Abstract
Oxygen metabolism is thought to impact on aging through the formation of reactive oxygen species (ROS) that are supposed to damage biological molecules. The study of p66Shc, a crucial regulator of ROS level involved in aging dysfunction, suggests that the incidence of degenerative disease and longevity are determined by a specific signaling function of ROS other than their unspecific damaging property.
Keywords: Aging, Life span, degenerative disease, oxidative stress
What we can learn from longevity mutants
The reason why we age seems obvious: entropy increases. The reason why different species are differently affected by passing of equal time should be apparent as well: genetic and epigenetic variability. Organism modification with time has been mainly explained by the production of free radicals as well as by immunological theories of aging. However, what we still miss is a list of genes responsible for aging; the study of these genes would tell us what aging is.
Senectus ipsa morbus est (Old age is in itself a disease), ancient romans said. However, the incidence of disease decreases in the extreme elderly, when aging expression reaches its maximum, whereas progeric syndromes associate to disease. Therefore, it is not clear whether aging itself is a disease and how it would impact on life span in a protected environment.
Our contribution to this field arises from the study of p66Shc, the first protein identified whose deletion in mouse prolongs life span and protects from a variety of aging-associated diseases without showing apparent negative effects.
P66Shc is a redox signaller P66Shc is a vertebrate protein. It is present in Xenophus, Botia Dario and mammals, while it is absent in Saccaromyces, Drosophila or Caenorhabditis [1]. P66Shc is one of three isoforms encoded by the ShcA locus [2].
The other two isoforms, p46Shc and p52Shc, with molecular weights of 46 and 52 KDa respectively, were first described as ‘adaptor' proteins that specifically bind to phosphorylated tyrosines on the cytoplasmic motif of growth factor receptors. Upon growth factor stimulation, p52Shc/p46Shc proteins are rapidly and efficiently tyrosine-phosphorylated by all the tyrosine kinase receptors tested in three major tyrosine residues, and recruit the Grb2-Sos complex on the plasma membrane [3]. In turn SOS, through its GEF activity, stimulates the conversion of the inactive Ras GDP into an active Ras GTP that subsequently activates the mitogen-activated protein kinase (MAPK) cascade. Recruitment of the Grb2/Sos complex by p52Shc/p46Shc and membrane relocalization of Sos are events considered sufficient to induce Ras activation [3]. The hypothesis that Shc proteins are involved in the regulation of Ras is further supported by the finding that over-expression of p52Shc/p46Shc increases proliferative response and enhances MAP kinase and Fos activation upon stimulation with EGF, GM-CSF and PDGF [2,4,5]. Notably, the shortest isoforms of Shc appeared early in evolution since their orthologues have been found in flies and nematods [1].
At molecular level, p66Shc, p52Shc and p46Shc largely share the same amino acid sequence at the C-terminus including the Src homologous type two domain (SH2), phosphotyrosine binding domain (PTB) responsible for the binding to phosphorylated tyrosine, and a region highly enriched in glycine and proline residues named collagen homologous (CH1) since its homology with collagen protein [6]. The peculiarity of p66Shc is an additional CH region (CH2) at its N-terminus [2,4].
Despite the high similarity p66Shc functionally differentiates from the other ShcA isoforms. There is no indication that p66Shc activates the Ras signaling pathway. Indeed, evidence for divergent regulation of p66Shc versus p52Shc/p46Shc immediately emerged from studies demonstrating that although p66Shc, like p52Shc /p46Shc, is a target of receptor tyrosine kinases (EGFR, INSR, PDGFR) and binds the Grb2/SOS complex [4,7,8], p66Shc over-expression, unlike that of p52Shc/p46Shc, has a negative effect on the Ras-MAPK-Fos pathway in response to EGF or cytokines in lymphocytes [4,9]. In fact, p66Shc has been shown to exert an inhibitory effect on the Erk pathway, which is necessary for coordinated actin cytoskeleton polymerization [10], and normal IGF-1 responsiveness of the MEK/ERK pathway in myoblasts [11]. How p66Shc exerts this negative effect is not clear. It was proposed that it acts by competing with p52Shc for Grb2 binding, sequestering the Grb2/Sos complex and therefore terminating Ras signaling [11].
