Abstract
Humans die from age-related diseases, which are deadly manifestations of the aging process. In order to extend life span, an anti-aging drug must delay age-related diseases. All together age-related diseases are the best biomarker of aging. Once a drug is used for treatment of any one chronic disease, its effect against other diseases (atherosclerosis, cancer, prostate enlargement, osteoporosis, insulin resistance, Alzheimer's and Parkinson's diseases, age-related macular degeneration) may be evaluated in the same group of patients. If the group is large, then the anti-aging effect could be validated in a couple of years. Startlingly, retrospective analysis of clinical and preclinical data reveals four potential anti-aging modalities.
Keywords: anti-aging drugs, diseases, cancer, atherosclerosis, resveratrol, rapamycin, metformin
Problem
The discovery of anti-aging drugs is no longer a fantasy. Numerous genes for aging and longevity have been identified in diverse organisms, revealing potential targets for potential anti-aging drugs. But how could potential anti-aging drug be introduced to humans? There are two problems. First, the effect of anti-aging agents on human aging may require almost a lifetime to determine [1]. Second, it is seemingly desirable to test anti-aging drugs in healthy individuals. However, all drugs have side effects. And, in healthy individuals, side effects would preclude clinical trials. How might these problems be solved? How could we validate anti-aging drugs in humans without life-long trials in healthy individuals?
Solution
The solution includes two steps. First, we must find an indication for a drug to treat at least one chronic disease. Then this drug could be tested in humans, not as an anti-aging drug, but as therapy for a particular disease. In fact this approach has been suggested for introduction of activators of sirtuins to the clinic [2,3].
Second, we must find a biomarker of aging that absolutely predicts longevity. Then using this biomarker, the anti-aging effect could be evaluated in the same patients.
Aging and age-related diseases
Aging can be defined as an increase in the probability of death. This is how the rate of aging can be measured. Humans die not from ‘healthy' aging but from age-related diseases. Healthy aging (a late onset of disease) is associated with longevity. For example, centenarians show significant delay in the onset of age-related diseases, including cardiovascular disease, type 2 diabetes, cancer and Alzheimer's disease. In other words, those who live longer are healthier and vice versa [4,5]. Since, by definition, all age-dependent diseases are connected with aging, these diseases are connected to each other. In fact, aging humans often suffer from many diseases simultaneously: diabetes, atherosclerosis, hypertension, macular degeneration, prostate enlargement and prostate cancer (in men) or breast cancer (in women), Alzheimer's disease and osteoarthritis. This is why elimination of one disease (e.g., cancer) will not radically extend maximal human lifespan. And as calculated, "the complete resolution of Alzheimer's disease would add about 19 days onto average life expectancy" [6]. But if a drug delays or stops all diseases, a person must live longer. Otherwise what would be the cause of death, if all causes were delayed? Since human longevity is limited by death from age-related diseases, a true anti-aging drug must delay age-related diseases. In other words, unless a drug delays age-related diseases, it will not extend lifespan. And vice versa, if a drug prevents age-related diseases, it must extend life span.
Biomarker of organismal aging
Given that (a) an increase in the death rate is a measure of aging and (b) the death rate is determined diseases taken together, then we can conclude that the sum of all age-related diseases is the best biomarker of aging. Any one age-related disease is not a biomarker of aging because, in addition to aging, numerous factors contribute to the incidence of a particular disease. For example, smoking increases the risk for lung cancer but not for Parkinson's disease. Yet, aging is a risk factor for both diseases. And, even for lung cancer, aging is a bigger risk factor than is smoking. Aging is the biggest risk factor for all age-related diseases. Whether aging and disease have a common mechanism or whether aging simply increases vulnerability to diseases, in any case, the inhibition of aging will delay diseases, thus extending life span.
Disease-specific drugs versus anti-aging agents
Slowing aging would delay all age-related diseases. If a drug is effective against one particular disease only, such a drug is not anti-aging. And current drugs are not anti-aging. For example, insulin compensates diabetes. Yet, insulin does not treat cancer. And vice versa chemotherapy may treat cancer but does not treat diabetes. So neither chemotherapy nor insulin is an anti-aging modality. Furthermore, both insulin and chemotherapy may accelerate aging.
