Abstract
Each year, millions of monarch butterflies from eastern North America migrate to overwinter in 10–13 discrete colonies located in the Oyamel forests of central Mexico. For decades efforts to track monarch migration have relied on observations and tag-recapture methods, culminating with the discovery of the wintering colonies in 1975. Monarch tag returns from Mexico, however, are few and primarily from two accessible colonies, and therefore tag-recapture techniques have not quantified natal origins or distinctiveness among monarch populations at wintering sites. Such information would be invaluable in the conservation of the monarch and its migration phenomenon since the wintering sites currently are threatened by habitat alteration. Here we show that stable hydrogen (δD) and carbon (δ13C) isotope ratios of wintering monarchs can be used to evaluate natal origins on the summer breeding range. Stable-hydrogen and carbon isotopic values of 597 wintering monarchs from 13 wintering roost sites were compared with isotopic patterns measured in individuals at natal sites across their breeding range over a single migration cycle. We determined that all monarch wintering colonies were composed of individuals originating mainly from the Midwest, United States, thereby providing evidence for a panmictic model of wintering colony composition. However, two colonies showed more northerly origins, suggesting possible priority colonies for conservation efforts.
More than 100 million monarch butterflies (Danaus plexippus) migrate annually from eastern North America to overwinter in 10–13 discrete (<2 ha) colonies located in remote Oyamel forests of central Mexico (refs. 1–6; Fig. 1). Monarchs returning to these wintering sites are separated by several generations from those that left the previous year, yet the same overwintering locations are used every year. Although monarch migration has been studied for more than 50 years (1, 6), how butterflies navigate to these locations from specific areas in North America (7) and whether colony composition is panmictic have long been mysteries.
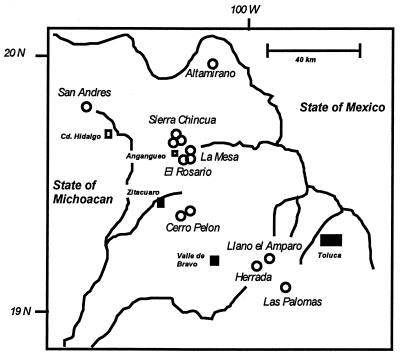
Figure 1.
Location of the 13 monarch wintering colonies (circles) in the Mexican States of Michoacán and México.
Previously, monarch migration to Mexico has been studied by using tag-recapture techniques, which involves the placement of small identification tags on monarchs captured and released in the breeding range (ref. 3; Monarch Watch Organization, University of Kansas, http://MonarchWatch.org). Hundreds of thousands of monarchs have been tagged over the past five decades, with only 125 recoveries from Mexico occurring since 1975 (Monarch Watch Organization, University of Kansas, http://MonarchWatch.org). Most (83%) tags recovered between 1975 and 1998 were found at colonies with public viewing or research access (El Rosario and Sierra Chincua), with few or no tags recovered from the remaining 11–12 remote sites (Monarch Watch Organization, University of Kansas, http://MonarchWatch.org). Other difficulties include the fact that most tagged migrants are recaptured within the breeding range, and the number of individuals tagged does not reflect monarch production in any given area. Thus, tagging has not yielded quantitative information on proportions of monarchs originating from various parts of the breeding range. Clearly, a better tool is needed to assess the origins of wintering monarchs in Mexico. Such knowledge is required to focus conservation efforts in critical portions of the North American breeding range and at wintering sites in Mexico.
