Abstract
Differentiated neurons display specific biochemical, physiological and morphological properties that apparently prevent them from further cell division. Nevertheless, expression of cell cycle modulators persists after neuronal differentiation and is upregulated under stress conditions, such as trophic factor deprivation, oxidative stress and the presence of DNA damaging agents. This apparent reactivation of the cell cycle has been postulated as a sine qua non for neuronal death in response to those stress conditions, particularly in Alzheimer's disease. However, the physiological and pathogenic implications of a putative neuronal cell cycle are far from clear. Here, we discuss the notion of the neuronal cell cycle as a mediator of cell death, with particular emphasis on Alzheimer's disease.
Keywords: cell cycle, neuron, Alzheimer's disease
Introduction
Once a neuron is born, it loses its capacity for cell division and differentiates, contributing uniquely to the plasticity of the basic wiring pattern that defines a neuronal system. The preservation of this pattern is necessary for the overall generation and storage of memories, as well as the acquisition of other higher brain skills. Differentiated neurons appear to be irreversibly post-mitotic, perhaps because a hypothetical cell division would result in cytoskeletal and synaptic disruption in order to prepare the cell for mitosis and cytokinesis, which would in turn impair neuronal connectivity and function. Hence, it is reasonable to hink that, once a neuron differentiates, it resides out of the reach of cell division control. However, this notion was first questioned when some researchers surprisingly observed that neuronal programmed cell death was accompanied by the expression of cell cycle markers. Specifically, cyclins and cyclin-dependent kinases (CDKs), key components of the cell cycle machinery (see Figure 1) were found upregulated after exposure to severe conditions, such as oxidative stress and trophic factors deprivation [1-10]. Based on the premise that "neurons do not divide", the notion that has emerged from this evidence is that activation of a neuronal cell cycle does exist but it is abortive, the final result being the initiation of apoptosis. As we discuss below, this aberrant phenotype has also been postulated as a mechanism of neuronal loss in neurodegenerative diseases, particularly Alzheimer's disease (AD).
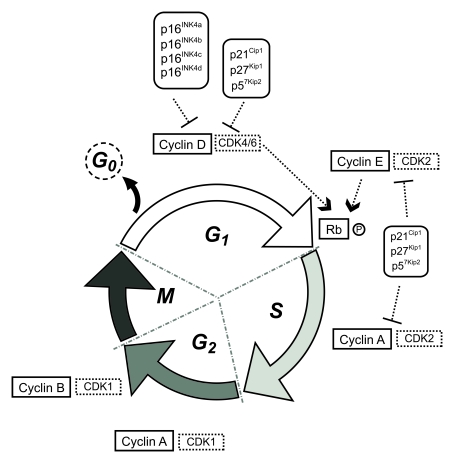
Figure 1. Schematic representation of the eukaryotic cell cycle.
Regulation of the cell cycle
The cell cycle of eukaryotic cells comprises four main successive phases: G1 phase (first gap), S phase (DNA synthesis), G2 phase (second gap) and M phase (mitosis) (Figure 1). Transition between the different phases and subsequent progression through the mitotic cycle is driven by a group of protein kinases whose activity is central to this process, the cyclin-dependent kinase (CDKs), and requires the binding of their activating partners cyclins, whose levels of expression varies throughout the cycle.
During G1 phase, mitogenic signals, such as extracellular growth factors or intercellular contact, trigger the activation of D-type cyclins that, jointly with CDK4 or CDK6, phosphorylate the retinoblastoma protein (Rb), inhibiting its affinity to bind the transcriptor factor E2F- 1. E2F-1 is released and directs the transcription of specific genes that code for proteins required in the next stages of the cell cycle. In late G1, an increase in cyclin E-CDK2 activity ensures the G1/S transition by completing Rb phosphorylation and irreversibly committing cells to enter the division process. Throughout S phase, cyclin A-CDK2 phosphorylates various substrates allowing DNA replication. After completion of S phase, DNA replication ceases and cells enter the G2 phase of the cycle. CDK2 is then replaced by CDK1 that associates with cyclin A and regulates the phosphorylation of proteins specific to the G2 and M phases of the cell cycle together with cyclin B-CDK1, that appears in late G2 and triggers the G2/M transition. Cyclin A is degraded and the system is reset, re-establishing the requirement for mitogenic cues to induce D-type cyclins for the next cycle. In M phase, cells physically divide originating two separate daughter cells (reviewed in [11]).