Metformin
The underlying cause of age-related type II diabetes is insulin resistance. Insulin treatment does not ‘treat' the cause, it just compensates for resistance. Unlike insulin, metformin, an oral anti-diabetic drug, restores insulin sensitivity in type diabetes type II. Remarkably, metformin decreases the incidence of breast cancer [7,8]. Also, metformin is considered for cancer treatment [9] and inhibits atherosclerosis in diabetic mice [10]. Metformin is used to induce ovulation in patients with polycystic ovary syndrome (PCOS). Six months of 1700 mg/d metformin treatment improved fertility in anovulatory PCOS women [11,12]. Given such effects on infertility, type II diabetes, cancer and atherosclerosis, it is plausible that metformin slows aging. In fact, it extends life span in rodents [13-15].
Calorie restriction
Calorie restriction (CR) extends life span from yeast and worms to rodents and perhaps humans [16-18]. If we did not already know that CR slows aging, how might we figure that out based solely on clinical data? Unrestricted food consumption leads to obesity associated with diabetes, atherosclerosis, thrombosis, hypertension, cancer (especially breast, prostate and colon cancer), coronary heart disease, stroke, osteoporosis and Alzheimer's disease [19-25]. In other words, unrestricted eating in humans (ad libitum in rodents) accelerates most, if not all, diseases of aging. So we can conclude that CR delays all diseases of aging. This suggests that CR is an anti-aging modality. And it is known that CR extends life span in almost all organisms from yeast to mammals.
From metformin and calorie restriction to rapamycin
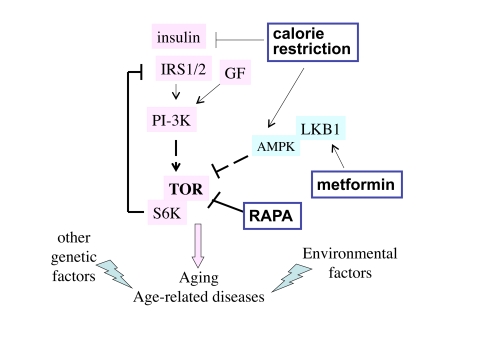
Numerous factors including insulin, glucose and amino acids activate the nutrient-sensing TOR (target of rapamycin) pathway. When the TOR pathway is activated, it acts via S6K to deplete the insulin-receptor-substrate (IRS1/2), causing insulin resistance (Figure 1). As shown in Figure 1, metformin indirectly (by activating AMPK) inhibits TOR and thereby restores insulin sensitivity [26].
Figure 1. The TOR intracellular signaling pathway.
Nutrients, GF (growth factors) and insulin activate the TOR pathway, which is involved in aging and age-related diseases. Other genetic factors and environmental factors (e.g., smoking) contribute to specific age-related diseases. Three potential anti-aging modalities (metformin, calorie restriction and rapamycin) all inhibit the TOR pathway.
CR decreases levels of nutrients and insulin and thus de-activates TOR (Figure 1). It is possible that the anti-aging effects of CR and metformin are due to inhibition of the TOR pathway. Like CR, rapamycin decreases size of fat cells and animal weight. When rats (15 weeks old) were either treated 1 mg/kg rapamycin 3 times per week for 12 weeks, rapamycin decreased their weight. Mean adipocyte diameter was decreased from 36 μm to 25 μm. At the end of the study, mean body weight in the rapamycin-treated rats was 356 g instead of 507 g, in spite of comparable food intake [27]. So rapamycin imitated CR. CR may also extend life span by activating sirtuins. Probably, sirtuins, AMPK and mTOR are linked in the common network [28].
Genetic inhibition of the TOR pathway slows down aging in diverse organisms, including yeast, worms, flies and mice [29-33]. If genetic inhibition of the TOR pathway slows aging, then rapamycin, a drug that inhibits TOR, must slow aging too. Once used for any indication, even unrelated to age-related diseases (such as renal transplantation, for instance), an anti-aging drug should slow down age-related diseases such as cancer, osteoporosis and atherosclerosis. Rapamycin is already used in renal transplant patients.