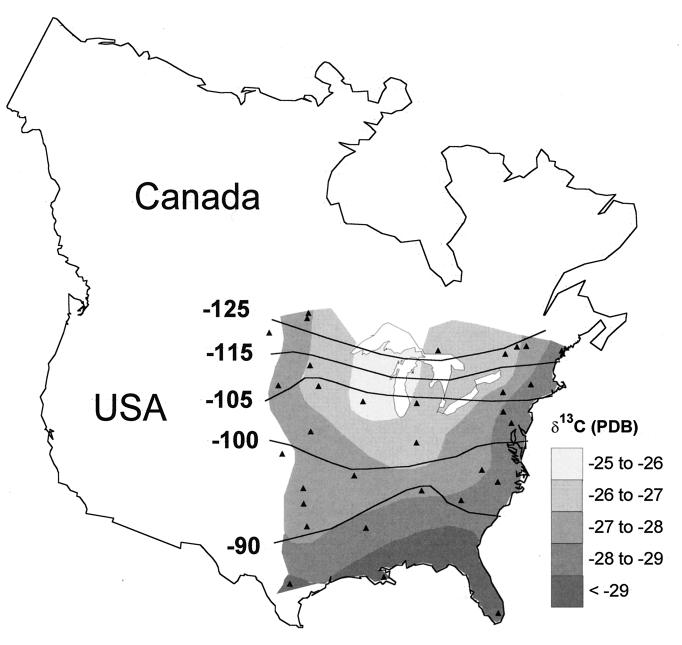
Based on laboratory and extensive field-rearing experiments across the eastern breeding range we showed that the stable-hydrogen (δD) and carbon (δ13C) isotopic composition of adult monarch wing membranes closely resembles, and permanently records, the isotopic composition of its natal (larval) food source (15). The isotopic composition of the monarch larval milkweed host plant (Asclepia sp.) is, in turn, controlled by continental isotopic patterns in rainfall for δD (8, 15) and other climatic and physiological factors for δ13C (8, 9). The δD and δ13C values of adult monarch wings from natal sites show isotopic trends across eastern North America, with increasingly depleted δD values found toward the northerly limits of the breeding range and 13C enrichment toward more northerly latitudes (15) (Fig. 2). These results show that natural signals of δD and δ13C are intrinsic markers of natal origins of monarchs. By sampling and measuring the isotopic composition of wintering monarchs in Mexico, we reasoned that it should be possible to infer their geographic natal origins and to establish whether wintering colonies are composed of monarchs from different regions.
Figure 2.
Geographic patterns of δD and δ13C in monarch wings from natal sites across the breeding range of eastern North America (synthesized from ref. 15). Solid triangles depict field-rearing sites.
METHODS
We measured δD and δ13C values of 597 monarch butterflies collected from natural mortality at 13 wintering colonies (44–50 per colony) during February of 1997 (Table 1). These were individuals that migrated from North America in late summer and fall of 1996, and so could be related directly back to our 1996 breeding range isotopic study (15) (Fig. 2). Fifty male and 50 females were taken at random from each colony and stored in paper envelopes. Monarch wing membranes were separated from the abdomen, placed in glass vials, solvent-cleaned, air-dried, and stored.
Table 1.
Mean and 95% confidence interval (CI) for δD and δ13C values of monarch butterflies collected from 13 wintering colonies in Mexico
| Wintering colony | δD, ‰ | 95% CI, ‰ | n | δ13C, ‰ | 95% CI, ‰ | n |
|---|---|---|---|---|---|---|
| Llano el Amparo | −104 ± 11 | −107 to −100 | 47 | −27.6 ± 1.1 | −27.9 to −27.3 | 47 |
| Altamirano | −104 ± 8 | −106 to −101 | 45 | −27.6 ± 1.1 | −27.9 to −27.3 | 47 |
| Sierra Chincua Barranca Hondon | −110 ± 10 | −113 to −107 | 44 | −27.5 ± 1.3 | −27.9 to −27.2 | 47 |
| Sierra Chincua Llano el Toro | −105 ± 8 | −108 to −103 | 46 | −27.3 ± 1.1 | −27.7 to −27.0 | 48 |
| Sierra Chincua Barranca la Meurto | −106 ± 11 | −110 to −103 | 48 | −27.4 ± 0.9 | −27.7 to −27.2 | 50 |
| Cerro Pelon La Gota de Agua | −110 ± 8 | −112 to −107 | 45 | −27.7 ± 1.3 | −28.1 to −27.3 | 48 |
| Cerro Pelon Los Cedrales | −107 ± 9 | −109 to −104 | 45 | −27.6 ± 1.4 | −28.0 to −27.2 | 49 |
| Herrada | −106 ± 10 | −109 to −103 | 48 | −27.5 ± 1.1 | −27.8 to −27.2 | 48 |
| Las Palomas | −104 ± 9 | −107 to −101 | 48 | −27.4 ± 1.1 | −27.8 to −27.1 | 46 |
| La Mesa | −104 ± 9 | −107 to −102 | 49 | −27.4 ± 1.3 | −27.8 to −27.0 | 48 |
| El Rosario El Campanario | −108 ± 11 | −111 to −104 | 48 | −27.7 ± 1.0 | −28.0 to −27.4 | 50 |
| El Rosario Planos de los Conecos | −106 ± 11 | −110 to −103 | 44 | −27.6 ± 1.2 | −28.1 to −27.4 | 49 |
| San Andres | −106 ± 10 | −109 to −103 | 45 | −27.7 ± 1.2 | −28.1 to −27.4 | 50 |
Italicized sites refer to discrete subcolonies of monarchs that are generally considered part of the larger colony indicated.
