC | Total OPPS | Total PFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPT Code |
Beneficiary | Medicare | Beneficiary with pathology review† |
Medicare with pathology review† |
Beneficiary | Medicare | Beneficiary with pathology review |
Medicare with pathology review |
Beneficiary | Medicare | Beneficiary with pathology review |
Medicare with pathology review |
| Colonoscopy without polypectomy | ||||||||||||
| 45378 | 129 | 514 | 129 | 514 | 226 | 511 | 226 | 511 | 74 | 298 | 74 | 298 |
| G0105 | 151 | 492 | 151 | 492 | 151 | 492 | 151 | 492 | 74 | 298 | 74 | 298 |
| G0121 | 151 | 492 | 151 | 492 | 151 | 492 | 151 | 492 | 74 | 298 | 74 | 298 |
| Colonoscopy with polypectomy | ||||||||||||
| 45380 | 136 | 545 | 165 | 659 | 233 | 541 | 259 | 612 | 88 | 354 | 117 | 467 |
| 45381 | 134 | 534 | 162 | 648 | 231 | 531 | 256 | 602 | 86 | 343 | 114 | 457 |
| 45382 | 149 | 596 | 177 | 710 | 246 | 592 | 271 | 663 | 118 | 472 | 146 | 586 |
| 45383 | 151 | 602 | 179 | 716 | 248 | 599 | 273 | 670 | 105 | 419 | 133 | 533 |
| 45384 | 139 | 554 | 167 | 668 | 236 | 551 | 261 | 622 | 87 | 349 | 116 | 463 |
| 45385 | 145 | 580 | 173 | 694 | 242 | 576 | 267 | 647 | 100 | 398 | 128 | 512 |
All values shown in 2007 dollars.
In the ASC setting pathology review is farmed out to external labs, for which PFS Office rates apply
Multiple polyps requiring the same type of polypectomy removal within a single colonoscopy do not add an incremental charge to the procedure. However if different types of polypectomy are required in removing multiple polyps then CMS reimburses 100% for the most expensive procedure and 50% of the facility cost for the second procedure. As a simplifying assumption we use the weights of procedures by CPT type and do not consider different fees for different combinations of endoscopy CPT codes for polyp removal.
The total costs per CPT code were calculated based on the frequencies for points of service as weights for the costs (Table A2-5). The total costs per screening procedure are based on the total costs per CPT code that are part of the procedure. The costs were weighted by the frequencies of the CPT codes (Table A2-6).
Table A2-5.
Percent of procedures by place of service (PoS), weights per place of service, and cost of individual procedures weighted by place of service
| CPT Code | % of procedures ASC (a) |
% of procedures OPPS (b) |
% of procedures Office (c) |
Total % (d=a+b+c) |
ASC Weight* (a/d) |
OPPS Weight* (b/d) |
Office Weight* (c/d) |
Beneficiary Weighted Cost by PoS** (B) |
Medicare Weighted Cost by PoS** (M) |
Total Weighted Cost by PoS (B+M) |
|---|---|---|---|---|---|---|---|---|---|---|
| Colonoscopy without polypectomy | ||||||||||
| 45378 | 42.8 | 40.3 | 4.2 | 87.2 | 0.49 | 0.46 | 0.05 | 171 | 502 | 673 |
| G0105 | 53.1 | 43.3 | 2.8 | 99.3 | 0.54 | 0.44 | 0.03 | 149 | 487 | 635 |
| G0121 | 51.0 | 44.5 | 3.2 | 98.7 | 0.52 | 0.45 | 0.03 | 148 | 486 | 634 |
| Colonoscopy with polypectomy | ||||||||||
| 45380 | 47.3 | 38.1 | 3.3 | 88.7 | 0.53 | 0.43 | 0.04 | 192 | 631 | 824 |
| 45381 | 46.0 | 40.8 | 2.3 | 89.1 | 0.52 | 0.46 | 0.03 | 192 | 622 | 814 |
| 45382 | 20.4 | 29.8 | 1.8 | 52.0 | 0.39 | 0.57 | 0.03 | 216 | 679 | 894 |
| 45383 | 42.3 | 46.9 | 4.5 | 93.6 | 0.45 | 0.50 | 0.05 | 211 | 684 | 895 |
| 45384 | 47.8 | 44.6 | 3.0 | 95.4 | 0.50 | 0.47 | 0.03 | 197 | 640 | 837 |
| 45385 | 48.5 | 41.5 | 3.8 | 93.7 | 0.52 | 0.44 | 0.04 | 202 | 666 | 868 |
Out of ASC, OPPS, and office.
