Abstract
Chemokines and chemokine receptors play an important role in immune homeostasis and surveillance. Altered or defective expression of chemokines and/or chemokine receptors could lead to a disease state including autoimmune disorder or cancer. Tumors from glioblastoma, melanoma, and neuroblastoma secrete high levels of chemokines that can promote tumor growth and progression or induce stromal cells present in the tumor microenvironment to produce cytokines or chemokines which, in turn, can regulate angiogenesis, tumor growth, and metastasis. On the other hand, chemokines secreted by tumor or stromal cells can also attract leukocytes such as dendritic cells, macrophages, neutrophils, and lymphocytes which may downmodulate tumor growth. New therapies that are aimed at limiting tumor growth and progression by attracting immune effector cells to the tumor site with chemokines may hold the key to the successful treatment of cancer, although this approach may be hampered by possible tumor growth-stimulating effects of chemokines.
Keywords: Chemokines, chemokine receptors, leukocyte migration, stroma, tumor cells
1. Introduction
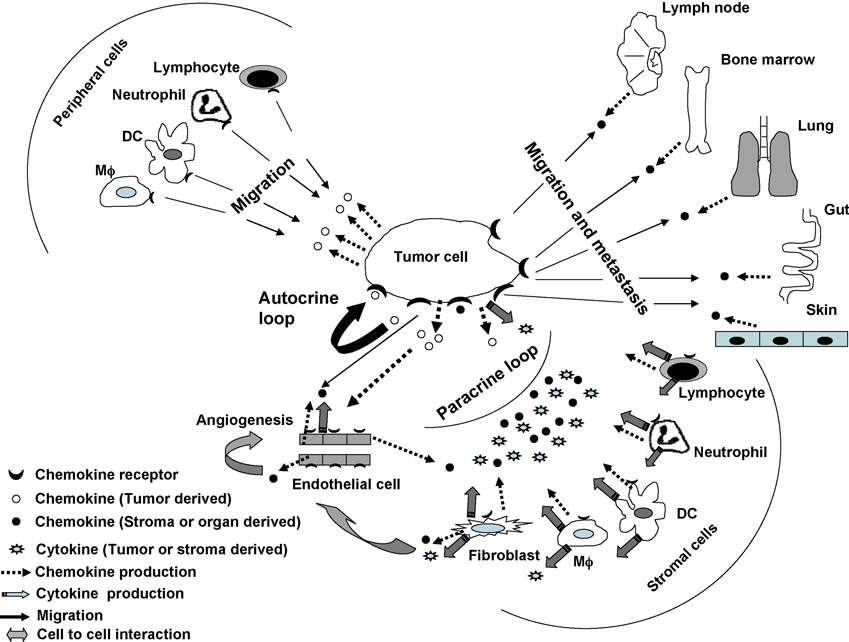
Chemokines are a subset of cytokines comprised of small peptides (8–11kDa) with four conserved cysteine residues [1, 2]. They are classified into four sub-groups (C, CC, CXC and CX3C) based on the location of the first two cysteine residues and bind to specific G-protein coupled cell surface receptors [1]. Binding of a chemokine to its receptor leads to a wide array of functional signals that includes development of lymphocytes (T and B cells), recruitment and migration of leukocytes, and control of infection, inflammation, autocrine tumor growth stimulation, angiogenesis and metastasis (Fig. 1, reviewed in [1–4]). Recruitment and localization of leukocytes are the key elements of immune surveillance. Leukocytes primarily depend on the expression of integrins, chemokine and chemokine receptors for transmigration through endothelial cell venules to lymphoid organs or sites of inflammation or infection [3, 5]. Chemokines are known to modulate expression of integrins which in turn facilitates transmigration of leukocytes. Type and level of chemokine receptor expression depend on the activation and differentiation state of each leukocyte cell type. Dendritic cells (DCs), Langerhans cells, monocytes/macrophages (Mϕ), neutrophils and lymphocytes all express chemokine receptors (Fig. 1). Immature DCs express CCR1, CCR5, CCR6, CCR9 and CXCR4 and mature DCs express CCR7, CCR9 and CXCR4 (reviewed in [2]). Naïve lymphocytes generally express CCR7, CCR9 and CXCR4, and activated lymphocytes CXCR3 [2]. Mature DCs and naïve lymphocytes expressing CCR7 are attracted towards lymph nodes that are known to be a rich source of the chemokine CCL21 [2, 6]. T helper (Th) type 1 cells express primarily CXCR3, and Th2 and regulatory T (Treg) cells express CCR4 among other chemokine receptors [5, 6]. Any defect in the expression of chemokines and/or their receptors results in immune imbalance which could give rise to a disease state that includes autoimmune disorder and cancer.