CDK activity is regulated through posttranslational modifications and subcellular translocations of specific CDK inhibitors (CDKIs), which are organized in two families, INK4 and Cip/Kip. The INK4 family (inhibitors of cyclin D-dependent kinases) consists of four members: p16INK4a, p15INK4b, p18INK4c and p19INK4d, and the Cip/Kip family (inhibitors of cyclin D-, cyclin E-, and cyclin A-dependent kinases) comprises p21Cip1, p27Kip1 and p57Kip2.
Two important checkpoints (G1/S and G2/M) coordinate CDKs activity and control the order and timing of cell-cycle transitions ensuring that DNA replication and chromosome segregation are completed correctly before allowing further progress through the cycle. The checkpoints allow alternative decisions between progression, growth arrest or induction of apoptosis. (See [12] for a detailed review addressing the regulation of the cell cycle in proliferating cells).
Differentiated neurons express cell cycle proteins
Neurogenesis, the birth of differentiated, functional neurons, takes place at two germinal compartments that line the lateral ventricles - the ventricular zone (VZ) and the subventricular zone (SVZ). Most neurons are originated prenatally through a process of migration to shape a complex pattern of layers. The deep layers are formed from earlier-born neurons originated in the VZ, while later-generated neurons from the SVZ occupy higher layers [13]. The journey is meant to cease proliferation and start neuronal differentiation. However, although terminally differentiated neurons seem to irreversibly withdraw from division, expression of cell cycle proteins is not completely silenced. Thus, cytoplasmic cyclin D1 was detected in mature neurons associated to the CDKIs p21Cip1 and p27Kip1, suggesting an impairment of its nuclear transport and a possible role in cell cycle withdrawal [14-16]. Indeed, cyclin D1 is downregulated [17], but also becomes predominantly cytoplasmic, in neuronal progenitor cells undergoing terminal differentiation [18]. Similarly, cyclin E expression was identified in the cytoplasm of postmitotic neurons [19,20]. More recently, Thomas Arendt's lab reported that, within the neocortex of the adult mouse, there is constitutive expression of cyclins D, E, A and B; of CDKs 4, 2 and 1; and of their inhibitors p16INK4a, p15INK4b, p18INK4c, p19INK4d, p21Cip1, p27Kip1 and p57Kip2 [21]. Furthermore, CDKs were found to be properly complexed to cyclins and exhibit kinase activity.
These findings have led to speculate that, in the absence of detectable neuronal cell division, there may be additional, cell cycle independent roles for cell cycle regulators in adult neurons. Indeed, there is evidence to suggest that cyclins and CDKs may participate in synaptic plasticity [22,23] and neuronal differentiation [24,25]. Similarly, CDH1 and APC (anaphase-promoting complex), which are found ubiquitously expressed in the nuclei of terminally differentiated neurons [26], and form a complex involved in cellular division at the end of mitosis and G1 through cyclin B degradation, also appear to play a role in regulating axonal growth and patterning in the developing brain [27]. Furthermore, CDK5, a cyclin-dependent kinase whose exact role in the cell cycle, if any, still remains elusive, is highly active in postmitotic neurons and is involved in the coordination of complex neuronal properties including synaptic plasticity, learning and memory (reviewed in [28]).
Thus, the presence of cell division mediators in differentiated neurons where the cell cycle is absent is well documented, and it does not appear to be the consequence of abnormal regulatory events. Rather, it appears as if at least some cell cycle proteins have adapted to life in a non-dividing neuron by learning and taking up additional, cell cycle-independent roles that are presumably crucial to neuronal function. The use of mouse conditional knockout models of these proteins should help us to unveil both the identity and importance of these putative functions.
Cell cycle abnormalities in differentiated neurons
There is also a substantial body of evidence pointing to a role for neuronal cell cycle proteins in the modulation of stress-induced apoptosis through a mechanism involving the initiation of a cell cycle. For example, rat cerebellar granule neurons plated in culture medium without trophic factors, such as brain-derived neurotrophic factor (BDNF), undergo apoptosis but also present up-regulated expression of both mRNA and protein levels of cyclin D1. Immunostaining confirmed cyclin D1 immunoreactivity prior to cell shrinkage and nuclear condensation. Furthermore, blocking the cell cycle with the CDKs inhibitors ciclopirox, mimosine and olomoucine was sufficient to suppress immunoreactivity and, more importantly, cell death [6]. Herrup et al. showed that two mouse neurological mutants, staggerer (sg/sg) and lurcher (+/Lc), that model the absence of trophic support in the brain, present significant numbers of cerebellar granule cells and inferior olive neurons degenerating after elevation of Cyclin D and proliferating cell nuclear antigen (PCNA) levels and bromodeoxyuridine (BrdU) incorporation [1]. RNA alphavirus Sindbis-driven expression of p16INK4a, p21Cip1 and p27Kip1, and of dominant negative forms of CDK4 and CDK6, protected rat primary neuronal cultures from apoptosis evoked by withdrawal of nerve growth factor (NGF) [2] and neuronal death as a result of DNA-damaging agents treatment, such as camptothecin, AraC and UV radiation [3]. The CDK inhibitors flavopiridol and olomoucine also protected the neurons from these conditions, suggesting that these cell cycle elements might mediate death signalling as a result of DNA-damaging environments [4]. Kruman et al. hypothesized that cell cycle reentry is a critical component of the DNA damage response in postmitotic neurons. Suppression of ataxia telangiectasia mutated (ATM), a component of DNA damage-induced checkpoint, by caffeine and wortmannin, attenuated both cell cycle reentry and apoptosis triggered by the genotoxic compounds etoposide, methotrexate, and homocysteine [7].