Weighted average of costs from table 5 including pathology (if applicable) by PoS
Table A2-6.
Costs of colonoscopy with and without polyps*
| CPT Code |
Beneficiary Weighted Cost by PoS (B) |
Medicare Weighted Cost by PoS (M) |
Total Weighted Cost by PoS (B+M) |
Total number of procedures per CPT code |
Weights by CPT code (w) |
Weighted Beneficiary Costs by PoS and CPT code (w*B) |
Weighted Medicare Costs by PoS and CPT code (w*M) |
Total Weighted Costs by PoS and CPT code (w*(B+M)) |
|---|---|---|---|---|---|---|---|---|
| Colonoscopy without polypectomy | ||||||||
| 45378 | 171 | 502 | 673 | 1,270,881 | 0.71 | 122 | 358 | 480 |
| G0105 | 149 | 487 | 635 | 208,073 | 0.12 | 17 | 57 | 74 |
| G0121 | 148 | 486 | 634 | 302,860 | 0.17 | 25 | 83 | 108 |
| Total | 164 | 498 | 662 | |||||
| Colonoscopy with polypectomy | ||||||||
| 45380 | 192 | 631 | 824 | 879,279 | 0.38 | 74 | 242 | 316 |
| 45381 | 192 | 622 | 814 | 33,907 | 0.01 | 3 | 9 | 12 |
| 45382 | 216 | 679 | 894 | 12,530 | 0.01 | 1 | 4 | 5 |
| 45383 | 211 | 684 | 895 | 89,884 | 0.04 | 8 | 27 | 35 |
| 45384 | 197 | 640 | 837 | 381,305 | 0.17 | 33 | 106 | 139 |
| 45385 | 202 | 666 | 868 | 896,966 | 0.39 | 79 | 260 | 339 |
| Total | 198 | 649 | 846 | |||||
COSTS OF TREATING COMPLICATIONS OF COLONOSCOPY
The costs of complications with colonoscopy were based on DRG codes of similar procedures. We assumed that all perforations and bleeds with transfusion would entail a hospitalization. We assumed that bleeds without transfusion would be handled by an emergency room visit ($320). The cost of perforation was based on DRG 442 for other OR procedures for injuries with complications ($12,446); bleeding with transfusion (all of whom are considered to require hospitalization) was based on DRG 452 for complications of treatment with complications or comorbidities ($5,208); and serosal burn generally requires a two-day hospitalization, which was assumed to be the same cost as that as for the bleed with transfusion.
COSTS FOR COLORECTAL CANCER TREATMENT
The cost of CRC treatment was derived from comparison of costs for CRC cases relative to those of matched controls in the SEER-Medicare files.26 Cost data were reported in 2004 dollars and subsequently updated to 2007 dollars using the medical care component of the Consumer Price Index.
Patients with a diagnosis of invasive CRC between 1973 and 2002 and aged 65 or above at some time between 1998 and 2003 were selected from SEER-Medicare (N = 124,793). Cancer patients with a prior cancer diagnosis (N= 20,277), or who were identified as having cancer through a death certificate or autopsy were excluded (N=623). An additional 24,920 patients were excluded because they were enrolled in managed care throughout the observation period or did not have both Medicare part A and part B at any point during the observation period. The remaining 76,722 CRC patients were included.
Potential controls were individuals without any cancer diagnoses recorded by SEER and aged 65 or above during the observation period, 1998-2003. A total of 170,491 controls were selected from a 5% random sample of Medicare enrollees and frequency matched to cases by gender, 5-year age strata (65-69, 70-74, 75-79, 80+) and SEER registry areas.
Phase of care definitions
Phase of care definitions were based on prior studies of direct medical costs. For cancer patients, months of observation and cost of care between 1998 and 2003 were divided into three clinically relevant phases of care - initial, last year of life, and continuing care based on the month of service on the Medicare claim. Date of death (or its absence) in the Medicare enrollment file through 2004 was used to determine vital status. Cause of death (cancer, non-cancer) was identified from SEER. The initial phase was defined as the first 12 months following diagnosis, the last year of life phase was defined as the final 12 months of life, and the continuing phase was defined as all months between the initial and last year of life phases of care. Not all cancer patients contributed to all phases of care, however. For patients surviving less than 24 months after diagnosis, the final 12 months of observation and costs of care were then allocated first to the last year of life phase, because the content of care for patients with short survival is more similar to the last year of life phase than the initial phase. The remainder of months of observation and costs were allocated to the initial phase, with no contribution to the continuing phase. Patients diagnosed prior to 1997 who survived beyond 2003 contributed months and costs of care only to the continuing phase. Within each tumor site and phase of care, average monthly estimates of cost of care were calculated.