Fig. 1. Role of chemokines and chemokine receptors in tumor microenvironment.
Chemokines secreted by tumor cells can induce autocrine tumor growth stimulation by binding to chemokine receptors on tumor cells, induce angiogenesis by activating endothelial cells, or attract leukocytes such as dendritic cells (DC), lymphocytes, macrophages (Mϕ) and neutrophils from the periphery to the tumor site [1–4]. Stromal cells within the tumor, including fibroblasts, DC, lymphocytes, Mϕ and neutrophils, may be activated by tumor cells through cell-cell interactions (not shown) or cytokines or chemokines produced by tumor cells [7, 15, 19, 20, 23, 43]. The activated stromal cells may then secrete cytokines (VEGF, TNF-α, TGF-β, IL-1, IL-10) or chemokines (CCL2, CXCL8, CXCL12) that can directly or indirectly promote tumor growth (paracrine tumor growth stimulation), angiogenesis, and metastasis [14–16, 23]. Cytokines produced by stromal cells can act on tumor cells directly to promote growth or indirectly by inducing tumor cells to secrete chemokines, such as CXCL8 or VEGF [16, 23]. CXCL8 can bind to tumor cells to promote autocrine tumor growth stimulation, and VEGF can induce tumors to produce CXCL12 and upregulate CXCR4 promoting autocrine tumor growth stimulation and metastasis. Fibroblast-derived VEGF or CXCL12 can also act on endothelial cells to promote angiogenesis, or CXCL12 can bind to tumor cells directly supporting tumor growth [16, 23]. Thus, tumor cell growth stimulation can occur through autocrine and/or paracrine loops. Chemokine receptor-positive tumor cells can migrate toward stromal derived chemokines produced in distant organs (bone marrow, gut, lung, lymph node and skin) resulting in disease progression and metastasis [1–4].
2. Role of chemokines and chemokine receptors in neuroectodermal tumor-host interaction
a. Factors produced by tumor and stromal cells
Stromal cells which are mainly comprised of fibroblasts, pericytes, macrophages, neutrophils, eosinophils, mast cells, lymphocytes, dendritic cells and endothelial cells, form part of the tumor microenvironment which supports and regulates growth of tumor cells (Fig. 1; [7–11]). Interactions between tumor and stromal cells can occur via cell-cell interactions or by cytokine- or chemokine-mediated signaling [7–12]. Tumor cells can influence stromal cells such as fibroblasts and macrophages present in the tumor microenvironment to produce growth factors such as vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, cytokines such as interleukin (IL)-1, or chemokines such as CCL2, CXCL8 or CXCL12 which in turn can directly or indirectly promote angiogenesis, tumor growth and metastasis (Fig. 1; [8, 10, 12, 13]). Alternatively, stromal cells are also known to stimulate tumor cells to produce chemokines that can influence angiogenesis, tumor growth and metastasis [7, 10, 12–24].