Oxidative stress-related cell death has also been associated with apparent cell cycle induction in post-mitotic neurons. Induction of cyclin B prior to the commitment of neurons to both dopamine- and peroxide-triggered apoptosis was reported in primary cultures of post-mitotic sympathetic neurons. Both neuronal death and rise in cyclin B were inhibited by antioxidant treatment [5].
In summary, the evidence available to us suggests that exposure of post-mitotic neurons to a wide range of stress stimuli triggers the expression of cell cycle proteins as part of a well regulated programmed cell death response. The most widely accepted scenario is that, in response to stress signals, neurons can be driven into the cell cycle but their array of cell cycle proteins may not suffice to allow for its completion, leading to a situation in which the cell cannot reverse course or complete division, rendering it non-functional and ready to trigger a programmed cell death response. In other words, neurons may have learned to translate stress signals into an irreversibly damaging incomplete cell cycle from which the cell has no choice but to trigger apoptosis. It is also noteworthy in this context that, despite the well-characterized presence of active apoptotic pathways in both in vitro and animal models of AD, the presence of classic apoptotic pathways in the human AD brain is not universally accepted [29]. Thus, it remains formally possible that the cell cycle-linked cell death response in AD, although well documented, may differ in nature from classic apoptosis pathways.
Additional support for this notion is provided by the demonstration of a direct causality link between overexpression of cell cycle mediators and neuronal death. Kranenburg et al showed that artificial elevation of cyclin D1 was sufficient to induce apoptosis and could be inhibited by the CDKI p16INK4 [30]. More recently, McShea et al. used adenoviral-mediated expression of c-myc and mutationally active ras oncogenes to force non-dividing cortical neurons into the cell cycle leading to their death [31]. Transgenic mouse models characterized by conditional expression of the simian virus 40 T antigen oncogene in postmitotic neurons clearly presented a neurodegenerative phenotype, consequence of forced cell cycle activation [32].
Nevertheless, even if cell cycle activation is a sine qua non for apoptosis in neurons, we still do not know whether the low constitutive levels of cell cycle proteins in neurons may exist to facilitate a fast response to stress or their presence simply reflects their role in unrelated functions.
Loss of neuronal cell cycle control in AD
If exposure to stress may trigger an abortive cell cycle in neurons, it is reasonable to ask whether such mechanism may exist in the AD brain, which is exposed to a wide range of stress stimuli. Substantial, although mostly descriptive, evidence suggests that this is indeed the case. Cyclins, CDKs and other cell cycle proteins are expressed in the AD brain [9,33-36]. In addition, Ranganathan et al. reported high levels of hyperphosphorylated Rb and observed altered subcellular distribution of E2F-1 to the cytoplasm [37] in brain and spinal cord tissues from Alzheimer's disease (AD). In another study, phosphorylated histone H3, a key component involved in chromosome compaction during cell division, was found increased in the cytoplasm of hippocampal neurons in AD, rather than within the nucleus as in actively dividing cells [38]. Cdk7, an activator of major cyclin-CDK complexes, constantly expressed during the cell cycle and indispensable for cell cycle progression, is also upregulated in susceptible hippocampal neurons of AD patients [39].
Further experiments from the Herrup's lab went further in their approach to the study of the neuronal cell cycle and, using fluorescent in situ hybridization, demonstrated that a significant fraction of the hippocampal pyramidal and basal forebrain neurons in AD have fully or partially replicated four separate genetic loci on three different chromosomes [40]. Mosch et al.[41] also quantified the DNA amount of identified cortical neurons in AD and reported a population of cyclin B1-positive tetraploid neurons that had entirely passed through a functional interphase with a complete DNA replication. These experiments are particularly important because, unlike evidence showing the presence of cell cycle markers in neurons, which could be dismissed as epiphenomena of suspect physiological relevance, they demonstrate that the DNA replication machinery is functional and capable of completing S phase in post-mitotic neurons.