Retrospective analysis of the clinical use of rapamycin
Rapamycin has been used in renal-transplant patients for several years. Since rapamycin was viewed as an immunossupressive drug (not as an anti-aging drug) it was expected that it would cause cancer.
Unexpectedly, it turned out that rapamycin prevented cancer, and even cured pre-existing cancer and Kaposi's sarcoma in renal transplant patients [34-44]. Furthermore, temsirolimus, an analog of rapamycin, has recently been approved for cancer therapy [45]. Also, everolimus, a TOR inhibitor, markedly delayed tumor development in transgenic mice that spontaneously develop ovarian carcinomas [46]. Would TOR inhibitors extend life span in transgenic mice? Since rapamycin delays cancer, it must prolong the life span of cancer-prone mice, who would otherwise die from cancer. Of course, humans die from a variety of age-related diseases, not from just one disease. To prolong life span dramatically, rapamycin must delay most of them.
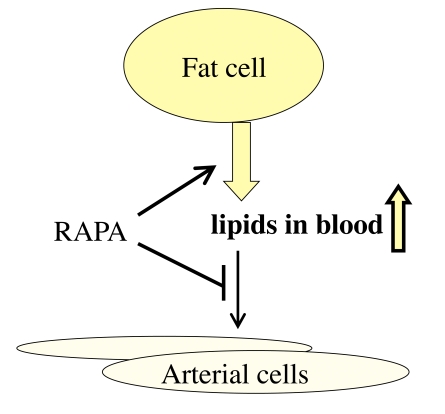
In renal transplant patients, rapamycin increases blood lipoproteins [47]. This is considered to be a negative side effect. Yet, this results from mobilization of fat from the fat tissue (lipolysis) [48,49]. This is exactly what happens during starvation or calorie restriction (CR). And CR extends life span. Furthermore, rapamycin reduces the accumulation of cholesterol within the arterial wall [50,51]. Thus, lipolysis of fat tissue and decreased uptake of cholesterol by tissues both contribute to high levels of lipids in blood (Figure 2). Despite hypercholesterolemia, rapamycin prevents atherosclerosis in animals [52]. In animal models, systemic administration of rapamycin reduces neointimal thickening and slows the progression of atherosclerosis in apoE-deficient mice with elevated levels of cholesterol [53-55]. In patients with coronary atherosclerosis, oral rapamycin prevents re-stenosis after implantation of metal stents [56]. As a case report, it has been described that conversion to everolimus (an analog of rapamycin) resulted in decrease in blood pressure [57]. In kidney transplant patients, 2 years after transplantation, body-mass index was significantly lower in the rapamycin-based treatment arm compared to cyclosporine [27].
Figure 2. Re-interpretation of the hyperlipidemic side effect of rapamycin.
Rapamycin activates adipose tissue lipase, thus mobilizing lipids from the fat tissue (lipolysis). This effect imitates starvation. Also, rapamycin inhibits lipoprotein lipase thus preventing utilization of lipids by the fat tissue and blocking lipid uptake by the arterial wall. This results in increase in blood lipids.
Multiple indications for a single drug
If a drug is indicated to treat most age-related diseases, then this drug could be defined as an anti-aging drug. The probability that a non-anti-aging drug would have independent activities against all diseases is exceedingly low.
Rapamycin analogs are approved to treat certain cancers [45]. Based on preclinical data, rapamycin has been considered in such pathologies as obesity [58], atherosclerosis [53-55], cardiac hypertrophy [59-64], aortic aneurysm [65], osteoporosis [66-68], organ fibrosis (liver, renal, cardiac fibrosis) [64,69,70-75], neurodegeneration [76,77], Alzheimer's disease [78,79], Parkinson's disease [80-82], psoriasis [80], skin scars and keloids [83], multiple sclerosis [84], arthritis [85,86], and renal hypertrophy in diabetes [87].
May rapamycin increase human life span?