b. Autocrine and Paracrine tumor growth stimulation
The expression of certain chemokines such as CCL5, CXCL1, CXCL3, CXCL8, CXCL10, CXCL12 and the corresponding chemokine receptors on tumor cells, including malignant melanomas, meningiomas, neuroblastomas and glioblastomas, is associated with autocrine or paracrine tumor growth stimulation (Fig. 1 and Table 1; [14, 25–31]). Autocrine tumor growth stimulation was demonstrated either in vivo by injecting human tumor cells into immunodeficient mice (nude or severe combined immunodeficient [SCID mice]) [30, 32, 33] or in in vitro proliferation assays, and was correlated with the amount of chemokine secreted by the tumor cells [25, 27–29, 34, 35]. Thus, melanoma cells secreting high amounts of CCL5 showed greater tumor formation in nude mice compared to cells secreting low amounts of the chemokine [33]. In a SCID mouse model, melanoma cells formed tumors following transfection with CXCL1-3 or CXCL8 [30, 32]. In both studies, the presence of CXCR2 on the tumor cells was responsible for autocrine tumor growth stimulation, and in vivo tumor formation in mice was inhibited by the use of anti-CXCL1-3 or anti-CXCL8 antibodies. In in vitro studies, the exogenous addition of CXCL10 or CXCL12 enhanced the proliferation of glioma, meningioma or neuroblastoma cells, and the use of anti-sense CXCL8 or antibodies to CXCL8 or CXCL12 or CXCR3 (chemokine receptor for CXCL10) inhibited tumor growth and proliferation [25, 27–29, 35–37].
Table 1.
Involvement of tumor host-derived chemokines and their receptors in tumor metastasis and/or disease progression
| Tumor type | Chemokine | Chemokine origin |
Chemokine receptor |
Involvement in | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Metastasis (site) |
Tumor progression |
Angiogenesis | Malignant transformation |
|||||
| Melanoma | CCL2 | Tumor, fibroblasts |
NT | NT | Yes | Yes | Yes | [15, 18] |
| Melanoma | CCL5 | Tumor | NT | NT | Yes | NT | NT | [33] |
| Melanoma | CCL21 | Tumor, endothelial cells |
CCR7 | Yes (LN) |
NT | NT | NT | [20, 22] |
| Melanoma | CXCL8 | Tumor, endothelial cells, fibroblasts, monocytes |
CXCR1, CXCR2 |
Yes (LN) |
Yes | Yes | Yes | [11, 19, 29, 30, 47] |
| Melanoma | CXCL1-3 | Tumor, fibroblasts |
CXCR2 | NT | Yes | Yes | Yes | [14, 39] |
| Glioblastoma | CXCL10 | Tumor | CXCR3 | NT | Yes | NT | NT | [27] |
| Melanoma, Glioblastoma |
CXCL12 | Tumor, astrocytes, fibroblasts, micro glial cells |
CXCR4 | Yes (Liver, Lungs) |
Yes | Yes | NT | [7, 10, 34, 35, 38] |
LN: lymph node; NT: not tested.
Paracrine tumor growth stimulation of melanoma, glioblastoma and astrocytoma cells is generally due to the secretion of chemokines such as CXCL1-3, CXCL8 and CXCL12 by tumor activated fibroblasts, infiltrating macrophages or microglial cells (Table 1; [7, 10, 14, 38]). The activation of fibroblasts by tumor cells has been demonstrated in vitro by gene expression analysis of cells grown in co-culture and further confirmed by immunohistological analysis of tumor biopsy specimens [14]. In melanoma, paracrine tumor growth stimulation can also occur indirectly through secretion of cytokines such as TNF-α and IL-1 by infiltrating macrophages that can act on tumor cells to produce CXCL8 and VEGF [23]. CXCL8 can directly stimulate autocrine tumor growth, whereas VEGF is known to act on tumor cells to produce CXCL12 and upregulate CXCR4 expression resulting in tumor growth stimulation and metastasis [16, 23]. CCR2+ microglial cells may migrate toward glioma and astrocytoma cells and induce the tumor cells to produce IL-10 that in turn causes autocrine tumor growth stimulation [10, 38].
c. Neoplastic transformation
Chemokines such as CCL2, CXCL1-3 and CXCL8 play a role in neoplastic transformation of normal human melanocytes or non-tumorigenic melanoma cells [18, 30, 39]. In these studies, cells transfected with CCL2, CXCL1-3 or CXCL8 formed tumors when transplanted into SCID or nude mice. The role of chemokines in neoplastic transformation was confirmed by the use of antibodies to chemokines (anti-CCL2, anti-CXCL1-3 or anti-CXCL8) which inhibited tumor formation in mice.