Because control subjects did not have a date of cancer diagnosis, they were randomly assigned a “pseudo-diagnosis date” that corresponded to the date of diagnosis of one of the pool of cancer cases. Months of observation and costs of care were assigned to phases of care in the same manner used with cases. In addition to frequency matching by gender, 5-year age group and SEER area strata, controls were also matched to cases by phase of care in up to a 1:5 case: control ratio based on the number of controls available for each stratum. To reflect costs associated with cancer care in the last year of life, cancer patients who died of cancer were matched to continuing controls, and cancer patients who died of other causes were matched to last year of life controls. As with cancer patients, average monthly estimates of cost of care were calculated for each phase of care for each of group of controls.
For months in which patients received coverage through managed care or were without both Medicare part A and part B, costs and months of observation time were excluded because these data would not completely capture the care received during this period.
Cost estimates
Cancer-related medical costs were estimated as differences in costs for cases and controls by phase of care. The analysis used Medicare payments to reflect costs of care, rather than billed charges. Payments for Medicare Part A (inpatient services) and Part B (outpatient services) were calculated separately. The Hospital Wage Index and the Medicare Economic Index were used to adjust for inflation in Medicare Parts A and B estimates, respectively, during 1998-2003. We also adjusted for geographic variability in costs of care across SEER sites. New treatments including biologicals, such as Avastin and Erbitux,27 have come into use in the past 3 years; these new drugs are markedly more expensive than the previous drugs. However the cost of these new drugs would not be captured by the 2004 reimbursement base available for this case-control study. Estimated patient deductibles and coinsurance expenses were added by adjusting Part A and Part B payments with Medicare reimbursement ratios provided by CMS's Office of the Actuary. Over the time period studied, these averaged about 8% for Part A and about 30% for Part B (Table A2-7).
Table A2-7.
Net payments for CRC care during 1998-2003 (in $2007)*
| Last Year of Life |
||||
|---|---|---|---|---|
| AJCC Stage | Initial Phase | Continuing Phase | Died of Cancer | Died of Other Causes |
| Direct Medical Costs | ||||
| I | 28,668 | 2,395 | 51,935 | 12,703 |
| II | 39,700 | 2,237 | 51,712 | 11,035 |
| III | 48,951 | 3,249 | 54,776 | 14,708 |
| IV | 64,801 | 10,419 | 73,522 | 39,679 |
The initial phase of care is the first 12 months following diagnosis, the last year of life phase is the final 12 months of life, and the continuing phase is all the months between the initial and last year of life phases. Cancer-related costs in the continuing phase of care are an annual estimate.
Appendix 3: Detailed overview of efficient screening policies for the total population and each population subgroup
APPENDIX 3A. total population (uniform).
Efficient screening policies characterized by age range, screening interval and number of scheduled examinations and expressed as the 3% discounted life-time costs and life-expectancy of the policy.
| Policy | Resources and benefits | |||
|---|---|---|---|---|
| No. of scheduled exams | Interval between exams (years) | Age range | Costs ($)*,† | Life-expectancy at age 40* |
| 1 | n.a. | 70-70 | 1,701 | 22.4063 |
| 1 | n.a. | 65-65 | 1,720 | 22.4120 |
| 1 | n.a. | 64-64 | 1,725 | 22.4131 |
| 1 | n.a. | 63-63 | 1,731 | 22.4142 |
| 1 | n.a. | 62-62 | 1,737 | 22.4151 |
| 1 | n.a. | 61-61 | 1,747 | 22.4158 |
| 2 | 14 | 60-74 | 1,827 | 22.4213 |
| 2 | 14 | 59-73 | 1,845 | 22.4223 |
| 2 | 14 | 58-72 | 1,866 | 22.4233 |
| 3 | 9 | 57-75 | 2,010 | 22.4282 |
| 3 | 10 | 53-73 | 2,125 | 22.4315 |
| 3 | 9 | 53-71 | 2,151 | 22.4322 |
| 4 | 8 | 52-76 | 2,301 | 22.4353 |
| 4 | 8 | 51-75 | 2,349 | 22.4362 |
| 5 | 6 | 51-75 | 2,563 | 22.4390 |
| 5 | 7 | 47-75 | 2,733 | 22.4407 |
| 6 | 6 | 47-77 | 2,923 | 22.4426 |
| 7 | 5 | 48-78 | 3,077 | 22.4436 |
| 7 | 5 | 47-77 | 3,170 | 22.4441 |
| 8 | 5 | 47-82 | 3,223 | 22.4443 |
| 8 | 5 | 46-81 | 3,329 | 22.4448 |
| 8 | 5 | 43-78 | 3,659 | 22.4462 |
| 9 | 5 | 43-83 | 3,707 | 22.4464 |
| 10 | 5 | 43-88 | 3,732 | 22.4465 |
| 10 | 5 | 42-87 | 3,860 | 22.4466 |
Average of results for population subgroups, weighted by size of population subgroup
Lifetime per person cost for CRC screening and treatment after age 40
APPENDIX 3B. White Men.