In principle, life-extending effect of anti-aging drug might be limited by side effects. Although chronic administration of rapamycin is associated with some undesirable effects in transplant patients (see for references [88]), they might be avoided by administrating rapamycin in pulses (for example, once a week). For example, chronic administration of rapamycin impairs wound healing. In theory, a pulse treatment might rejuvenate wound-healing cells [88]. A single dose of rapamycin reverses insulin resistance, whereas chronic administration of rapamycin may precipitate diabetes in certain conditions. Clinical trials will be needed to determine benefits of pulse treatment with rapamycin. Alternatively, rapamycin can be combined with ‘complementary' drugs. Thus, hyperlipidemia caused by rapamycin may deteriorate insulin-resistance. Yet, hyperlipidemia caused by rapamycin can be controlled by lipid-lowering drugs. A combination of rapamycin with resveratrol may be especially intriguing.
Resveratrol
Resveratrol, an activator of SIRT1 in mammals, extends life span in diverse species [89,90]. Resveratrol was shown to prevent cancer, atherosclerosis, neuro-degeneration and insulin-resistance (diabetes type II) [10,91-100]. Resveratrol also indirectly inhibits PI-3K/mTOR/S6K pathway [101-105]. SIRT1 and mTOR could be members of the same sirtuin/TOR network. The link between TOR and sirtuins has been suggested [28]. It is likely that TOR (pro-aging pathway) and sirtuins (anti-aging pathway) antagonize each other [106]. However, inhibition of the TOR pathway by resveratrol occurs at near-toxic concentrations [107].
The ability of resveratrol to extend life span may be limited by its toxicity at high doses due to off-target effects. Therefore, more selective activators of SIRT1 undergo clinical trials [3]. Importantly, these drugs will be developed to treat age-related diseases such as type 2 diabetes [3]. This is the only possible strategy for a drug to enter the clinic. But here is an additional aspect: this is the only practical way of how anti-aging effect can be evaluated too. Once used for treatment of diabetes, sirtuin activators might delay heart diseases, cancer, neurodegeneration and other age-related diseases in the same patients. And delaying of all diseases must extend life span, thus validating a drug as anti-aging.
Conclusion
It was previously assumed that anti-aging drugs should be tested in healthy individuals. Ironically, the best biomarker of aging is the occurrence of age-related diseases. And this is how anti-aging drugs can be validated in the clinic (by showing that a putative anti-aging drug can prevent or delay the onset of all age-related diseases). Then such drugs could be approved for prevention of any particular age-related disease in healthy individuals. Thus, potential anti-aging drugs should be introduced to the clinical trials for therapy of a particular disease but be ultimately approved for prevention of all age-related diseases in healthy individuals. And this is synonymous to the approval of a drug as anti-aging.
Acknowledgments
This work was not funded by any sources. The author is a founder of Oncotarget but is not employed by the company and declares no conflicts of interests.
References
- 1.Hadley EC, lakatta EG, Morrison-Bogorad M, Warner HR, Hodes RJ. The future of aging therapies. Cell. 2005;120:557–567. doi: 10.1016/j.cell.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair DA, Guarente L. Unlocking the secrets of longevity genes. Sci Am. 2006;294:48–51. doi: 10.1038/scientificamerican0306-48. [DOI] [PubMed] [Google Scholar]
- 3.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perls T, Kunkel L, Puca A. The genetics of aging. Curr Opin Genet Dev. 2002;12:362–369. doi: 10.1016/s0959-437x(02)00310-6. [DOI] [PubMed] [Google Scholar]
- 5.Curtis R, Geesaman BJ, DiStefano PS. Ageing and metabolism: drug discovery opportunities. Nat Rev Drug Discov. 2005;4:569–580. doi: 10.1038/nrd1777. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick L. The future of ageing. Nature. 2000;408:267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- 7.Evans JM. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreasedmortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002;25:2244–2248. doi: 10.2337/diacare.25.12.2244. [DOI] [PubMed] [Google Scholar]
- 9.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 10.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 11.Cheang KI, Sharma ST, Nestler JE. Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:595–604. doi: 10.1080/09513590601005847. [DOI] [PubMed] [Google Scholar]