d. Angiogenesis
Chemokines secreted by tumor cells are indirectly involved in angiogenesis by activating stromal cells to produce CCL2 or CXCL8 that can act on endothelial cells to indirectly promote angiogenesis (reviewed in [1, 2, 4, 40]; Fig. 1; Table 1). Interaction between tumor cells and fibroblasts in angiogenesis was demonstrated in vitro in 3-dimensional organotypic cultures [15]. Fibroblasts grown in the presence of tumor cells showed enhanced production of CCL2 and CXCL8 which stimulated endothelial cells in the organotypic culture. In another study, the indirect role of infiltrating macrophages in promoting angiogenesis has been demonstrated [23]. Tumor activated macrophages secreted TNF-α and IL-1 α which in turn induced VEGF production by the tumor cells resulting in angiogenesis. Neutralization of cytokines by the addition of anti-TNF-α or anti-IL-1α antibodies resulted in the blockade of VEGF production [23].
e. Tumor metastasis
Expression of chemokine receptors on tumor cells is associated with disease progression and organ specific metastasis (Fig. 1; [26, 36, 37, 40–42]). Many organs such as bone marrow, liver, lung, lymph node and small intestine are rich sources of CCL21, CCL25 and CXCL12 and hence, they can attract chemokine receptor-positive tumor cells (reviewed in [1, 2, 4]). Stromal cells such as endothelial cells, macrophages, microglial cells and fibroblasts present in the tumor environment can play a direct or indirect role in metastasis (Fig. 1; Table 1; [7, 8, 11, 17, 38, 43]). Endothelial cells grown in the presence of melanoma and glioma cells showed increased production of CCL21 and CXCL8 that resulted in transmigration of chemokine receptor positive tumor cells towards lymphatic vessels [7, 19, 20, 43]. Use of anti-CXCL8 or anti-CXCR1 antibody in vitro effectively blocked migration of tumor cells towards endothelial cells in transmigration experiments [19, 20]. These observations were further confirmed in a mouse tumor model [20]. Moreover, stromal cells can also indirectly facilitate tumor migration by stimulating tumor cells to express chemokine receptors or produce chemokines such as CCL2 or CXCL8, or growth factors such as VEGF resulting in tumor progression and angiogenesis [11, 19, 24]. In addition, infiltrating stromal cells can stimulate tumor cells to produce matrix metalloproteinase which in turn induces matrix degradation facilitating tumor escape [7, 8, 11, 17].
f. Leukocyte migration
Melanoma, neuroblastoma and glioblastoma cells produce CCL2, CCL5, CCL19, CXCL8, CXCL10, CXCL12 and CX3CL chemokines (Table 2; [33, 44–56]). These chemokines attract a large number of chemokine receptor-positive leukocytes to the tumor site, including T (Th and CTL) cells, natural killer-like T (NKT) cells, Tregs, DCs, Mϕ and neutrophils (Fig. 1; [26, 41, 57]). Infiltration of leukocytes into the tumor site might result in tumor cell apoptosis or enhancement of tumor cell growth [18, 45, 46, 54–56, 58]. Nesbit et al., [18] have compared the tumorigenicity of human melanoma cells producing low or high amounts of CCL2. Tumors producing high amounts of CCL2 attracted macrophages inhibiting tumor growth; however, tumors producing low amounts of the chemokine promoted tumor growth by stimulating angiogenesis. Using an organotypic melanoma/skin reconstruct model, we have shown that melanoma-reactive CTL (CCR2+, CCR4+ or CXCR4+) can migrate through a layer of collagen and fibroblasts into a layer of tumor cells and that migration was mediated by CCL2 or CXCL12 [55, 56, 58]. Using the same model, we have also shown that migration of Th cells toward melanoma cells was mediated by CXCR3 and CXCL10 (Somasundaram et al., unpublished observations). Thus, chemokines produced by tumor cells can attract a wide variety of leukocytes which may have a positive or negative impact on tumor growth. Chemokines produced by tumor cells or infiltrating stromal cells can also downmodulate immune responses resulting in tumor escape from immune surveillance (reviewed in [10, 12, 21, 57]).