Finally, studies on p66Shc knock down did not demonstrated any role for p66Shc in growth factor response or Ras signaling whereas they revealed an unexpected function of p66Shc in regulating intracellular redox balance and oxidative stress levels [12]. Indeed, compared to WT, the amount of reactive oxygen species (ROS) was shown to be decreased in p66Shc- depleted cultivated cells, as revealed by the reduced oxidation of ROS sensitive probes as well as by the reduced accumulation of endogenous markers of oxidative stress [9,13-17]. Likewise, p66Shc-/- mice show diminished levels of both systemic (isoprostane) and intracellular (nytrotyrosines, 8-oxo-dG) oxidative stress [14,18,19].
Mechanisms of p66 Shc - redox activity regulation
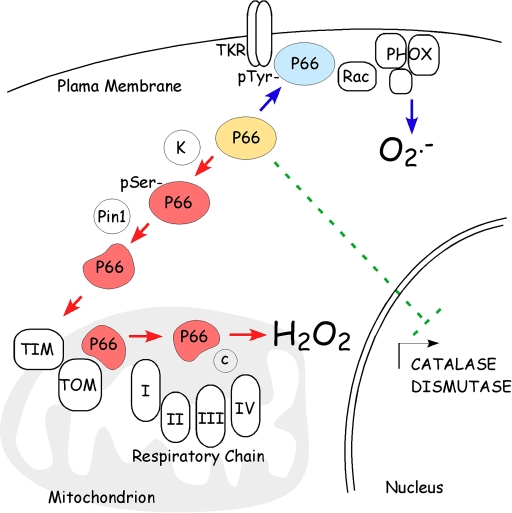
Basically, intracellular ROS levels can be increased by three main mechanisms: reducing ROS scavenging, increasing membrane oxidases activity, or by mitochondrial respiratory chain leakage. P66Shc has been reported to act through all of them. In fact, p66Shc silencing by RNAi or gene targeting deletion was found to increase levels of superoxide dismutases and catalases in a variety of cells. In particular, p66Shc appeared to decrease the expression of ROS scavenging enzymes through the inhibition of FOXO transcription factors [13] (Figure 1). In addition, p66Shc has been proposed to mask the growth factor receptor bound protein Grb2 from Sos1, favoring the rac1-specific GEF activity of Sos1, rac1 activation and triggering of NADPH membrane oxidase ROS production [20] (Figure 1).
Figure 1. P66 Shc controls intracellular ROS metabolism.
at multiple sites. P66Shc (in blue) stimulates ROS production by plasma membrane oxidases through the association with membrane receptor and Rac activation of phagocitic oxidases. Upon phosphorylation and consequent Pin-1-mediated conformational changes, p66Shc (in red) translocates, through the TIM/TOM mitochondrial import machinery, within the mitochondrial inter-membrane space where it oxidizes reduced cytochrome c and catalyzes the partial reduction of O2 to H2O2. Then, p66Shc decreases the expression of ROS scavenging enzymes.
Finally, a fraction of p66Shc has been observed within the mitochondrial inter-membrane space (IMS) [16]. Notably, electrochemical experiments demonstrated that the amino terminal portion of p66Shc contains a redox active region able to mediate electron transfer from reduced cytochrome c to molecular oxygen, thus producing hydrogen peroxide (Figure 1).
As reported, all proteins of the mitochondrial inter-membrane space are synthesized in the cytosol and are then imported into the mitochondria [21]. Most of them do not contain any cleavable sequences and are targeted to IMS by as yet unidentified import signals.
The import of p66shc into mitochondrial IMS is not still understood at a mechanistic level. However, a mechanism that depends on p66Shc post-translational modifications, including serine phosphorylation by stress kinases like Jnk-1 and Pkc-B and prolilisomerization by Pin-1, has been described, which allows p66Shc increase within the mitochondria during apoptosis [22]. A second level of activation of p66Shc mitochondrial function is represented by the effective amount of p66Shc within mitochondrial vesicles. In fact, mitochondrial p66Shc has been observed to associate to a high molecular weight complex of about 670 KDa and to the mitochondrial chaperon mtHsp70 [23]. Notably, treatment of cells with pro-apoptotic stimuli such as UVC or H2O2 induces the dissociation of this complex and the consequent release of monomeric p66Shc,which is then free to react with cytochrome c [23].