- 12.Palomba S, Orio FJ, Falbo A, Russo T, Tolino A, Zullo F. Clomiphene citrate versus metformin as first-line approach for the treatment of anovulation in infertile patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:3498–3503. doi: 10.1210/jc.2007-1009. [DOI] [PubMed] [Google Scholar]
- 13.Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–246. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 14.Anisimov VN, Egormin PA, Bershtein LM, Zabezhinskii MA, Piskunova TS, Popovich IG, Semenchenko AV. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139:721–723. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 15.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 16.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy BK, Steffen KK, Kaeberlein M. Ruminations on dietary restriction and aging. Cell Mol Life Sci. 2007;64:1323–1328. doi: 10.1007/s00018-007-6470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everitt AV, Le Couteur DG. Life extension by calorie restriction in humans. Ann N Y Acad Sci. 2007;1114:428–433. doi: 10.1196/annals.1396.005. [DOI] [PubMed] [Google Scholar]
- 19.Anisimov VN. Premature ageing prevention: limitations and perspectives of pharmacological intervention. Curr Drug Targets. 2006;7:1485–1504. doi: 10.2174/1389450110607011485. [DOI] [PubMed] [Google Scholar]
- 20.Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 21.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–12. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson PW, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in the elderly. Am J Geriatr Cardiol. 2002;11:119–123. doi: 10.1111/j.1076-7460.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 23.Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–1029. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 24.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2594. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 26.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovira J, Marcelo Arellano E, Burke JT, Brault Y, Moya-Rull D, Bañón-Maneus E, Ramírez-Bajo MJ, Gutiérrez-Dalmau A, Revuelta I, Quintana LF, Campistol JM, Diekmann F. Effect of mTOR inhibitor on body weight: from an experimental rat model to human transplant patients. Transpl Int. 2008;21:992–998. doi: 10.1111/j.1432-2277.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 28.Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 Link Calorie Restriction and TOR to Sirtuin-Mediated Lifespan Extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 30.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 31.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 32.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17:33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Law BK. Rapamycin: an anti-cancer immunosuppressant. Crit Rev Oncol Hematol. 2005;56:47–60. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Nungaray N, Arriola M, Gutierrez MJ, Oliva E, Hernandez E, Gonzalez E, Andres A, Morales JM. Rapamycin at six years can exhibit normal renal function without proteinuria or neoplasia after renal transplantation. A single-center experience. Transplant Proc. 2005;37:3727–3728. doi: 10.1016/j.transproceed.2005.09.125. [DOI] [PubMed] [Google Scholar]
- 36.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 37.Zmonarski SC, Boratynska M, Rabczynski J, Kazimierczak K, Klinger M. Regression of Kaposi's sarcoma in renal graft recipients after conversion to sirolimus treatment. Transplant Proc. 2005;37:964–966. doi: 10.1016/j.transproceed.2004.12.172. [DOI] [PubMed] [Google Scholar]
- 38.Rizell M, Cahlin C, Friman S, Hafstrom L, Lonn L, Olausson M, Lindner P. Impressive regression of primary liver cancer after treatment with sirolimus. Acta Oncol. 2005;44:496. doi: 10.1080/02841860510044610. [DOI] [PubMed] [Google Scholar]
- 39.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 40.Yakupoglu YK, Buell JF, Woodle S, Kahan BD. Individualization of Immunosuppressive Therapy. III. Sirolimus Associated With a Reduced Incidence of Malignancy. Transplant Proc. 2006;38:358–361. doi: 10.1016/j.transproceed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, Kreis H, Burke JT, Brault Y, Scarola JA, Neylan JF. Sirolimus Therapy after Early Cyclosporine Withdrawal Reduces the Risk for Cancer in Adult Renal Transplantation. J Am Soc Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 42.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446–449. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 43.Mohsin N, Budruddin M, Pakkyara A, Darweesh A, Nayyer M, Amitabh J, Daar AS. Complete regression of visceral Kaposi's sarcoma after conversion to sirolimus. Exp Clin Transplant. 2005;3:366–369. [PubMed] [Google Scholar]