Table 2.
Involvement of chemokines expressed by tumor cells and chemokine receptors expressed by leukocytes in leukocyte migration into tumors
| Tumor type | Chemokine | Chemokine receptor |
Effector cell | Reference |
|---|---|---|---|---|
| Melanoma, neuroblastoma, glioblastoma |
CCL2 | CCR2, CCR4 |
CTL, NKT, Treg, Mϕ |
Somasundaram, Gross et al. unpublished observations, [44, 45, 49, 50, 55, 60] |
| Melanoma | CCL5 | NT | DC, T, Mϕ | [33] |
| Glioblastoma | CCL19 | NT | CD4+, CD8+T | [46] |
| Neuroblastoma | CXCL8 | NT | Lymphocytes, neutrophils |
[53] |
| Melanoma | CXCL9, CXCL10 |
NT | Mϕ, T | [48] |
| Melanoma | CXCL10 | CXCR3 | Th | Somasundaram et al. unpublished |
| Melanoma | CXCL12 | CXCR4 | CTL | [56, 58] |
| Glioblastoma | CXCL12 | CXCR4 | Mϕ | [52] |
| Neuroblastoma | CX3CL | CX3CR1 | T, NK | [54] |
CTL: cytotoxic T lymphocyte; DC: dendritic cell; Mϕ: macrophage; NK: natural killer cell; NKT: natural killer like T cell; Th: helper T cell; Treg: regulatory T cell; NT: not tested.
g. Immunoregulation
The presence of Treg cells at the tumor site can downmodulate the immune response and provide an immune escape mechanism for the tumor. Glioblastoma, gastric carcinoma and Hodgkin lymphoma cells attract Treg cells by secreting CCL2, CCL17 or CCL22 chemokines [59–61]. Immunohistological staining of tumor infiltrating lymphocytes or analysis of peripheral blood lymphocytes from patients with these tumors indicated elevated levels of CCR4+, FoxP3+, CD4+CD25+ T cells in the vicinity of the tumor or the peripheral blood when compared to healthy controls. Subsequent analysis of the tumor cells from these patients revealed high expression of the chemokines CCL2, CCL17 and CCL22 that are known to attract CCR4+ Treg cells. Chemoattraction of CCR4+ Treg cells by tumor-derived chemokines was further confirmed in an in vitro migration assay in which lymphocyte migration toward tumor cell supernatant was blocked by anti-chemokine antibodies. Selective Treg cell infiltration of progressive melanomas may have been mediated by chemokines as analysis of tumor ascites fluid indicated the presence of CCL15, CCL18 and CXCL10 chemokines [62]. However, it is unclear from the above study whether macrophages that were also shown to infiltrate the tumors played an indirect role in the recruitment of Treg cells.
3. Conclusions
Chemokine receptors on tumor cells are important mediators of metastasis. Expression of both chemokines and their receptors by tumors may lead to autocrine tumor growth stimulation or expression of cytokines or chemokines by stromal cells which can influence paracrine tumor growth stimulation and metastasis. Chemokine secretion by tumors can also affect tumor growth and metastasis by recruitment of leukocytes such as macrophages, dendritic cells, T and B cells, into the tumor bed and/or promotion of angiogenesis. Although it is well known that tumors use a host of factors other than chemokines to regulate immune responses resulting in tumor growth stimulation or inhibition, selective modulation of chemokine activity at the tumor site to shift the balance in favor of a sustained growth-inhibitory immune response could lead to successful tumor regression and elimination. Approaches to gene therapy with chemokines or administration of antibody-chemokine fusion proteins, using chemokines selected for their potential to attract effector leukocytes to the tumor site, may provide novel cancer therapies that could also be used in combination with active specific immunotherapy with cancer vaccines. Thus, in vivo gene therapy of experimental mouse tumors with CCL5 or CCL21 genes or administration of tumor-specific antibody-chemokine fusion proteins (CCL5-anti-Her2/neu, CCL16-anti-chTNT-3, CXCL9-anti-KSA, CCL7 or CXCL10-anti-lymphoma Ig variable region) are exciting examples of novel cancer therapies [63–67]. The efficacy of these treatments in cancer patients has yet to be determined. However, one has to keep in mind that enhancing the chemokine concentration at the tumor site may exert tumor growth-stimulating, rather than - inhibiting effects.