Interestingly, p66Shc half-life increases upon apoptotic stimulation in a p53-dependent way, thus linking the pro-apoptotic activity of p66Shc to the p53 pathway [14].
Function of p66 Shc - oxidative signal
Regardless of how p66Shc may shift the intracellular redox balance towards oxidation, it appears that p66Shc specifically evolved to increase intracellular ROS levels. In this view, different functions have been assigned to p66Shc- produced ROS. Initially it was reported that H2O2 produced by p66Shc within the mitochondria induces the opening of the mitochondrial permeability transition pore leading to swelling of the organelle [16]. The consequent rupture of mitochondrial integrity then triggers the release of various proapoptotic mitochondrial factors, including cytochrome c, into the cytosol, where they activate the apoptotic cascade leading to cell death [23]. Indeed, p66Shc-/- cells have been demonstrated to be resistant to apoptosis induced by a variety of different signals, including ultraviolet radiation, staurosporine, growth factor deprivation, calcium ionophore, CD3-CD4 cross-linking and taxol [9,12,23]. Likewise, p66Shc-/- mice were found resistant to apoptosis induced by paraquat, hypercholesterolemia, ischemia, angiotensin II, carbon tetrachloride and ethanol [12,15,16,18]. Notably, p66Shc deletion in mice was shown to improve resistance to hyperglycaemic damage in diabetic model of nephropathy and cardiovascular diseases due the reduction of apoptosis and cell loss [24,25].
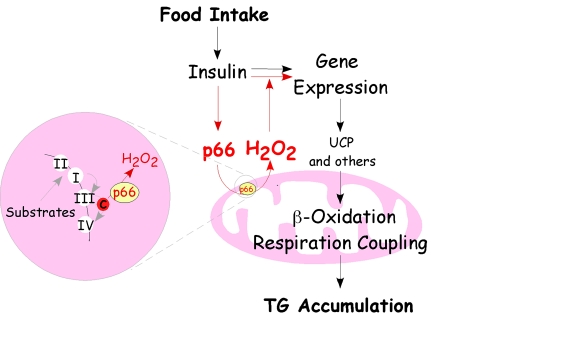
Recently, another role for p66Shc - mediated ROS has been described in the regulation of adipogenesis. In adipocytes, p66Shc was demonstrated to be involved in insulin-induced gene expression regulation and triglyceride accumulation. In fat cells insulin induces serine 36 specific phosphorylation of p66Shc thus stimulating p66Shc ROS production, which, in turn, potentiates insulin transduction signaling. Indeed, mutants unable to translocate to the mitochondria and to produce H2O2 do not sustain insulin-dependent signaling and triglyceride accumulation when reintroduced in p66Shc-/- cells [17]. Interestingly, some phosphatases inhibiting insulin signaling (e.g. PTEN) are inactivated by oxidation [26]. Thus, it appears that p66Shc-generated ROS play a crucial role in regulating insulin signaling and fat development, likely through the modulation of these redox-sensitive phosphatases. Indeed, p66Shc-/- mice are protected from diet-induced obesity, suggesting that this molecular pathway regulates diet-associated fat development [17]. But if p66Shc is able to convert signals from the diet into variations of the intracellular redox balance, affecting insulin sensitivity, critically, the process that triggers adipogenesis following food intake should stem from the integration of both intracellular (mitochondrial ROS production) and extracellular (circulating insulin) signals [17] (Figure 2).
Figure 2. Regulatory circuit of p66 Shc-mediated fat development.