Interestingly, CDK inhibitors p16INK4a, p15INK4b, p18INK4c and p19INK4d have also been found abnormally expressed in the temporal cortex and in pyramidal neurons of the hippocampus of AD patients [42-44]. An increase in the cytoplasmic levels of p27Kip1 was also identified in vulnerable neurons from individuals with histopathologically confirmed AD [45]. The signifycance of these findings is not immediately obvious. One could argue that expression of these inhibitors occurs as a defence mechanism against the untimely activation of cell cycle initiators. However, that would run counterintuitive to the notion that initiation of an abortive cell cycle is an adaptive response to stress. Clearly, much of the nature of cell cycle events in neurons, whether in response to stress situations or in basal conditions, is far from being understood.
Interestingly, although DNA replication and entry into S phase can be demonstrated to occur in dying neurons, progression through M phase has never been reported. Although the presence of binucleated neurons has been recently reported [46], no condensed chromosomes, formation of a mitotic spindle-like structure, or cytokinesis have ever been described, consistent with the idea that susceptible neurons may be arrested at the G2/M transition before they die. Therefore, activation of CDK1 at G2 might be a rate-limiting step before neurons undergo apoptosis. Indeed, activated CDK1 can phosphorylate and activate the pro-apoptotic BAD protein [47], thus providing a direct link between the cell cycle apparatus and the cell death machinery in neurons. It is also reasonable to suggest, in our opinion, that neuronal apoptosis at the G2 stage may simply be the result of permanent loss of ability to undergo chromosome segregation and cytokinesis due to a highly specialized cytoskeleton. In other words, cytoskeletal commitment to the plasticity of neuronal shape may come at the expense of its inability to dismantle dendrite and axonal structures to commit to mitotic spindle formation and cytokinesis. Indeed, the microtubule associated protein tau, which is phosphorylated during this phase of the cell cycle in a mitotic-competent cell, has also been consistently reported to be abnormally phosphorylated in AD and colocalizes with cell cycle regulators [32,33,45,48-50]. Moreover, tau can be phosphorylated by CDK1 [51] and CDK1-like protein [52,53]. Therefore, abnormally increased levels of tau phosphorylation could be explained in the context of an unsuccessful attempt to modulate G2 neuronal architecture and prepare it for mitosis, leading to programmed cell death.
Mechanisms of neuronal cell cycle reentry. Lessons from familial AD
Taken together, the available evidence pointing to a role for an abortive cell cycle in neurodegeneration in AD is reasonably strong. Nevertheless, the question remains: what mechanisms do neurons use to enter the cell cycle in the first place in response to a stress signal? If this is an adaptive response, there must be a well-defined molecular pathway that triggers an entry into an apoptotic cell cycle. Although nothing is known in this respect, some clues can be obtained from studies of familial AD (FAD) cases that, perhaps not surprisingly, also display cell cycle abnormalities [54-56].
Mutations in the genes for amyloid precursor protein (APP) and presenilins (PS1, PS2) associated to FAD lead in all cases to aberrant production of Aβ peptides [57], which in turn exacerbate cell cycle-related neuronal death [58-61]. In addition, increased Rb phosphorylation and E2F1 levels are measurable in areas surrounding a subset of Aβ-containing plaques [62]. Interestingly, Copani et al. reported that, unexpectedly, the reparative DNA polymerase β may act as a death signal when erratically expressed by differentiated neurons exposed to Aβ [63]. In short, exposure of post-mitotic neurons to the Aβ levels present in the AD brain may trigger a signalling pathway leading to the initiation of an abortive neuronal cell cycle.