Because a portion of the total hydrogen of monarch wing membrane (largely keratin) is available for isotopic exchange with ambient water vapor, it was necessary to quantify and eliminate the effect of this uncontrolled, temperature-dependent variable. Unfortunately, complete elimination of exchangeable hydrogen (i.e., hydrogen involved in O—H bonds) by chemical techniques such as nitration is not possible for complex organic matter (10). Hydrogen–isotope exchange between wing membrane and water vapor was first quantified by equilibrating samples with steam having a wide range of hydrogen-isotopic values (−135 to +525‰) at constant temperature (130 ± 0.1°C) and then measuring the total hydrogen δD values (10, 15). Hydrogen in monarch wing membranes available for isotopic exchange at this temperature was determined to be 19.5 ± 0.7% (r2 = 0.99, P < 0.001, n = 27). In all our samples, potential variability resulting from uncontrolled hydrogen-isotopic exchange was eliminated by controlled equilibration of all wing membrane samples with steam (δD = −135‰) at 130 ± 0.1°C for 2 hr. Sample reproducibility of repeated equilibrated samples was better than ±2‰ for δD. Thus, total hydrogen-isotopic results for equilibrated samples could be compared reliably among samples and sites. After steam equilibration in Vycor break-seal tubes, all water vapor was cryogenically removed, and samples were sealed under vacuum, combusted at 850°C in the presence of cupric oxide, and followed by cryogenic separation of CO2 from H2O. Waters of combustion were reduced to H2 gas on hot zinc (15). Stable-isotope analyses were performed on a Micromass Optima dual-inlet isotope-ratio mass spectrometer. Stable-carbon isotope analyses are reported in parts per thousand (‰) deviation from the Pee Dee belemnite (PDB) standard, with a sample reproducibility of better than ±0.1‰. Stable-hydrogen isotope results are reported in parts per thousand deviation from the SMOW standard and normalized on the Vienna Standard Mean Ocean Water/Standard Light Antarctic Precipitation (VSMOW/SLAP) scale, with a sample reproducibility of better than ±2.0‰.
RESULTS AND DISCUSSION
No effect of sex on the distribution of stable isotopes in monarch butterflies was observed (MANOVA F2,557 = 1.6, P = 0.2), and populations from all colonies showed normal distributions for both δ13C and δD (Kolmogorov–Smirnov, P < 0.01 in all cases). In general, δD and δ13C values of monarch wings overlapped considerably among wintering colonies (Table 1, MANOVA F24,1114 = 1.5, P = 0.05), but some difference in the distribution of δD values among sites (ANOVA F12,558 = 2.2, P = 0.01) was apparent (Table 1). The Barranca Hondon and La Gota de Agua monarch colonies were more depleted in δD values and did not overlap at the 95% confidence interval with the more enriched δD distributions of Llano el Amparo, Altamirano, Las Palomas, and La Mesa, suggesting more northern natal origins of monarchs at these two colonies.
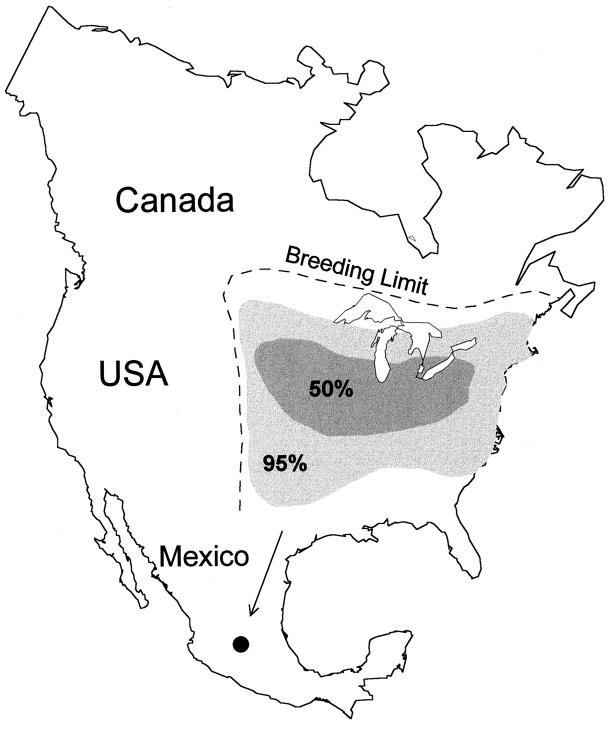
We inferred natal origins of wintering monarchs from Mexico by comparing δD and δ13C data of wintering individuals with isotopic values found in monarchs field raised at natal sites throughout their breeding range (Fig. 2). Our δD and δ13C data show that 95% of wintering monarchs originated from throughout the known breeding range (Fig. 3). However, 50% of wintering monarchs originated from a fairly restricted geographic part of the breeding range, including the states of Kansas, Nebraska, Iowa, Missouri, Wisconsin, Illinois, Michigan, Indiana, and Ohio. This corresponds to an area of intense corn, soybean, and dairy production in the midwestern United States. Possibly, larger numbers of monarchs are produced in such areas of cultivation since milkweed host plants are persistent there despite pesticide and weed control measures. Proportionately fewer monarchs originated from the most southern or northern reaches of the breeding range (Fig. 3). Fewer wintering monarchs from the extremes of the breeding range were expected since relatively fewer individuals are produced there. Lack of representation from southern parts of the breeding range also is a result of the lack of larval host plant availability in the southern United States in late summer (11).