- 44.Cullis B, D'Souza R, McCullagh P, Harries S, Nicholls A, Lee R, Bingham C. Sirolimus-induced remission of posttransplantation lymphoproliferative disorder. Am J Kidney Dis. 2006;47:e67–72. doi: 10.1053/j.ajkd.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 45.Hudes G. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 46.Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, Hensley HH, Hamilton TC, Testa JR. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–2413. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 47.Legendre C, Campistol JM, Squifflet JP, Burke JT. Cardiovascular risk factors of sirolimus compared with cyclosporine: early experience from two randomized trials in renal transplantation. Transplant Proc. 2003;35:151S–153S. doi: 10.1016/s0041-1345(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 48.Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- 49.Morrisett JD, Abdel-Fattah G, Kahan BD. Sirolimus changes lipid concentrations and lipoprotein metabolism in kidney transplant recipients. Transplant Proc. 2003;35:143S–150S. doi: 10.1016/s0041-1345(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 50.Basso MD, Nambi P, Adelman SJ. Effect of sirolimus on the cholesterol content in ApoE knockout mice. Transplant Proc. 2003;35:3136–3138. doi: 10.1016/j.transproceed.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 51.Ma KL, Ruan XZ, Powis SH, Moorhead JF, Varghese Z. Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2721–2728. doi: 10.1152/ajpheart.01174.2006. [DOI] [PubMed] [Google Scholar]
- 52.Mueller MA, Beutner F, Teupser d, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimis in LDLR-/- mice despite hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 53.Pakala R, Stabile E, Jang GJ, Clavijo L, Waksman R. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol. 2005;46:481–486. doi: 10.1097/01.fjc.0000177985.14305.15. [DOI] [PubMed] [Google Scholar]
- 54.Elloso MM, Azrolan N, Sehgal SN, Hsu PL, Phiel KL, Kopec CA, Basso MD, Adelman SJ. Protective effect of the immuno-suppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3:562–569. doi: 10.1034/j.1600-6143.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 55.Waksman R, Pakala R, Burnett MS. Oral rapamycin inhibits growth of atherosclerotic plaque in apoE knock-out mice. Cardiovasc Radiat Med. 2003;4:34–38. doi: 10.1016/s1522-1865(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez AE, Granada JF, Rodriguez-Alemparte M, Vigo CF, Delgado J, Fernandez-Pereira C, Pocovi A, Rodriguez-Granillo AM, Schulz D, Raizner AE, Palacios I, O'neill W, Kaluza GL, Stone G, Investigators OI. Oral Rapamycin After Coronary Bare-Metal Stent Implantation to Prevent Restenosis The Prospective, Randomized Oral Rapamycin in Argentina (ORAR II) Study. J Am Coll Cardiol. 2006;47:1522–1529. doi: 10.1016/j.jacc.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 57.Pascual J, Fernández AM, Marcén R, Ortuño J. Conversion to everolimus in a patient with arterial hypertension and recurrent cutaneous neoplasia - a case report. Nephrol Dial Transplant. 2006;21 Suppl 3:iii38–iii41. doi: 10.1093/ndt/gfl299. [DOI] [PubMed] [Google Scholar]
- 58.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 59.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Tu VC, Bahl JJ, Chen QM. Signals of oxidant-induced cardiomyocyte hypertrophy: key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther. 2002;300:1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
- 61.Sadoshima J, Izumo S. Rapamycin selectively inhibits angiotensin II-induced increase in protein synthesis in cardiac myocytes in vitro. Potential role of 70-kD S6 kinase in angiotensin II-induced cardiac hypertrophy. Circ Res. 1995;77:1040–1052. doi: 10.1161/01.res.77.6.1040. [DOI] [PubMed] [Google Scholar]
- 62.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha T, Li Y, Gao X, McMullen JR ST, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med. 2005;39:1570–1580. doi: 10.1016/j.freeradbiomed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 64.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 65.Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR. Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg. 2004;40:334–338. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 66.Kneissel M, Luong-Nguyen NH, Baptist M, Cortesi R, Zumstein-Mecker S, Kossida S, O'Reilly T, Lane H, Susa M. Everolimus suppresses cancellous bone loss, bone resorption, and cathepsin K expression by osteoclasts. Bone. 2004;35:1144–1156. doi: 10.1016/j.bone.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- 68.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280:3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- 69.Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279:23166–23175. doi: 10.1074/jbc.M401238200. [DOI] [PubMed] [Google Scholar]