Mutations in Presenilin 1 (PS1) account for the majority of all FAD cases, and one of its functions is precisely the APP γ-secretase-dependent cleavage responsible for Aβ generation. However, PS1 is a multifunctional protein and participates in many other signalling pathways, involving Notch, MEK/ERK, PI3K/Akt, β-catenin and others (reviewed in [64]). Relevant to the present discussion, PS1 is involved in β-catenin proteolysis, coupling its stepwise phosphorylation by PKA and GSK3-β prior to degradation [65-67]. Thus, in the absence of PS1 or in the presence of PS1 FAD mutations, this function is impaired and β-catenin is translocated to the nucleus, leading to hyperproliferation in mitotically competent cells [66-68], and tumorigenesis in peripheral tissue lacking PS1 [69]. Data from our lab points to a β-catenin-dependent aberrant cell cycle reactivation in cultured primary neurons from mice harbouring the knock-in PS1 mutation M146V (PS1 KIM146V), as determined by increased BrdU incorporation. This accelerated entry into the cell cycle appeared to be abortive, initiating an apoptotic response. Furthermore, treatment with quercetin, a disruptor of the β-catenin/TCF transcription complex, reduced cyclin D1 levels and reversed the cell cycle/cell death phenotype, consistent with a role for β-catenin in this cell cycle-driven apoptosis [70]. Thus, it is possible that the elevated levels of β-catenin that are present in the PS1 FAD brain accelerate cell cycle entry simply by upregulating cyclin D1 transcription. In further support of this notion, we found that levels of cyclin D1 are elevated in the hippocampus of PS1 FAD patients when compared to sporadic AD patients and non-demented controls (Currais, Hortobagyi and Soriano, unpublished results).
Recently, Repetto et al. demonstrated a critical role for PS1 in the trafficking and turnover of the epidermal growth factor receptor (EGFR), a key signaling receptor tyrosine kinase [71]. As with β-catenin, mutations that enhance EGFR expression can serve as oncogenic signals that promote hyperplasia and neoplastic transformation in human tissues, including skin. EGFR is important for development of the nervous system and maintenance of neural stem cells growth and differentiation. However, excess of EGF induces neuronal death, and strong EGFR immunoreactivity has been detected in neurites surrounding neuritic plaques in AD. Thus, the authors hypothesize that activation of EGFR and β-catenin pathways by the loss of PS1 can mutually reinforce each other and may contribute to neurodegeneration and aberrant cell cycle re-entry by stabilizing both EGFR and β-catenin while simultaneously driving Aβ42 deposition (discussed in [71]).
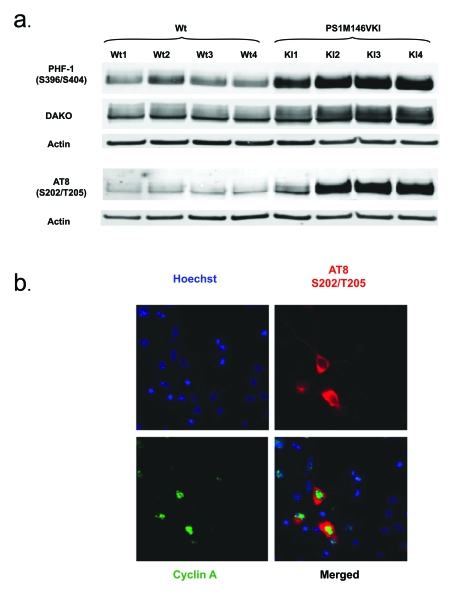
Interestingly, and consistent with the notion that a highly specialized cytoskeleton may be the origin of cell cycle-driven apoptosis by simply preventing a cycling neuron from undergoing chromosome segregation and cytokinesis, we have found profound abnormalities in tau homeostasis in our PS1 FAD mouse model. Specifically, tau is hyperphosphorylated in mitotic epitopes in these mice (Figure 2a) and, perhaps more importantly, nuclear expression of cyclin A appears to correlate with the tau phosphorylation at S202/T205 (Figure 2b).
Figure 2.
(a) Tau accumulates and is hyperphosphorylated at S202/T205 and S396/S404 in primary neurons from PS1 M146V mice compared to wild-type controls. Shown is a Western blot analysis of Triton X-100 soluble lysates. Antibodies used were AT8 (phosphorylated S202/T205), PHF-1 (phosphorylated S396/S404) and DAKO (total tau); (b) Tau phosphorylation at S202/T205 is detectable exclusively in neurons expressing cyclin A, highlighting the importance of tau phosphorylation dynamics in the neuronal cell cycle.