The scheme recapitulates the pathway of p66Shc that drives mitochondrial H2O2 and its relationship with insulin receptor signaling leading to fat accumulation. Food intake determines energetic substrate availability and insulin stimulates intracellular transduction pathways that regulate gene transcription in order to favor triglyceride accumulation. P66Shc-mediated ROS production is directly boosted by insulin and in turn potentiates insulin receptor signaling, suppresses the expression of uncoupling proteins and beta oxidation enzymes leading to triglyceride accumulation.
Notably, p66 Shc - produced H2O2 might control intracellular signaling events also in tissues other than fat. In particular, the response of myocytes and endothelial cells to glycaemia and ischemia, as well as the renewal control of breast stem cells upon hypoxia, has been linked to p66Shc- redox activity [24,27-29].
Therefore, p66Shc behaves like an atypical signal transducer that tunes membrane receptor signaling or intracellular glucose/oxygen sensing via the regulation of intracellular re-dox balance.
P66Shc impacts on overall energy metabolism and aging
Was the p66Shc gene conserved during mammals development, in spite of its deleterious effects on life-span and disease, because of p66Shc- mediated ROS signaling function in fat tissues? P66Shc-/- mice have reduced body weight, due to reduced fat mass of both white and brown adipose tissues [17]. This leanness is not explainable by changes in food intake, intestinal absorption of nutrients or locomotor activity. Rather, it may reflect defective lipogenesis in adipocytes, as suggested by the reduced lipid accumulation of p66Shc-/- adipocytes transplanted into WT recipient mice [17]. However, this interpretation of the mechanisms leading to decreased fat mass in p66Shc-/- mice poses the question of how energy balance is maintained in the absence of p66Shc, and why energy storage is reduced. As p66Shc-/- mice showed increased basal body temperature and increased basal metabolic rate, this suggests that increased uncoupled respiration in the fat mitochondria of p66Shc-/- mice leads to increased energy expenditure, which contributes to resistance to body weight gain [17].
Fat has a crucial role in the thermoregulation of mammals. It protects from body heat loss (thermoinsulation) and generates heat for the maintenance of body temperature when animals are exposed to cold (thermo-genesis). Notably, p66Shc-/- mice were found to be more sensitive to cold due to the reduced thermal insulation effect of fat pads [17]. Therefore, adaptation to cold as well as optimization of energy storage when food is available, both altered in the lean p66Shc-/- mice, have been proposed as possible evolutionary functions whose fitness pressure preserves the p66Shc gene in mammals.
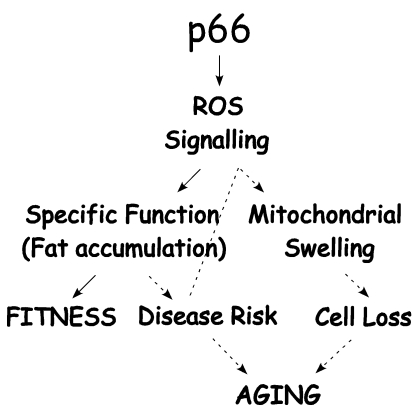
These findings of reduced adiposity in p66Shc-/- mice might have important implications for the effect of p66Shc on lifespan. Aging is associated with a pathological trait, often associated with obesity (metabolic syndrome), which predisposes to diabetes and cardiovascular diseases [30-34]. In humans, these diseases strongly affect morbidity and mortality, especially among the elderly [30,35]. Oxidative stress has been implicated in a number of chronic disease states usually grouped under the umbrella of the metabolic syndrome [36-42], and it is thought to contribute to the aging process [43]. It has been hypothesized that the production of free radicals is dependent on metabolic rate [44], and that this may have an impact on the aging process. In p66Shc-/- mice, like in caloric restriction and FIRKO mice, fat deposits are moderately decreased [17,45], suggesting that reduced oxidative stress in p66Shc-/- mice might increase longevity through the direct effect of reduced adiposity. Notably, p66Shc-/- mice are more resistant to diabetes and have reduced risk of atherosclerosis and cardiovascular damage upon HF-diet [18,25]. Therefore, the effect of p66Shc on aging might be considered a sort of chronic decay like the metabolic syndrome progression, although the contribution of the metabolic syndrome to life span is still not clear (Figure 3).