Efficient screening policies characterized by age range, screening interval and number of scheduled examinations and expressed as the 3% discounted life-time costs and life-expectancy of the policy.
| Policy | Resources and benefits | |||
|---|---|---|---|---|
| No. of scheduled exams | Interval between exams (years) | Age range | Costs ($)† | Life-expectancy at age 40 |
| 1 | n.a. | 70-70 | 1,824 | 21.9125 |
| 1 | n.a. | 65-65 | 1,831 | 21.9186 |
| 1 | n.a. | 63-63 | 1,836 | 21.9211 |
| 1 | n.a. | 62-62 | 1,840 | 21.9221 |
| 1 | n.a. | 61-61 | 1,848 | 21.9229 |
| 2 | 14 | 60-74 | 1,916 | 21.9276 |
| 2 | 14 | 59-73 | 1,933 | 21.9288 |
| 2 | 14 | 58-72 | 1,953 | 21.9297 |
| 3 | 9 | 57-75 | 2,082 | 21.9343 |
| 3 | 8 | 57-73 | 2,104 | 21.9350 |
| 3 | 8 | 56-72 | 2,141 | 21.9360 |
| 3 | 9 | 53-71 | 2,220 | 21.9383 |
| 4 | 7 | 53-74 | 2,361 | 21.9412 |
| 4 | 7 | 52-73 | 2,413 | 21.9421 |
| 5 | 6 | 51-75 | 2,609 | 21.9445 |
| 6 | 6 | 47-77 | 2,962 | 21.9478 |
| 7 | 5 | 48-78 | 3,103 | 21.9488 |
| 8 | 5 | 47-82 | 3,241 | 21.9493 |
| 8 | 5 | 43-78 | 3,684 | 21.9511 |
| 9 | 5 | 43-83 | 3,723 | 21.9512 |
| 10 | 5 | 43-88 | 3,742 | 21.9513 |
| 10 | 5 | 42-87 | 3,871 | 21.9513 |
Lifetime per person cost for CRC screening and treatment after age 40
APPENDIX 3C. Black Men.
Efficient screening policies characterized by age range, screening interval and number of scheduled examinations and expressed as the 3% discounted life-time costs and life-expectancy of the policy.
| Policy | Resources and benefits | |||
|---|---|---|---|---|
| No. of scheduled exams | Interval between exams (years) | Age range | Costs ($)† | Life-expectancy at age 40 |
| 1 | n.a. | 64-64 | 1,746 | 19.8487 |
| 1 | n.a. | 63-63 | 1,750 | 19.8503 |
| 1 | n.a. | 62-62 | 1,754 | 19.8519 |
| 1 | n.a. | 61-61 | 1,760 | 19.8533 |
| 1 | n.a. | 60-60 | 1,767 | 19.8546 |
| 1 | n.a. | 59-59 | 1,777 | 19.8558 |
| 2 | 15 | 58-73 | 1,840 | 19.8612 |
| 2 | 14 | 58-72 | 1,846 | 19.8616 |
| 2 | 14 | 57-71 | 1,866 | 19.8629 |
| 3 | 11 | 53-75 | 2,035 | 19.8721 |
| 3 | 10 | 53-73 | 2,056 | 19.8731 |
| 3 | 10 | 52-72 | 2,090 | 19.8745 |
| 3 | 9 | 52-70 | 2,116 | 19.8754 |
| 4 | 9 | 48-75 | 2,342 | 19.8822 |
| 4 | 8 | 48-72 | 2,401 | 19.8834 |
| 5 | 7 | 47-75 | 2,582 | 19.8871 |
| 5 | 7 | 46-74 | 2,652 | 19.8882 |
| 6 | 6 | 47-77 | 2,735 | 19.8894 |
| 6 | 6 | 46-76 | 2,817 | 19.8904 |
| 6 | 6 | 45-75 | 2,906 | 19.8913 |
| 7 | 5 | 44-74 | 3,249 | 19.8945 |
| 8 | 5 | 43-78 | 3,402 | 19.8956 |
| 8 | 5 | 42-77 | 3,527 | 19.8961 |
| 9 | 5 | 42-82 | 3,556 | 19.8962 |
| 10 | 5 | 42-87 | 3,570 | 19.8962 |
Lifetime per person cost for CRC screening and treatment after age 40
APPENDIX 3D. White Women.