In summary, although the molecular events in a neuron converting a stress stimulus into a signal to enter an abortive cell cycle remain unknown, results from experiments using PS1 FAD models point to the accidental triggering of oncogenic pathways (i.e. aberrant expression of cyclin D1 and EGFR). In that context, tau hyperphosphorylation could be interpreted as a by-product of the attempt by the affected neuron to achieve a mitosis-ready configuration. If this is representative of what occurs in the more widespread non-familial AD cases, we would favour the hypothesis that, rather than an abortive cell cycle being an early event in a regulated cell death response to stress, upregulation of cell cycle proteins in the AD brain may simply reflect the activation of oncogenic pathways that cannot be translated into cell division because of impaired cytoskeletal dynamics, rendering the cell dysfunctional and ready to be eliminated by apoptosis. In further support of this notion, work from the Smith lab has shown that forcing post-mitotic neurons to re-enter the cell cycle through the expression of MYC results in tau changes similar to those seen in AD neurons. More importantly, MYC expression in forebrain neurons of a transgenic model results in cell death and cognitive deficits [31,72]
Concluding remarks
After differentiation, neurons become post-mitotic, acquiring a structural and functional plasticity at the apparent expense of a permanent exit from the cell cycle. Therefore, the expression of cell cycle markers in the adult brain has always been a subject of controversial debate. Clearly, although neurons are terminally differentiated cells, they do express a wide range of cell cycle proteins and are known to be capable of replicating their DNA, although no cases of a neuronal cell division have ever been reported. This, together with the finding that the expression of cell cycle proteins is necessary to execute apoptosis in response to certain stress signals, has led to the proposition that a neuronal cell cycle does exist and is part of a well-regulated response to stress signals. Whether this interpretation is correct will probably depend on the nature of the initial signal triggering a neuron into the cell cycle in the first place. The fact that cell cycle proteins in neurons are capable of performing non-cell cycle functions and that, at least in PS1-associated FAD, oncogenic signals are readily generated, argue, in our opinion, for a neuronal cell cycle being no different from other oncogenic signals in proliferative cells. The reason for the absence of neuronal division and, indeed, tumors of neuronal origin, would simply reflect the impossibility of a fully mature neuronal cytoskeleton to revert to a mitosis-ready configuration. Clearly, more research is needed before we can begin to understand the physiological and pathogenic implications of a neuronal cell cycle.
Footnotes
The authors of this manuscript have no conflict of interests to declare.
References
- 1.Herrup K, Busser JC. The induction of multiple cell cycle events precedes target-related neuronal death. Development, 1995;121:2385–2395. doi: 10.1242/dev.121.8.2385. [DOI] [PubMed] [Google Scholar]
- 2.Park DS. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci. 1997;17:8975–8983. doi: 10.1523/JNEUROSCI.17-23-08975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park DS. Cyclin-dependent kinases participate in death of neurons evoked by DNA-damaging agents. J Cell Biol. 1998;143:457–467. doi: 10.1083/jcb.143.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park DS. Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress. J Neurosci. 1998;18:830–840. doi: 10.1523/JNEUROSCI.18-03-00830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirvan A. Expression of cell cycle-related genes during neuronal apoptosis: is there a distinct pattern. Neurochem Res. 1998;23:767–777. doi: 10.1023/a:1022415611545. [DOI] [PubMed] [Google Scholar]
- 6.Sakai K. Up-regulation of cyclin D1 occurs in apoptosis of immature but not mature cerebellar granule neurons in culture. J Neurosci Res. 1999;58:396–406. [PubMed] [Google Scholar]
- 7.Kruman II. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 8.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagy Z. Cell cycle markers in the hippocampus in Alzheimer's disease. Acta Neuropathol (Berl) 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- 10.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer's disease. J Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 12.Arendt T, Synaptic plasticity, cell cycle activation in neurons are alternative effector pathways: the 'Dr. Jekyll and Mr. Hyde concept' of Alzheimer's disease or the yin and yang of neuroplasticity. Prog Neurobiol. 2003;71(2-3):83–248. doi: 10.1016/j.pneurobio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci. 2007;8:438–450. doi: 10.1038/nrn2097. [DOI] [PubMed] [Google Scholar]
- 14.Tamaru T. Identification of cells expressing a D type G1 cyclin in matured brain: implication for its role in neuronal function. Neurosci Lett. 1993;153:169–172. doi: 10.1016/0304-3940(93)90314-b. [DOI] [PubMed] [Google Scholar]