Figure 3. P66 Shc/ROS signaling determines fitness and aging associated dysfunctions.
P66Shc/ROS signals to specific functions that improve fitness whilst these same functions may increase disease risk chronically (such as obesity related disorders) and contribute to trigger p66Shc-mediated cell death. Then, increased disease risk and cell loss rate contribute to aging dysfunctions.
The life-prolonging action of caloric restriction (CR) offers an excellent chance for investigating the connection between stress and aging. The anti-aging action of CR can be viewed as "nutritional stress," because the organism's reduced caloric intake seems to be a stimulatory metabolic response for survival. Thus, as an omnipotent intervention, CR provides a unique opportunity to probe the organism's ability to withstand age-related stress as a survival strategy. Recent geriatric research has provided sufficient experimental data supporting the anti-aging property of CR [46-48].
What kills mammals is a "p66 Shc syndrome"
Finally, the study of p66Shc confirms that very close links exist between energetic metabolism, oxidative stress and aging. P66Shc represents a clear example of an antagonistic pleiotropic function, which generates both beneficial and detrimental phenomena in an organism.
Darwin might say that aging expresses fitness (senectus robur est), at least as much as one is able to face illness. However, it remains unclear whether aging is also a disease or whether life span is regulated by energetic metabolism disorders that could eventually result in lethal effects or sub-pathological multiple dysfunctions.
In a series of WT and p66Shc-/- very old moribund mice, significant recurring cause of death were not identified. Indeed, it is known that in mice as in humans even accurate autopsy might often remain "blank", in the absence of masses, haemorrhages, abscesses or other evident septic conditions. Mice presented only sporadic terminal emphysema (mainly in WT mice), occasional lymphocytic pneumonia and very rare malignant tumors. On the other hand, it is impossible to rule out other causes of death, such as cardiac fibrillation or acute myocardial infarction, which score negative for morphological investigation (unpublished data).
In conclusion, whether aging determines life span through diseases or through the acceleration of a fatal physiological decline remains puzzling. It is expected that further, more intense investigations in the cause of death in mammals might contribute to the solution.
P66Shc story suggests that necessary regulators of oxygen and energetic metabolism may be involved both in the onset of the acute phase of diseases and in the induction of aging related detrimental changes that ultimately kill the organism.
Acknowledgments
We thank Paola Dalton for the preparation of the manuscript. This work was supported by National Institute of Health Grant 1P01AG025532-01A1.
References
- 1.Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Curr Opin Genet Dev. 2000;10:668–674. doi: 10.1016/s0959-437x(00)00146-5. [DOI] [PubMed] [Google Scholar]
- 2.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Grignani F, Pawson T, Pelicci PG. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 3.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, Pawson T, Di Fiore PP, Lanfrancone L, Pelicci PG. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. Embo J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanfrancone L, Pelicci G, Brizzi MF, Aronica MG, Casciari C, Giuli S, Pegoraro L, Pawson T, Pelicci PG, Arouica MG. Over-expression of Shc proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SoS and Grb2/p140 complexes to the beta receptor subunit. Oncogene. 1995;10:907–917. [PubMed] [Google Scholar]
- 6.Pelicci G, Dente L, De Giuseppe A, Verducci-Galletti B, Giuli S, Mele S, Vetriani C, Giorgio M, Pandolfi PP, Cesareni G, Pelicci PG. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene. 1996;13:633–41. [PubMed] [Google Scholar]
- 7.Yokote K, Mori S, Hansen K, McGlade J, Pawson T, Heldin CH, Claesson-Welsh L. Direct interaction between Shc and the platelet-derived growth factor beta-receptor. J Biol Chem. 1994;269:15337–15343. [PubMed] [Google Scholar]
- 8.Skolnik EY, Lee CH, Batzer A, Vicentini LM, Zhou M, Daly R, Myers MJ Jr, Backer JM, Ullrich A, White MF. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. Embo J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacini S, Pellegrini M, Migliaccio E, Patrussi L, Ulivieri C, Ventura A, Carraro F, Naldini A, Lanfrancone L, Pelicci P, Baldari CT. p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Mol Cell Biol. 2004;24:1747–1757. doi: 10.1128/MCB.24.4.1747-1757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natalicchio A, Laviola L, De Tullio C, Renna LA, Montrone C, Perrini S, Valenti G, Procino G, Svelto M, Giorgino F. Role of the p66Shc isoform in insulin-like growth factor I receptor signaling through MEK/Erk and regulation of actin cytoskeleton in rat myoblasts. J Biol Chem. 2004;279:43900–43909. doi: 10.1074/jbc.M403936200. [DOI] [PubMed] [Google Scholar]