Efficient screening policies characterized by age range, screening interval and number of scheduled examinations and expressed as the 3% discounted life-time costs and life-expectancy of the policy.
| Policy | Resources and benefits | |||
|---|---|---|---|---|
| No. of scheduled exams | Interval between exams (years) | Age range | Costs ($)† | Life-expectancy at age 40 |
| 1 | n.a. | 72-72 | 1,563 | 23.4026 |
| 1 | n.a. | 71-71 | 1,567 | 23.4035 |
| 1 | n.a. | 70-70 | 1,573 | 23.4045 |
| 1 | n.a. | 65-65 | 1,606 | 23.4091 |
| 1 | n.a. | 64-64 | 1,614 | 23.4099 |
| 1 | n.a. | 63-63 | 1,624 | 23.4106 |
| 2 | 14 | 61-75 | 1,721 | 23.4167 |
| 2 | 14 | 60-74 | 1,742 | 23.4177 |
| 2 | 14 | 59-73 | 1,762 | 23.4185 |
| 2 | 14 | 58-72 | 1,785 | 23.4192 |
| 3 | 9 | 57-75 | 1,950 | 23.4242 |
| 3 | 10 | 53-73 | 2,069 | 23.4265 |
| 4 | 8 | 53-77 | 2,221 | 23.4293 |
| 4 | 8 | 52-76 | 2,267 | 23.4301 |
| 4 | 8 | 51-75 | 2,314 | 23.4309 |
| 5 | 7 | 51-79 | 2,458 | 23.4326 |
| 5 | 6 | 51-75 | 2,551 | 23.4336 |
| 6 | 6 | 47-77 | 2,925 | 23.4365 |
| 7 | 6 | 46-82 | 3,074 | 23.4374 |
| 8 | 5 | 46-81 | 3,363 | 23.4387 |
| 9 | 5 | 44-84 | 3,634 | 23.4396 |
| 9 | 5 | 43-83 | 3,750 | 23.4399 |
| 10 | 5 | 43-88 | 3,785 | 23.4399 |
| 10 | 5 | 42-87 | 3,911 | 23.4400 |
Lifetime per person cost for CRC screening and treatment after age 40
APPENDIX 3E. Black Women.
Efficient screening policies characterized by age range, screening interval and number of scheduled examinations and expressed as the 3% discounted life-time costs and life-expectancy of the policy.
| Policy | Resources and benefits | |||
|---|---|---|---|---|
| No. of scheduled exams | Interval between exams (years) | Age range | Costs ($)† | Life-expectancy at age 40 |
| 1 | n.a. | 63-63 | 1,716 | 21.9029 |
| 1 | n.a. | 62-62 | 1,721 | 21.9045 |
| 1 | n.a. | 61-61 | 1,729 | 21.9059 |
| 1 | n.a. | 60-60 | 1,736 | 21.9071 |
| 1 | n.a. | 59-59 | 1,748 | 21.9081 |
| 2 | 15 | 59-74 | 1,819 | 21.9139 |
| 2 | 14 | 58-72 | 1,842 | 21.9157 |
| 2 | 15 | 57-72 | 1,857 | 21.9167 |
| 2 | 14 | 57-71 | 1,865 | 21.9171 |
| 3 | 11 | 53-75 | 2,060 | 21.9277 |
| 3 | 10 | 52-72 | 2,117 | 21.9307 |
| 4 | 8 | 51-75 | 2,299 | 21.9359 |
| 4 | 8 | 49-73 | 2,403 | 21.9385 |
| 5 | 7 | 47-75 | 2,671 | 21.9435 |
| 6 | 6 | 47-77 | 2,851 | 21.9464 |
| 6 | 6 | 46-76 | 2,934 | 21.9475 |
| 8 | 5 | 43-78 | 3,567 | 21.9525 |
| 9 | 5 | 43-83 | 3,613 | 21.9527 |
| 9 | 5 | 42-82 | 3,737 | 21.9530 |
| 10 | 5 | 42-87 | 3,765 | 21.9530 |
Lifetime per person cost for CRC screening and treatment after age 40
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman D. Race, gender, and colorectal cancer screening. Am J Gastroenterol. 2005;100:2756–8. doi: 10.1111/j.1572-0241.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal S, Bhupinderjit A, Bhutani MS, Boardman L, Nguyen C, Romero Y, Srinivasan R, Figueroa-Moseley C. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100:515–23. doi: 10.1111/j.1572-0241.2005.41829.x. discussion 14. [DOI] [PubMed] [Google Scholar]
- 5.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BKe. SEER Cancer Statistics Review, 1975-2001. National Cancer Institute; 2004. Accessed at: http://seer.cancer.gov/csr/1975_2001/ [Google Scholar]
- 6.Goldie SJ. Chapter 15: Public health policy and cost-effectiveness analysis. J Natl Cancer Inst Monogr. 2003:102–10. doi: 10.1093/oxfordjournals.jncimonographs.a003471. [DOI] [PubMed] [Google Scholar]
- 7.Koretz RL. Malignant polyps: are they sheep in wolves' clothing? Ann Intern Med. 1993;118:63–8. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 8.DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–5. [PubMed] [Google Scholar]
- 9.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL., Jr. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85:969–74. [PubMed] [Google Scholar]
- 10.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86:946–51. [PubMed] [Google Scholar]
- 11.Rex DK, Lehman GA, Hawes RH, Ulbright TM, Smith JJ. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology. 1991;100:64–7. doi: 10.1016/0016-5085(91)90583-7. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance Research Program . National Cancer Institute SEER*Stat Software. version 5.3.1. 2003. [Google Scholar]
- 13.Gyrd-Hansen D, Sogaard J, Kronborg O. Analysis of screening data: colorectal cancer. Int J Epidemiol. 1997;26:1172–81. doi: 10.1093/ije/26.6.1172. [DOI] [PubMed] [Google Scholar]