- 15.Sumrejkanchanakij P. Prevention of cyclin D1 nuclear localization in terminally differentiated neurons. Kokubyo Gakkai Zasshi. 2003;70:131–139. doi: 10.5357/koubyou.70.131. [DOI] [PubMed] [Google Scholar]
- 16.Sumrejkanchanakij P. Role of cyclin D1 cytoplasmic sequestration in the survival of postmitotic neurons. Oncogene. 2003;22:8723–8730. doi: 10.1038/sj.onc.1206870. [DOI] [PubMed] [Google Scholar]
- 17.Kranenburg O. Inhibition of cyclin-dependent kinase activity triggers neuronal differentiation of mouse neuroblastoma cells. J Cell Biol. 1995;131:227–234. doi: 10.1083/jcb.131.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumrejkanchanakij P, Eto K, Ikeda MA. Cytoplasmic sequestration of cyclin D1 associated with cell cycle withdrawal of neuroblastoma cells. Biochem Biophys Res Commun. 2006;340:302–308. doi: 10.1016/j.bbrc.2005.11.181. [DOI] [PubMed] [Google Scholar]
- 19.Miyajima M, Nornes HO, Neuman T. Cyclin E is expressed in neurons and forms complexes with cdk5. Neuroreport. 1995;6:1130–1132. doi: 10.1097/00001756-199505300-00014. [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga Y. Expression of cyclin E in postmitotic cells in the central nervous system. Kokubyo Gakkai Zasshi. 2000;67:169–181. doi: 10.5357/koubyou.67.169. [DOI] [PubMed] [Google Scholar]
- 21.Schmetsdorf S, Gartner U, Arendt T. Constitutive expression of functionally active cyclin-dependent kinases and their binding partners suggests noncanonical functions of cell cycle regulators in differentiated neurons. Cereb Cortex. 2007;17:1821–1829. doi: 10.1093/cercor/bhl091. [DOI] [PubMed] [Google Scholar]
- 22.Richter JD. Think globally, translate locally: what mitotic spindles and neuronal synapses have in common. Proc Natl Acad Sci U S A. 2001;98:7069–7071. doi: 10.1073/pnas.111146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowser R, Smith MA. Cell cycle proteins in Alzheimer's disease: plenty of wheels but no cycle. J Alzheimers Dis. 2002;4:249–254. doi: 10.3233/jad-2002-4316. [DOI] [PubMed] [Google Scholar]
- 24.Ross ME, Risken M. MN20, a D2 cyclin found in brain, is implicated in neural differentiation. J Neurosci. 1994;14(11 Pt 1):6384–6391. doi: 10.1523/JNEUROSCI.14-11-06384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross ME, Carter ML, Lee JH. MN20, a D2 cyclin, is transiently expressed in selected neural populations during embryogenesis. J Neurosci. 1996;16:210–9. doi: 10.1523/JNEUROSCI.16-01-00210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gieffers C. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci U S A. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi Y. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science. 2004;303:1026–1030. doi: 10.1126/science.1093712. [DOI] [PubMed] [Google Scholar]
- 28.Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- 29.Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12(6A):2263–2280. doi: 10.1111/j.1582-4934.2008.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kranenburg O, van der Eb AJ, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. Embo J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 31.McShea A. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta. 2007;1772:467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Park KH. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci. 2007;27:2969–2978. doi: 10.1523/JNEUROSCI.0186-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent I. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci. 1997;17:3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith MZ, Nagy Z, Esiri MM. Cell cycle-related protein expression in vascular dementia and Alzheimer's disease. Neurosci Lett. 1999;271:45–48. doi: 10.1016/s0304-3940(99)00509-1. [DOI] [PubMed] [Google Scholar]
- 35.Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998;18:2801–2807. doi: 10.1523/JNEUROSCI.18-08-02801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranganathan S, Scudiere S, Bowser R. Hyperphosphorylation of the retinoblastoma gene product and altered subcellular distribution of E2F-1 during Alzheimer's disease and amyotrophic lateral sclerosis. J Alzheimers Dis. 2001;3:377–385. doi: 10.3233/jad-2001-3403. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa O. Ectopic localization of phosphorylated histone H3 in Alzheimer's disease: a mitotic catastrophe. Acta Neuropathol (Berl) 2003;105:524–528. doi: 10.1007/s00401-003-0684-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X. Neuronal CDK7 in hippocampus is related to aging and Alzheimer disease. Neurobiol Aging. 2000;21:807–813. doi: 10.1016/s0197-4580(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosch B. Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci. 2007;27:6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arendt T. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer's disease. Neuroreport. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- 43.McShea A. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer's disease. Am J Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 44.Arendt T, Holzer M, Gartner U. Neuronal expression of cycline dependent kinase inhibitors of the INK4 family in Alzheimer's disease. J Neural Transm. 1998;105(8-9):949–960. doi: 10.1007/s007020050104. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa O. Increased p27, an essential component of cell cycle control, in Alzheimer's disease. Aging Cell. 2003;2:105–110. doi: 10.1046/j.1474-9728.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X. Neuronal binucleation in Alzheimer disease hippocampus. Neuropathol Appl Neurobiol. 2008;34:457–465. doi: 10.1111/j.1365-2990.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 47.Konishi Y. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell. 2002;9:1005–1016. doi: 10.1016/s1097-2765(02)00524-5. [DOI] [PubMed] [Google Scholar]