- 11.Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22:2162–2175. doi: 10.1210/me.2008-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 13.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 14.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 15.Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, Biglioli P, Giorgio M, Martin-Padura I, Pelicci PG, Capogrossi MC. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 16.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species thattrigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Berniakovich I, Trinei M, Stendardo M, Migliaccio E, Minucci S, Bernardi P, Pelicci PG, Giorgio M. p66Shc-generated oxidative signal promotes fat accumulation. J Biol Chem. 2008;283:34283–34293. doi: 10.1074/jbc.M804362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 20.Khanday FA, Yamamori T, Mattagajasingh I, Zhang Z, Bugayenko A, Naqvi A, Santhanam L, Nabi N, Kasuno K, Day BW, Irani K. Rac1 leads to phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress. Mol Biol Cell. 2006;17:122–129. doi: 10.1091/mbc.E05-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann JM, Neupert W. Protein transport into mitochondria. Curr Opin Microbiol. 2000;3:210–214. doi: 10.1016/s1369-5274(00)00077-1. [DOI] [PubMed] [Google Scholar]
- 22.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 23.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 24.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, Tanner FC, Pelicci P, Volpe M, Anversa P, Luscher TF, Cosentino F. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci U S A. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G. Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes. 2006;55:1642–1650. doi: 10.2337/db05-1477. [DOI] [PubMed] [Google Scholar]
- 26.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 27.Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 28.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi G, Di Giulio C, Rapino C, Rapino M, Antonucci A, Cataldi A. p53 and p66 proteins compete for hypoxia-inducible factor 1 alpha stabilization in young and old rat hearts exposed to intermittent hypoxia. Gerontology. 2006;52:17–23. doi: 10.1159/000089821. [DOI] [PubMed] [Google Scholar]
- 30.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 31.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 32.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 35.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 36.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 37.Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF, Schiffrin EL. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40:866–871. doi: 10.1161/01.hyp.0000037969.41360.cc. [DOI] [PubMed] [Google Scholar]
- 38.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 39.Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. 2008;46:499–505. doi: 10.1515/CCLM.2008.096. [DOI] [PubMed] [Google Scholar]
- 40.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuzawa-Nagata N, Takamura T, Ando H, Nakamura S, Kurita S, Misu H, Ota T, Yokoyama M, Honda M, Miyamoto K, Kaneko S. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57:1071–1077. doi: 10.1016/j.metabol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Kumashiro N, Tamura Y, Uchida T, Ogihara T, Fujitani Y, Hirose T, Mochizuki H, Kawamori R, Watada H. Impact of oxidative stress and peroxisome proliferator-activated receptor gamma coactivator-1alpha in hepatic insulin resistance. Diabetes. 2008;57:2083–2091. doi: 10.2337/db08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harman D. The Free Radical Theory of Aging: Effect of Age on Serum Copper Levels. J Gerontol. 1965;20:151–3. doi: 10.1093/geronj/20.2.151. [DOI] [PubMed] [Google Scholar]
- 44.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact. Antioxid Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 45.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 46.Sohal RS,Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masoro EJ. Caloric restriction. Aging. 1998;10:173–174. [PubMed] [Google Scholar]
- 48.Bodkin NL, Ortmeyer HK, Hansen BC. Long-term dietary restriction in older-aged rhesus monkeys: effects on insulin resistance. J Gerontol A Biol Sci Med Sci. 1995;50:B142–147. doi: 10.1093/gerona/50a.3.b142. [DOI] [PubMed] [Google Scholar]