- 14.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 15.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 16.Aslinia F, Uradomo L, Steele A, Greenwald BD, Raufman JP. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721–31. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 17.Cotterill M, Gasparelli R, Kirby E. Colorectal cancer detection in a rural community. Development of a colonoscopy screening program. Can Fam Physician. 2005;51:1224–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD, Church J, Marshall JB, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 21.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–73. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 22.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 23.Jentschura D, Raute M, Winter J, Henkel T, Kraus M, Manegold BC. Complications in endoscopy of the lower gastrointestinal tract. Therapy and prognosis. Surg Endosc. 1994;8:672–6. doi: 10.1007/BF00678564. [DOI] [PubMed] [Google Scholar]
- 24.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 25.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 26.Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Oortmarssen GJv, Loeve F, Habbema JDF. Model Profiler of the MISCAN-Colon microsimulation model for colorectal cancer. Department of Public Health; Erasmus MC: 2004. Accessed at: https://cisnet.flexkb.net/mp/pub/cisnet_colorectal_sloankettering_profile.pdf. [Google Scholar]
- 27.Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–7. doi: 10.1177/00359157740676P115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–70. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 29.Loeve F, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Final Report MISCAN-Colon Microsimulation Model for Colorectal Cancer. Department of Public Health, Erasmus MC. 1998 [Google Scholar]
- 30.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, Habbema JD. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111:633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 31.Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, Zauber AG. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin. vol. 2005. U.S. Census Bureau; Wahington DC: 2004. [Google Scholar]
- 33.Hollingsworth B, Dawson PJ, Maniadakis N. Efficiency measurement of health care: a review of non-parametric methods and applications. Health Care Manag Sci. 1999;2:161–72. doi: 10.1023/a:1019087828488. [DOI] [PubMed] [Google Scholar]
- 34.Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ. 1993;12:459–67. doi: 10.1016/0167-6296(93)90005-y. [DOI] [PubMed] [Google Scholar]
- 35.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–59. doi: 10.3322/canjclin.56.3.143. quiz 84-5. [DOI] [PubMed] [Google Scholar]
- 36.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, Ballegooijen Mv, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. 2007. Accessed at: https://www.cms.hhs.gov/mcd/viewtechassess.asp?where=index&tid=52. [PubMed] [Google Scholar]
- 37.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 38.Gold MRSJ, Russell LB, Weinstein MG, editors. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York, NY: 1996. [Google Scholar]
- 39.Anderson JC, Gonzalez JD, Messina CR, Pollack BJ. Factors that predict incomplete colonoscopy: thinner is not always better. Am J Gastroenterol. 2000;95:2784–7. doi: 10.1111/j.1572-0241.2000.03186.x. [DOI] [PubMed] [Google Scholar]
- 40.Cotton PB, Connor P, McGee D, Jowell P, Nickl N, Schutz S, Leung J, Lee J, Libby E. Colonoscopy: practice variation among 69 hospital-based endoscopists. Gastrointest Endosc. 2003;57:352–7. doi: 10.1067/mge.2003.121. [DOI] [PubMed] [Google Scholar]
- 41.Denis B, Weiss AM, Peter A, Bottlaender J, Chiappa P. Quality assurance and gastrointestinal endoscopy: an audit of 500 colonoscopic procedures. Gastroenterol Clin Biol. 2004;28:1245–55. doi: 10.1016/s0399-8320(04)95218-9. [DOI] [PubMed] [Google Scholar]
- 42.Harewood GC. Relationship of colonoscopy completion rates and endoscopist features. Dig Dis Sci. 2005;50:47–51. doi: 10.1007/s10620-005-1276-y. [DOI] [PubMed] [Google Scholar]
- 43.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72–7. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 44.Schoenfeld P, Cash B, Flood A, Dobhan R, Eastone J, Coyle W, Kikendall JW, Kim HM, Weiss DG, Emory T, Schatzkin A, Lieberman D, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–8. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 45.Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297–303. doi: 10.1053/j.gastro.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 46.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. Jama. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 47.Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000;16:799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 48.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–21. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 49.Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, Fendrick AM. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer. A cost-effectiveness analysis. Ann Intern Med. 2001;135:769–81. doi: 10.7326/0003-4819-135-9-200111060-00007. [DOI] [PubMed] [Google Scholar]