Acknowledgements
We thank Laura Gross and Marion Sacks for editorial assistance. These studies were supported by NIH grants CA25874, CA93372, CA10815, and CA114046.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- 1.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 2.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 4.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 5.Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–189. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- 6.D'Ambrosio D. Regulatory T cells: how do they find their space in the immunological arena? Semin Cancer Biol. 2006;16:91–97. doi: 10.1016/j.semcancer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 8.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Karnoub AE, Weinberg RA. Chemokine networks and breast cancer metastasis. Breast Dis. 2006;26:75–85. doi: 10.3233/bd-2007-26107. [DOI] [PubMed] [Google Scholar]
- 10.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 12.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 13.Varney ML, Olsen KJ, Mosley RL, Singh RK. Paracrine regulation of vascular endothelial growth factor--a expression during macrophage-melanoma cell interaction: role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J Interferon Cytokine Res. 2005;25:674–683. doi: 10.1089/jir.2005.25.674. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher PG, et al. Gene expression profiling reveals cross-talk between melanoma and fibroblasts: implications for host-tumor interactions in metastasis. Cancer Res. 2005;65:4134–4146. doi: 10.1158/0008-5472.CAN-04-0415. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LJ, Chen H, Bauer RJ, Bauer SM, Velazquez OC. Normal human fibroblasts enable melanoma cells to induce angiogenesis in type I collagen. Surgery. 2005;138:439–449. doi: 10.1016/j.surg.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Hong X, et al. SDF-1 and CXCR4 are up-regulated by VEGF and contribute to glioma cell invasion. Cancer Lett. 2006;236:39–45. doi: 10.1016/j.canlet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Labrousse AL, Ntayi C, Hornebeck W, Bernard P. Stromal reaction in cutaneous melanoma. Crit Rev Oncol Hematol. 2004;49:269–275. doi: 10.1016/j.critrevonc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. 2001;166:6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 19.Ramjeesingh R, Leung R, Siu CH. Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1. FASEB J. 2003;17:1292–1294. doi: 10.1096/fj.02-0560fje. [DOI] [PubMed] [Google Scholar]
- 20.Shields JD, et al. Chemokine-mediated migration of melanoma cells towards lymphatics--a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 21.Shurin MR, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 22.Streit M, Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003;22:3172–3179. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]
- 23.Torisu H, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- 24.Zhang L, Yeger H, Das B, Irwin MS, Baruchel S. Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9:36–46. doi: 10.1593/neo.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajetto A, et al. CXCR4 and SDF1 expression in human meningiomas: a proliferative role in tumoral meningothelial cells in vitro. Neuro Oncol. 2007;9:3–11. doi: 10.1215/15228517-2006-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyman O, Krieg C. The role of chemokines in cancer immune surveillance by the adaptive immune system. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.011. this issue. [DOI] [PubMed] [Google Scholar]
- 27.Maru SV, et al. Chemokine production and chemokine receptor expression by human glioma cells: Role of CXCL10 in tumour cell proliferation. J Neuroimmunol. 2008;199:35–45. doi: 10.1016/j.jneuroim.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 28.Meier R, et al. The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS ONE. 2007;2:e1016. doi: 10.1371/journal.pone.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–2675. [PubMed] [Google Scholar]
- 30.Schaider H, et al. Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer. 2003;103:335–343. doi: 10.1002/ijc.10775. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 32.Haghnegahdar H, et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mrowietz U, et al. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br J Cancer. 1999;79:1025–1031. doi: 10.1038/sj.bjc.6690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbero S, et al. Expression of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1 in human brain tumors and their involvement in glial proliferation in vitro. Ann N Y Acad Sci. 2002;973:60–69. doi: 10.1111/j.1749-6632.2002.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 35.Sehgal A, Keener C, Boynton AL, Warrick J, Murphy GP. CXCR-4, a chemokine receptor, is overexpressed in and required for proliferation of glioblastoma tumor cells. J Surg Oncol. 1998;69:99–104. doi: 10.1002/(sici)1096-9098(199810)69:2<99::aid-jso10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Gross N, Meier R. Chemokines in neuroectodermal cancers: The crucial growth signal from the soil. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.009. this issue. [DOI] [PubMed] [Google Scholar]