- 70.Gabele E, Reif S, Tsukada S, Bataller R, Yata Y, Morris T, Schrum LW, Brenner DA, Rippe RA. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374–13382. doi: 10.1074/jbc.M409444200. [DOI] [PubMed] [Google Scholar]
- 71.Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 2005;16:2063–2072. doi: 10.1681/ASN.2004030180. [DOI] [PubMed] [Google Scholar]
- 72.Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, Mauviel A, Verrecchia F. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin: A direct role as an anti-fibrotic agent. J Biol Chem. 2006;281:33045–52. doi: 10.1074/jbc.M606366200. [DOI] [PubMed] [Google Scholar]
- 73.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens. 2006;24:1663–1670. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 74.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 75.Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006;69:2029–2036. doi: 10.1038/sj.ki.5000161. [DOI] [PubMed] [Google Scholar]
- 76.Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 77.Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 78.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 79.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am J Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marsland AM, Griffiths CE. The macrolide immunosuppressants in dermatology: mechanisms of action. Eur J Dermatol. 2002;12:618–622. [PubMed] [Google Scholar]
- 81.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 82.Pannu J, Trojanowska M. Recent advances in fibroblast signaling and biology in scleroderma. Curr Opin Rheumatol. 2004;16:739–745. doi: 10.1097/01.bor.0000137894.63091.1a. [DOI] [PubMed] [Google Scholar]
- 83.Ong CT, Khoo YT, Mukhopadhyay A, Do DV, Lim IJ, Aalami O, Phan TT. mTOR as a potential therapeutic target for treatment of keloids and excessive scars. Exp Dermatol. 2007;16:394–404. doi: 10.1111/j.1600-0625.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 84.Farrell R, Heaney D, Giovannoni G. Emerging therapies in multiple sclerosis. Expert Opin Emerg Drugs. 2005;10:797–816. doi: 10.1517/14728214.10.4.797. [DOI] [PubMed] [Google Scholar]
- 85.Carlson RP, Hartman DA, Tomchek LA, Walter TL, Lugay JR, Calhoun W, Sehgal SN, Chang JY. Rapamycin, a potential disease-modifying antiarthritic drug. J Pharmacol Exp Ther. 1993;266:1125–1138. [PubMed] [Google Scholar]
- 86.Foroncewicz B, Mucha K, Paczek L, Chmura A, Rowinski W. Efficacy of rapamycin in patient with juvenile rheumatoid arthritis. Transpl Int. 2005;18:366–368. doi: 10.1111/j.1432-2277.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 87.Sakaguchi M, Isono M, Isshiki K, Sugimoto T, Koya D, Kashiwagi A. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem Biophys Res Commun. 2006;340:296–301. doi: 10.1016/j.bbrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res. 2008;11:801–808. doi: 10.1089/rej.2008.0722. [DOI] [PubMed] [Google Scholar]
- 89.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 90.Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 92.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 93.Westphal CH, Dipp MA, Guarente L. A therapeutic role for sirtuins in diseases of aging. Trends Biochem Sci. 2007;555-60:555–560. doi: 10.1016/j.tibs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 94.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;10:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 95.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 97.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 99.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10:5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 103.Brito PM, Devillard R, Nègre-Salvayre A, Almeida LM, Dinis TC, Salvayre R, Augé N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.11.011. In press . [DOI] [PubMed] [Google Scholar]
- 104.Fröjdö S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haider UG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol Pharmacol. 2002;62:772–777. doi: 10.1124/mol.62.4.772. [DOI] [PubMed] [Google Scholar]
- 106.Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill. Drug Discov Today. 2007;12:218–224. doi: 10.1016/j.drudis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 107.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8 doi: 10.4161/cc.8.12.8810. In press . [DOI] [PubMed] [Google Scholar]