- 50.Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact? Clin Gastroenterol Hepatol. 2004;2:554–63. doi: 10.1016/s1542-3565(04)00247-2. [DOI] [PubMed] [Google Scholar]
- 51.Lieberman DA. Cost-effectiveness model for colon cancer screening. Gastroenterology. 1995;109:1781–90. doi: 10.1016/0016-5085(95)90744-0. [DOI] [PubMed] [Google Scholar]
- 52.Ness RM, Holmes AM, Klein R, Dittus R. Cost-utility of one-time colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol. 2000;95:1800–11. doi: 10.1111/j.1572-0241.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 53.Sonnenberg A, Delco F. Cost-effectiveness of a single colonoscopy in screening for colorectal cancer. Arch Intern Med. 2002;162:163–8. doi: 10.1001/archinte.162.2.163. [DOI] [PubMed] [Google Scholar]
- 54.Sonnenberg A, Delco F, Bauerfeind P. Is virtual colonoscopy a cost-effective option to screen for colorectal cancer? Am J Gastroenterol. 1999;94:2268–74. doi: 10.1111/j.1572-0241.1999.01304.x. [DOI] [PubMed] [Google Scholar]
- 55.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133:573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 56.Vijan S, Hwang EW, Hofer TP, Hayward RA. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001;111:593–601. doi: 10.1016/s0002-9343(01)00977-9. [DOI] [PubMed] [Google Scholar]
- 57.Vijan S, Hwang I, Inadomi J, Wong RK, Choi JR, Napierkowski J, Koff JM, Pickhardt PJ. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102:380–90. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glick S, Wagner JL, Johnson CD. Cost-effectiveness of double-contrast barium enema in screening for colorectal cancer. AJR Am J Roentgenol. 1998;170:629–36. doi: 10.2214/ajr.170.3.9490943. [DOI] [PubMed] [Google Scholar]
- 59.Briggs AH, O'Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 60.Theuer CP, Taylor TH, Brewster WR, Anton-Culver H. Gender and race/ethnicity affect the cost-effectiveness of colorectal cancer screening. J Natl Med Assoc. 2006;98:51–7. [PMC free article] [PubMed] [Google Scholar]
- 61.Theuer CP, Wagner JL, Taylor TH, Brewster WR, Tran D, McLaren CE, Anton-Culver H. Racial and ethnic colorectal cancer patterns affect the cost-effectiveness of colorectal cancer screening in the United States. Gastroenterology. 2001;120:848–56. doi: 10.1053/gast.2001.22535. [DOI] [PubMed] [Google Scholar]
- 62.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 63.Kalow W. Pharmacogenetics and pharmacogenomics: origin, status, and the hope for personalized medicine. Pharmacogenomics J. 2006 doi: 10.1038/sj.tpj.6500361. [DOI] [PubMed] [Google Scholar]
- 64.Abrahams E, Ginsburg GS, Silver M. The Personalized Medicine Coalition: goals and strategies. Am J Pharmacogenomics. 2005;5:345–55. doi: 10.2165/00129785-200505060-00002. [DOI] [PubMed] [Google Scholar]
- 65.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the United States. Clin Gastroenterol Hepatol. 2005;3:798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 66.Chan AT, Giovannucci EL, Schernhammer ES, Colditz GA, Hunter DJ, Willett WC, Fuchs CS. A prospective study of aspirin use and the risk for colorectal adenoma. Ann Intern Med. 2004;140:157–66. doi: 10.7326/0003-4819-140-3-200402030-00006. [DOI] [PubMed] [Google Scholar]
- 67.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 68.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–63. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 69.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–14. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 70.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 71.Giovannucci E, Stampfer MJ, Colditz GA, Rimm EB, Trichopoulos D, Rosner BA, Speizer FE, Willett WC. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–84. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 72.Taupin D, Chambers SL, Corbett M, Shadbolt B. Colonoscopic screening for colorectal cancer improves quality of life measures: a population-based screening study. Health Qual Life Outcomes. 2006;4:82. doi: 10.1186/1477-7525-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970-1990: gender, racial, and educational differences. Soc Sci Med. 2001;52:1629–41. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 74.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med. 2003;139:959–65. doi: 10.7326/0003-4819-139-12-200312160-00005. [DOI] [PubMed] [Google Scholar]
- 76.Cancer Facts & Figures 2007. American Cancer Society. 2007 Accessed at: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf.