- 48.Dranovsky A. Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer's disease pathology. Neurobiol Aging. 2001;22:517–528. doi: 10.1016/s0197-4580(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhu X. Elevated expression of a regulator of the G2/M phase of the cell cycle, neuronal CIP-1-associated regulator of cyclin B, in Alzheimer's disease. J Neurosci Res. 2004;75:698–703. doi: 10.1002/jnr.20028. [DOI] [PubMed] [Google Scholar]
- 50.Hernandez-Ortega K, Ferrera P, Arias C. Sequential expression of cell-cycle regulators and Alzheimer's disease-related proteins in entorhinal cortex after hippocampal excitotoxic damage. J Neurosci Res. 2007;85:1744–1751. doi: 10.1002/jnr.21301. [DOI] [PubMed] [Google Scholar]
- 51.Bennecib M. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 52.Paudel H. K. Phosphorylation by neuronal cdc2-like protein kinase promotes dimerization of Tau protein in vitro. J Biol Chem. 1997;272:28328–28334. doi: 10.1074/jbc.272.45.28328. [DOI] [PubMed] [Google Scholar]
- 53.Sobue K. Interaction of neuronal Cdc2-like protein kinase with microtubule-associated protein tau. J Biol Chem. 2000;275:16673–16680. doi: 10.1074/jbc.M000784200. [DOI] [PubMed] [Google Scholar]
- 54.Tatebayashi Y. Cell-cycle-dependent abnormal calcium response in fibroblasts from patients with familial Alzheimer's disease. Dementia. 1995;6:9–16. doi: 10.1159/000106916. [DOI] [PubMed] [Google Scholar]
- 55.Janicki SM, Stabler SM, Monteiro MJ. Familial Alzheimer's disease presenilin-1 mutants potentiate cell cycle arrest. Neurobiol Aging. 2000;21:829–836. doi: 10.1016/s0197-4580(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oakley H. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Copani A. Mitotic signaling by beta-amyloid causes neuronal death. Faseb J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- 59.Copani A. DNA polymerase-beta is expressed early in neurons of Alzheimer's disease brain and is loaded into DNA replication forks in neurons challenged with beta-amyloid. J Neurosci. 2006;26:10949–10957. doi: 10.1523/JNEUROSCI.2793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biswas SC. Bim is elevated in Alzheimer's disease neurons and is required for beta-amyloid-induced neuronal apoptosis. J Neurosci. 2007;27:893–900. doi: 10.1523/JNEUROSCI.3524-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malik B, Currais A, Soriano S. Cell cycle-driven neuronal apoptosis specifically linked to amyloid peptide Abeta(1-42) exposure is not exacerbated in a mouse model of presenilin-1 familial Alzheimer's disease. J Neurochem. 2008; 2008;106:912–916. doi: 10.1111/j.1471-4159.2008.05446.x. [DOI] [PubMed] [Google Scholar]
- 62.Jordan-Sciutto KL, Malaiyandi LM, Bowser R. Altered distribution of cell cycle transcriptional regulators during Alzheimer disease. J Neuropathol Exp Neurol. 2002;61:358–367. doi: 10.1093/jnen/61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Copani A. Erratic expression of DNA polymerases by beta-amyloid causes neuronal death. Faseb J. 2002;16:2006–2008. doi: 10.1096/fj.02-0422fje. [DOI] [PubMed] [Google Scholar]
- 64.Venezia V. Amyloid precursor protein and presenilin involvement in cell signaling. Neurodegener Dis. 2007;4(2-3):101–111. doi: 10.1159/000101834. [DOI] [PubMed] [Google Scholar]
- 65.Kang DE. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19:4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soriano S. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152:785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang DE. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- 68.Chevallier NL. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia X. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10863–10868. doi: 10.1073/pnas.191284198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malik B. Loss of neuronal cell cycle control as a mechanism of neurodegeneration in the presenilin-1 Alzheimer's disease brain. Cell Cycle. 2008;7:637–646. doi: 10.4161/cc.7.5.5427. [DOI] [PubMed] [Google Scholar]
- 71.Repetto E. Presenilin-1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J Biol Chem. 2007;282:31504–31516. doi: 10.1074/jbc.M704273200. [DOI] [PubMed] [Google Scholar]
- 72.Lee HG. The neuronal expression of MYC causes a neurodegenerative phenotype in a novel transgenic mouse. Am J Pathol. 2009;174:891–897. doi: 10.2353/ajpath.2009.080583. [DOI] [PMC free article] [PubMed] [Google Scholar]