Figure 3.
Natal origins of monarch butterflies wintering in Mexico derived from δD and δ13C data (n = 597). The light-gray area indicates the range of natal origins for 95% of monarchs from all wintering sites. The darker-gray area indicates range of natal origins for 50% of monarchs from all wintering colonies. The dashed line is the approximate monarch breeding range limit. The Mexican monarch overwintering colonies are denoted by the solid circle.
Our isotopic evidence shows that the 13 discrete monarch wintering colonies in Mexico generally are well mixed and thus demonstrates the existence of a panmictic model of monarch wintering colony composition. With the exception of the two sites noted, our results further suggest that the loss of a single wintering roost site is unlikely to affect one part of the breeding population in eastern North America over another. However, because the primary geographic production area for monarchs is centered in the American Midwest, that area should be focal for conservation efforts in North America. The combination of δD and δ13C measurements of tissues of breeding and wintering populations of monarchs measured in a single year represents a new and powerful tool for understanding the ecology of this species and avoids interannual isotopic variability (12). Furthermore, our approach can be readily applied to other migratory organisms in North America and likely elsewhere and possibly refined through the assay of isotopes of other elements (13, 14).
Acknowledgments
We thank Constantino Orduña Trejo of the Mexican National Institute of Forestry and Agriculture (INIFAP) for coordinating field sampling in Mexico, as well as the numerous staff members at the Mexican monarch reserves for assistance in the field. We thank the INIFAP and Mexican National Institute of Ecology for permission to sample at all wintering colonies. S. Polischuk, B. Boldt-Leppin, and R. George assisted with lab analyses. This research was funded by Environment Canada and by grants from the Canada–Mexico International Partnerships Program and the Manitoba Model Forest Project.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Urquhart F A. Nat Geogr. 1976;150:160–173. [Google Scholar]
- 2.Brower L P. Nat Hist. 1977;84:40–53. [Google Scholar]
- 3.Urquhart F A. News Lepidopt Soc. 1978;1978:3–4. [Google Scholar]
- 4.Brower L P. J Lepidopt Soc. 1995;49:304–385. [Google Scholar]
- 5.Malcolm S B. Trends Ecol Evol. 1987;2:292–293. doi: 10.1016/0169-5347(87)90055-3. [DOI] [PubMed] [Google Scholar]
- 6.Urquhart F A. The Monarch Butterfly. Toronto: Univ. of Toronto Press; 1960. [Google Scholar]
- 7.Perez S O, Taylor O R, Jander R A. Nature (London) 1997;387:29. [Google Scholar]
- 8.White J W C. In: Stable Isotopes in Ecological Research. Rundel P W, Ehleringer J R, Nagy K A, editors. Berlin: Springer; 1988. pp. 142–162. [Google Scholar]
- 9.Körner C H, Farquar G D, Wong S C. Oecologia. 1991;88:30–40. doi: 10.1007/BF00328400. [DOI] [PubMed] [Google Scholar]
- 10.Schimmelmann A. Anal Chem. 1991;63:2456–2459. [Google Scholar]
- 11.Woodson R E., Jr Ann Miss Botan Gard. 1954;41:1–211. [Google Scholar]
- 12.Koch P L, Heisinger J, Moss C, Carlson R W, Fogel M L, Behrensmeyer A K. Science. 1995;267:1340–1343. doi: 10.1126/science.267.5202.1340. [DOI] [PubMed] [Google Scholar]
- 13.Hobson K A, Wassenaar L I. Oecologia. 1997;109:142–148. doi: 10.1007/s004420050068. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain C P, Blum J D, Holmes R T, Feng X, Sherry T W, Graves G R. Oecologia. 1997;109:132–141. doi: 10.1007/s004420050067. [DOI] [PubMed] [Google Scholar]
- 15.Hobson, K. A., Wassenaar, L. I. & Taylor, O. R. (1999) Oecologia, in press. [DOI] [PubMed]