- 77.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–77. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 78.Colorectal Cancer Facts & Figures Special Edition 2005. American Cancer Society. 2005 Accessed at: http://www.cancer.org/downloads/STT/CAFF2005CR4PWSecured.pdf.
- 1.Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–7. doi: 10.1177/00359157740676P115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Gyrd-Hansen D, Sogaard J, Kronborg O. Analysis of screening data: colorectal cancer. Int J Epidemiol. 1997;26:1172–81. doi: 10.1093/ije/26.6.1172. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Launoy G, Smith TC, Duffy SW, Bouvier V. Colorectal cancer mass-screening: estimation of faecal occult blood test sensitivity, taking into account cancer mean sojourn time. Int J Cancer. 1997;73:220–4. doi: 10.1002/(sici)1097-0215(19971009)73:2<220::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance Research Program . National Cancer Institute SEER*Stat Software. version 5.3.1. 2003. [Google Scholar]
- 7.DiSario JA, Foutch PG, Mai HD, Pardy K, Manne RK. Prevalence and malignant potential of colorectal polyps in asymptomatic, average-risk men. Am J Gastroenterol. 1991;86:941–5. [PubMed] [Google Scholar]
- 8.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL., Jr. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85:969–74. [PubMed] [Google Scholar]
- 9.Koretz RL. Malignant polyps: are they sheep in wolves' clothing? Ann Intern Med. 1993;118:63–8. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86:946–51. [PubMed] [Google Scholar]
- 11.Rex DK, Lehman GA, Hawes RH, Ulbright TM, Smith JJ. Screening colonoscopy in asymptomatic average-risk persons with negative fecal occult blood tests. Gastroenterology. 1991;100:64–7. doi: 10.1016/0016-5085(91)90583-7. [DOI] [PubMed] [Google Scholar]
- 12.Palitz AM, Selby JV, Grossman S, Finkler LJ, Bevc M, Kehr C, Conell CA. The Colon Cancer Prevention Program (CoCaP): rationale, implementation, and preliminary results. HMO Pract. 1997;11:5–12. [PubMed] [Google Scholar]
- 13.Aslinia F, Uradomo L, Steele A, Greenwald BD, Raufman JP. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721–31. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 14.Cotterill M, Gasparelli R, Kirby E. Colorectal cancer detection in a rural community. Development of a colonoscopy screening program. Can Fam Physician. 2005;51:1224–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD, Church J, Marshall JB, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 16.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 17.Levin TR, Palitz A, Grossman S, Conell C, Finkler L, Ackerson L, Rumore G, Selby JV. Predicting advanced proximal colonic neoplasia with screening sigmoidoscopy. Jama. 1999;281:1611–7. doi: 10.1001/jama.281.17.1611. [DOI] [PubMed] [Google Scholar]
- 18.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 20.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007;39:168–73. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 21.Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–72. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 22.Levin TR, Conell C, Shapiro JA, Chazan SG, Nadel MR, Selby JV. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002;123:1786–92. doi: 10.1053/gast.2002.37064. [DOI] [PubMed] [Google Scholar]
- 23.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jentschura D, Raute M, Winter J, Henkel T, Kraus M, Manegold BC. Complications in endoscopy of the lower gastrointestinal tract. Therapy and prognosis. Surg Endosc. 1994;8:672–6. doi: 10.1007/BF00678564. [DOI] [PubMed] [Google Scholar]
- 25.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, Ballegooijen Mv, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer. 2007. Accessed at: https://www.cms.hhs.gov/mcd/viewtechassess.asp?where=index&tid=52. [PubMed] [Google Scholar]
- 26.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 27.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–9. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]