- 37.Rubin J. Chemokine Signaling in Cancer: One Hump or Two? Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.001. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40:252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 39.Luan J, et al. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- 40.Fujii N, Shim H, Oishi S. Chemokine receptor CXCR4 as a therapeutic target for neuroectodermal tumors. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.11.004. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pistoia V, Raffaghello L, Cocco C, Corrias MV, Airoldi I. Chemokines in neuroectodermal tumour progression and metastasis. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.003. this issue. [DOI] [PubMed] [Google Scholar]
- 42.Sommer L, Civenni G. Chemokines in neuroectodermal development and cancer stem cells. Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.11.003. this issue. [DOI] [PubMed] [Google Scholar]
- 43.Strieter RM, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 44.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 45.Graves DT, Barnhill R, Galanopoulos T, Antoniades HN. Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am J Pathol. 1992;140:9–14. [PMC free article] [PubMed] [Google Scholar]
- 46.Kimura T, et al. Expression of lymphocyte-specific chemokines in human malignant glioma: Essential role of LARC in cellular immunity of malignant glioma. Int J Oncol. 2002;21:707–715. doi: 10.3892/ijo.21.4.707. [DOI] [PubMed] [Google Scholar]
- 47.Kunz M, et al. Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma. A potential mechanism for high tumor aggressiveness. Am J Pathol. 1999;155:753–763. doi: 10.1016/S0002-9440(10)65174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 49.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–527. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 50.Metelitsa LS, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rempel SA, Dudas S, Ge S, Gutierrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 52.Salmaggi A, et al. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol. 2004;67:305–317. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- 53.Yang KD, Shaio MF, Wang CL, Wu NC, Stone RM. Neuroblastoma cell-mediated leukocyte chemotaxis: lineage-specific differentiation of interleukin-8 expression. Exp Cell Res. 1994;211:1–5. doi: 10.1006/excr.1994.1050. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Y, et al. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Cancer Res. 2007;67:2331–2338. doi: 10.1158/0008-5472.CAN-06-3041. [DOI] [PubMed] [Google Scholar]
- 55.Zhang T, et al. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur J Immunol. 2006;36:457–467. doi: 10.1002/eji.200526208. [DOI] [PubMed] [Google Scholar]
- 56.Zhang T, et al. CXC chemokine ligand 12 (stromal cell-derived factor 1 alpha) and CXCR4-dependent migration of CTLs toward melanoma cells in organotypic culture. J Immunol. 2005;174:5856–5863. doi: 10.4049/jimmunol.174.9.5856. [DOI] [PubMed] [Google Scholar]
- 57.Conrad C, Navarini-Meury AA. Melanoma and innate immunity - active inflammation or just erroneous attraction? (Melanoma as the source of leukocyte-attracting chemokines) Semin Cancer Biol. 2008 doi: 10.1016/j.semcancer.2008.10.012. this issue. [DOI] [PubMed] [Google Scholar]
- 58.Zhang T, et al. Preferential involvement of CX chemokine receptor 4 and CX chemokine ligand 12 in T-cell migration toward melanoma cells. Cancer Biol Ther. 2006;5:1304–1312. doi: 10.4161/cbt.5.10.3153. [DOI] [PubMed] [Google Scholar]
- 59.Ishida T, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 60.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizukami Y, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–2293. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 62.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- 64.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kutubuddin M, et al. Eradication of pre-established lymphoma using herpes simplex virus amplicon vectors. Blood. 1999;93:643–654. [PubMed] [Google Scholar]
- 66.Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384–8392. [PubMed] [Google Scholar]
- 67.Ruehlmann JM, et al. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res. 2001;61:8498–8503. [PubMed] [Google Scholar]